Abstract
Seasonal variation in the rates of infection with certain Gram-negative organisms has been previously examined in tertiary-care centers. We performed a population-based investigation to evaluate the seasonal variation in Escherichia coli bloodstream infection (BSI). We identified 461 unique patients in Olmsted County, Minnesota, from 1 January 1998 to 31 December 2007, with E. coli BSI. Incidence rates (IR) and IR ratios (IRR) were calculated using Rochester Epidemiology Project tools. Multivariable Poisson regression was used to examine the association between the IR of E. coli BSI and average temperature. The age- and gender-adjusted IR of E. coli BSI per 100,000 person-years was 50.2 (95 % confidence interval [CI]: 42.9-57.5) during the warmest 4 months (June through September) compared to 37.1 (95% CI: 32.7-41.5) during the remainder of the year, resulting in a 35% (95% CI: 12-66%) increase in IR during the warmest four months. The average temperature was predictive of increasing IR of E. coli BSI (p=0.004); there was a 7% (95% CI: 2-12%) increase in the IR for each 10-degree Fahrenheit (c. 5.5 °C) increase in average temperature. To our knowledge, this is the first study to demonstrate seasonal variation in E. coli BSI, with a higher IR during the warmest four months than during the remainder of the year.
Keywords: bacteraemia, epidemiology, seasonal variation, incidence, Escherichia coli
INTRODUCTION
Seasonal variation in rates of infection with certain Gram-negative organisms including Acinetobacter species [1], Aeromonas hydrophila [2], and Burkholderia pseudomallei [3] has been previously described. A more recent study described seasonal variation in the incidence rates (IR) of infections due to Acinetobacter baumannii, Enterobacter cloacae, Escherichia coli, and Pseudomonas aeruginosa in hospitalized patients [4]. In addition, seasonal variation in bloodstream infection (BSI) due to Acinetobacter spp. [1] and Klebsiella pneumoniae [5] has been reported in studies from tertiary care centers.
To our knowledge, seasonal variation in E. coli BSI has not been previously described; therefore, we performed a population-based investigation to examine the association between seasonal variation and the IR of E. coli BSI. The primary aims of the study were two-fold: (i) to compare the IR of E. coli BSI in the population of Olmsted County, Minnesota, between the warmest four months of the year (June through September) to that during the remainder of the year, and (ii) to examine the association between IR and average temperature.
METHODS
Setting
Olmsted County is located in southeastern Minnesota with a population of 124,277 according to the 2000 census (US Census Bureau, Olmsted County QuickFacts [http://quickfacts.census.gov], accessed April 21, 2008). With the exception of a lower prevalence of injection drug use, a higher prevalence of middle-class individuals, and a higher proportion being employed in the health care industry, the population characteristics of Olmsted County residents are similar to those of US non-Hispanic whites [6, 7]. The Rochester Epidemiology Project (REP) is a unique medical records-linkage system that encompasses care delivered to residents of Rochester and Olmsted County, Minnesota. The microbiology laboratories at Mayo Medical Center and Olmsted Medical Center are the only two laboratories in Olmsted County. These two medical centers are geographically isolated from other urban centers as previously described [6, 8, 9]; therefore, local residents are able to obtain health care within the community rather than seeking health care at a distant geographic location.
Case Ascertainment
We used complete enumeration of the Olmsted County, Minnesota, population from 1 January 1998 through 31 December 2007. Using the microbiology databases at the Mayo Medical Center Rochester, and Olmsted Medical Center, we identified 461 unique patients with first episodes of monomicrobial E. coli BSI. Medical records were reviewed by the primary investigator (M.N.A.) to confirm the diagnosis, determine patient residency status, and obtain baseline clinical features. Patients with E. coli BSI who were living outside Olmsted County at the time of diagnosis were excluded. Blood cultures were performed using standard microbiology techniques according to CLSI. Both laboratories are certified by the College of American Pathologists. The study was approved by the institutional review boards of both institutions. The detailed case ascertainment and blood culture methods used were described previously [9, 10].
Case definition
Monomicrobial BSI was defined as growth of only one organism in a blood culture, excluding coagulase-negative staphylococci, Corynebacterium spp., and Propionibacterium spp. Cases were classified according to the site of acquisition into nosocomial, health care-associated and community-acquired, as previously defined [11]. The primary source of BSI was defined using the Centers for Disease Control and Prevention criteria [12].
Statistical Analysis
The IR, expressed as the number of new cases of BSI per 100,000 person-years, was calculated assuming that the entire population of Olmsted County was at risk of BSI. The 2000 Olmsted County census figures were used to compute the age-, gender- and calendar year-specific person-year denominator with a projected population growth rate after 2000 of 1.9% per year. The 2005 census figures for Olmsted County confirmed this annual projected growth rate as an accurate estimate. The 10-year study period was categorized into five two-year intervals (1998-1999, 2000-2001, 2002-2003, 2004-2005, and 2006-2007) and age was categorized into five groups (0-18, 19-39, 40-59, 60-79, and ≥ 80 years). The IR was directly adjusted to the US 2000 white population. The 95% confidence intervals (CI) for the IR were estimated using a Poisson distribution.
To evaluate the association between seasonal variation and IR of E. coli BSI, the IR for the four warmest months (June through September) and the IR for the eight remaining months were each calculated; the person-year denominator was multiplied by 1/3 and 2/3, respectively. The incidence rate ratio (IRR) is the ratio of the IR for the four warmest months relative to the IR for the remaining eight months. The 95% CI for the IRR were constructed using the bootstrap method.
Multivariable Poisson regression was used to evaluate the association between IR of E. coli BSI and average monthly temperatures for Rochester, Minnesota, adjusting for gender, age, and calendar year. Average monthly temperatures were obtained from historic city records (Weatherbase Historical Weather for Rochester, Minnesota, USA (http://www.weatherbase.com), accessed 24 July 2008). The IR was calculated for each of the 12 months assuming a fixed population within a given year. The SAS procedure GENMOD (version 8, SAS Institute Inc, Cary, NC) was used for all Poisson regression analyses. The two-sided level of significance for statistical testing was defined as p<0.05.
RESULTS
The median age of patients with E. coli BSI was 69 (Interquartile range: 50-81) years and 66% were female. Most cases were community-acquired (59%); the remaining 9% and 32% were nosocomial and health care-associated, respectively. The urinary tract was the most common primary source of infection (80%), followed by the gastrointestinal tract (9%), and the respiratory tract (4%). Seven percent of patients with E. coli BSI had an unknown primary source of infection.
The overall age- and gender-adjusted IR of E. coli BSI was 41.4 per 100,000 person-years (95% CI: 37.6-45.3; Table 1). The IR increased at a quadratic rate with age (p<0.001) and was higher in females than in males; the age-adjusted IR was 48.0 per 100,000 person-years (95% CI: 42.5-53.4) for females and 34.1 per 100,000 person-years (95% CI: 28.6-39.6) for males. There was no change in IR detected over calendar years 1998 to 2007 (p=0.487 for linear trend).
Table 1.
Incidence rates of Escherichia coli bloodstream infection by age group and gender, 1998-2007.
| Gender | Age group (years) |
||||||
|---|---|---|---|---|---|---|---|
| 0-18 | 19-39 | 40-59 | 60-79 | ≥ 80 | Age-adjusted* | Age- and gender-adjusted* |
|
| Female | 12 (6.7) | 50 (25.1) | 55 (31.1) | 94 (120.1) | 95 (329.1) | 48.0 (42.5-53.4) | |
| Male | 7 (3.7) | 10 (5.1) | 26 (15.5) | 78 (116.4) | 34 (263.6) | 34.1 (28.6-39.6) | |
| Overall | 19 (5.1) | 60 (15.3) | 81 (23.5) | 172 (118.4) | 129 (308.9) | 41.6 (37.8-45.4) | 41.4 (37.6-45.3) |
NOTE. Data are given as counts (incidence rates per 100,000 person-years), unless otherwise indicated.
Incidence rates (95% confidence intervals) are adjusted for US white 2000 census. There is a quadratic increase in the incidence rate of E. coli bloodstream infection with increasing age (p<0.001).
Figure 1 displays the relationship between the IR of E. coli BSI and average monthly temperature. There was a significant association between seasonal variation and the IR of E. coli BSI. The age- and gender-adjusted IR of E. coli BSI per 100,000 person-years was 50.2 (95% CI: 42.9-57.5) during the warmest four months of the year (June through September) compared to 37.1 (95% CI: 32.7-41.5) during the remaining eight months, resulting in a 35% (95% CI: 12-66%) increase in IR during the warmest four months (Table 2). When considering only the warmest two months (July and August), there was a 44% (95% CI: 16-79%) increase in the IR in comparison to the remaining ten months. Similarly, there was an association between the average monthly temperature and the IR of E. coli BSI (p=0.004). More specifically, there was a 7% (95% CI: 2-12%) increase in the IR for each 10-degree Fahrenheit (c. 5.5 °C) increase in average temperature.
Figure 1.
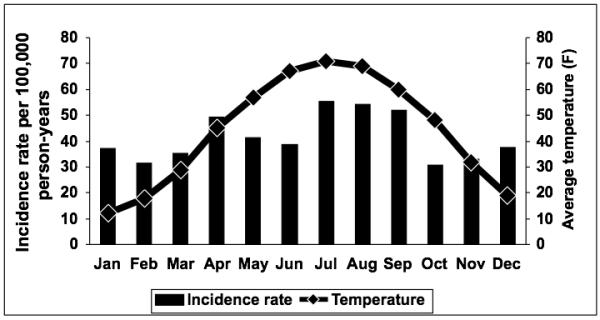
Monthly age- and gender-adjusted incidence rates of Escherichia coli bloodstream infection and average monthly temperatures, 1998-2007.
Table 2.
Age-adjusted incidence rates of Escherichia coli bloodstream infection for the warmest four months compared to the remainder of the year, 1998-2007.
| Gender | IR (95% CI) |
IRR (95% CI) | |
|---|---|---|---|
| June - September | Rest of the year | ||
| Female | 58.8 (48.3 – 69.2) | 42.6 (36.3 – 48.8) | 1.38 (1.09-1.75) |
| Male | 40.5 (30.2 – 50.8) | 30.9 (24.4 – 37.3) | 1.31 (0.95-1.81) |
| Overall* | 50.2 (42.9 – 57.5) | 37.1 (32.7 – 41.5) | 1.35 (1.12-1.66) |
NOTE. IR: incidence rate per 100,000 person-years, CI: confidence intervals, IRR: incidence rate ratio.
Overall incidence rates are gender-adjusted for US white 2000 census.
DISCUSSION
To our knowledge, this is the first study to describe a seasonal variation in E. coli BSI. We found a 35% increase in the IR of E. coli BSI during the warmest four months of the year (June through September) compared to that for the remainder of the year and a 7% increase in the IR of E. coli BSI for each 10-degree Fahrenheit (c. 5.5 °C) increase in average temperature.
The high IR of E. coli BSI during the four warmest months and the association between the IR and average temperature is intuitive because they characterize the in vitro temperature requirements for growth of E. coli. It has been shown that the doubling rate of E. coli increases with increasing temperature until an optimal growth temperature of 35-36 degrees Celsius is reached [13]. This growth pattern of E. coli was also seen in our local environment. Researchers at the University of Minnesota reported a higher density of E. coli in temperate soils from Lake Superior watersheds in Northern Minnesota during the warmest months compared to the remaider of the year [14]. This ecological association may provide a logical hypothetical explanation for our study results with an increased risk of human colonization with pathogenic strains of E. coli during the warmest months and hence an increased rate of infection. According to this hypothesis, the age- and gender-adjusted IR of E. coli BSI should continue to increase as the average temperatures approach the optimal growth temperature of E. coli. It would be interesting to compare the IR of E. coli BSI in other population-based settings with relatively similar population characteristics to ours, but at geographic locations with differing average temperatures.
A recent report from four tertiary-care centers in four continents described a seasonal variation in the IR of K. pneumoniae BSI and an association between the IR of K. pneumoniae BSI and average temperature [5]. It is conceivable that we would observe a similar pattern in the IR of E. coli BSI because the in vitro temperature requirements for growth of both E. coli and K. pneumoniae are relatively uniform. Due to the relatively small number of patients with BSI due to K. pneumoniae and other Gram-negative bacilli in our local population over the 10-year study period, an examination of seasonal variation for these organisms was not conducted.
The IR of E. coli BSI increased with age, similar to what we have previously observed in P. aeruginosa BSI in the same population [9]. In contrast to P. aeruginosa BSI that was more common in males and more likely to be nosocomial or health care-associated [9], E. coli BSI was more common in females and more likely to be community-acquired.
There are a few limitations to our study. First, the population of Olmsted County consists mainly of middle class whites; therefore, our study results may be generalized only to communities with similar population characteristics. Second, because of the relatively cold weather in Olmsted County, during the winter months, some retirees travel to spend the winter in warmer geographic locations. It is conceivable that E. coli BSIs in these individuals would not have been captured by our local microbiology laboratories and therefore, we could have underestimated the IR of E. coli BSI during winter months. Nevertheless, it is highly unlikely that this phenomenon in such a small portion of the general population explains the 35% increase in the IR of E. coli observed during the summer months.
In summary, we demonstrated higher IRs of E. coli BSI during the four warmest months than during the remainder of the year as well as an association between the IR of E. coli BSI and average temperature. These novel epidemiological observations should stimulate hypothesis-generated investigations to further evaluate the impact of climate on infectious diseases.
Acknowledgments
The authors thank E. Vetter and M. A. Butler for providing us with vital data from the microbiology laboratory databases at the Mayo Clinic, Rochester and Olmsted Medical Center.
The authors thank S. Schrage, S. Stotz, and all the staff at the Rochester Epidemiology Project for their administrative help and support.
MNA and BDL have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The study receieved funding from the Small Grants Program and the Baddour Family Fund at the Mayo Clinic, Rochester, MN. The funding source had no role in the study design. This work was made possible by research grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, U.S. Public Health Service).
Footnotes
A poster of this study was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America annual meeting, 27 October 2008,Washington, DC.
Transparency Declaration: The authors declare no conflicts of interest.
References
- 1.McDonald LC, Banerjee SN, Jarvis WR. Seasonal variation of Acinetobacter infections: 1987-1996. Nosocomial infections surveillance system. Clin Infect Dis. 1999;29:1133–1137. doi: 10.1086/313441. [DOI] [PubMed] [Google Scholar]
- 2.Picard B, Goullet P. Seasonal prevalence of nosocomial Aeromonas hydrophila infection related to Aeromonas in hospital water. J Hosp Infect. 1987;10:152–155. doi: 10.1016/0195-6701(87)90141-1. [DOI] [PubMed] [Google Scholar]
- 3.Currie BJ, Fisher DA, Howard DM, et al. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000;74:121–127. doi: 10.1016/s0001-706x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 4.Perencevich EN, McGregor JC, Shardell M, et al. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol. 2008;29:1124–1131. doi: 10.1086/592698. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DJ, Richet H, Chen LF, et al. Seasonal variation in klebsiella pneumoniae bloodstream infection on 4 continents. J Infect Dis. 2008;197:752–756. doi: 10.1086/527486. [DOI] [PubMed] [Google Scholar]
- 6.Melton LJ., 3rd History of the Rochester epidemiology project. Mayo Clinic proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 7.Steckelberg JM, Melton LJ, 3rd, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88:582–588. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 8.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hasan MN, Wilson JW, Lahr BD, Eckel-Passow JE, Baddour LM. Incidence of Pseudomonas aeruginosa bacteraemia: a population-based study. Am J Med. 2008;121:702–708. doi: 10.1016/j.amjmed.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 11.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 12.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 13.Bronikowski AM, Bennett AF, Lenski RE. Evolutionary adaptation to temperature. VIII. Effects of temperature on growth rate in natural isolates of Escherichia coli and Salmonella enterica from different thermal environments. Evolution. 2001;55:33–40. doi: 10.1554/0014-3820(2001)055[0033:EATTVE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Ishii S, Ksoll WB, Hicks RE, Sadowsky MJ. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl Environ Microbiol. 2006;72:612–621. doi: 10.1128/AEM.72.1.612-621.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


