Abstract
Purpose
Chronic myelogenous leukemia (CML) is characterized by the constitutive activation of Bcr-Abl tyrosine kinase. Bcr-Abl-T315I is the predominant mutation that causes resistance to imatinib, cytotoxic drugs, and the second-generation tyrosine kinase inhibitors. The emergence of imatinib resistance in patients with CML leads to searching for novel approaches to the treatment of CML. Gambogic acid, a small molecule derived from Chinese herb gamboges, has been approved for phase II clinical trial for cancer therapy by the Chinese Food and Drug Administration (FDA). In this study, we investigated the effect of gambogic acid on cell survival or apoptosis in CML cells bearing Bcr-Abl-T315I or wild-type Bcr-Abl.
Experimental Design
CML cell lines (KBM5, KBM5-T315I, and K562), primary cells from patients with CML with clinical resistance to imatinib, and normal monocytes from healthy volunteers were treated with gambogic acid, imatinib, or their combination, followed by measuring the effects on cell growth, apoptosis, and signal pathways. The in vivo antitumor activity of gambogic acid and its combination with imatinib was also assessed with nude xenografts.
Results
Gambogic acid induced apoptosis and cell proliferation inhibition in CML cells and inhibited the growth of imatinib-resistant Bcr-Abl-T315I xenografts in nude mice. Our data suggest that GA-induced proteasome inhibition is required for caspase activation in both imatinib-resistant and -sensitive CML cells, and caspase activation is required for gambogic acid–induced Bcr-Abl downregulation and apoptotic cell death.
Conclusions
These findings suggest an alternative strategy to overcome imatinib resistance by enhancing Bcr-Abl downregulation with the medicinal compound gambogic acid, which may have great clinical significance in imatinib-resistant cancer therapy.
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder characterized by a reciprocal translocation between chromosomes 9 and 22, resulting in the expression of a fusion oncoprotein, Bcr-Abl (1, 2). This aberrant tyrosine kinase is mainly responsible for malignant transformation by activating multiple signal transduction pathways, including the MAPK/ERK cascade, PI3K/Akt, and STATs (3–5). Activation of these pathways in Bcr-Abl cells results in increased expression of several antiapoptotic proteins, such as Bcl-2, Bcl-xL, Mcl-1, and XIAP, thus leading to advantaged cell survival (6–8). Bcr-Abl tyrosine kinase has been considered as an important target for CML therapeutics (9–11). Imatinib mesylate (imatinib) was the first selective tyrosine kinase inhibitor for cancer therapy approved by the U.S. Food and Drug Administration. Clinical studies show that imatinib is highly active in newly diagnosed patients with chronic phase CML, and to a less extent, in patients with accelerated and blastic-phase disease (12). Unfortunately, resistance to imatinib develops over time and is becoming an emerging problem for CML treatment (13). Approximately 50 point mutations have been identified to be associated with clinical resistance to imatinib, and T315I Bcr-Abl, accounting for about 20% of all the point mutations, is the most stubborn point mutation impacting on the binding of imatinib with Bcr-Abl kinase domain (13–15). Hence, novel strategies to overcome this resistance are required. Recent data suggest that inhibiting Bcr-Abl expression is a promising approach to overcome imatinib resistance (16).
Gambogic acid is a small molecule extracted from the traditional Chinese medicine gamboges, which has been used for hundreds of years in China (17). Gambogic acid has a strong cytotoxic effect on a variety of tumors (18, 19). Unlike other chemotherapeutics, gambogic acid has very weak effect on the hematologic system (20, 21). Of note, gambogic acid has been approved by the Chinese Food and Drug Administration for phase II clinical trial in solid cancer therapy. Several molecular targets of gambogic acid have been proposed (22, 23). Most recently, we have reported that gambogic acid is a novel tissue-specific proteasome inhibitor, with potency comparable to bortezomib but much less toxicity (24). We have also clarified that gambogic acid only gains proteasome-inhibitory function after being metabolized by intracellular CYP2E1 (24). Therefore, gambogic acid is a promising anticancer agent with less toxicity on the normal tissues. Although proteasome inhibitors such as bortezomib have been reported to downregulate Bcr-Abl expression and induce cell death in CML cells (25–27), the role of gambogic acid in Bcr-Abl hematopoietic malignancies remains unknown.
Here, we investigated the antineoplastic effects of gambogic acid in CML cell lines, mononuclear cells from patients with CML, including those resistant to imatinib-based therapies and in mouse imatinib-resistant xenograft models. The results show that gambogic acid could efficiently overcome imatinib resistance in vitro and in vivo.
Materials and Methods
Chemicals
Gambogic acid, diethyl dithiocarbamate (DDC), Annexin V, propidium iodide (PI), and rhodamine-123 were obtained from Sigma-Aldrich. C9–C10 disrupted gambogic acid (GA~) was synthesized by our laboratory (24). Bortezomib, cycloheximide, z-VAD-fmk, and z-DEVD-fmk were from BD Biosciences. CA-074Me and antibody against MMP2 (6E3F8) were from Abcam. Antibodies against c-Abl (C-19), Mcl-1 (S-19), ubiquitin (P4D1), caspases-3, -8, -9, apoptosis-inducing factor (AIF), Bcl-2, and Bax were from Santa Cruz Biotechnology. Antibodies against PARP (clone 4C10-5) and CXCR4 were from BD Biosciences. Antibodies against phospho-c-Abl at Y245, phospho-Erk1/2 (T202/Y204), Erk1/2, phospho-CrkL at Y207, CrkL, phospho-Akt, Akt, hsp70, hsp90, IκB-α, cleaved caspases-3, -9, Ki-67 (8D5), and XIAP were from Cell Signaling Technology. Antibodies against phospho-STAT5A/B (Y694/Y699, clone 8-5-2) and STAT5 were from Upstate Technology; mouse monoclonal antibody against actin from Sigma-Aldrich. Antibody against CD11b/c (NB110-40766) was from Novus Biologicals. Enhanced chemiluminescence reagents were purchased from Amersham Biosciences. The molecular weight of proteins used in this study was labeled in Supplementary Fig. S1.
Cell sample collection and cell culture
The culture of KBM5 and KBM5-T315I cells was described previously (15, 28). In brief, KBM5 cell line expressing the 210 kDa wild-type Bcr-Abl was derived from a female patient with CML. The KBM5-T315I cells, harboring a threonine to isoleucine substitution at position 315 of Abl, were originally established by exposure to increasing concentrations of imatinib and became imatinib-resistant. KBM5 cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone). KBM5-T315I cells were routinely maintained in the same medium but in the presence of 1 µmol/L imatinib, which was removed before experiments were started by a wash-out period of 2 to 3 days. K562 cells were cultured in RPMI-1640 medium (Invitrogen) supplemented with 10% FCS (Hyclone). Short tandem repeat profiles of all the three cell lines have been confirmed by Beijing Microread Genetics Co., Ltd. in recent 3 months. It was found that in K562 cells there are three alleles in one loci (D21S11); no cross-contamination of other human cells is found in this test; this K-562 line is 100% matched with the American Type Culture Collection, DSMZ, and JCRB data bank. The other two lines are not available in the cell data bank.
Bone marrow and blood samples of patients with CML were obtained from discarded material utilized for routine laboratory tests at the Department of Hematology, Guangzhou First Municipal People’s Hospital (Guangzhou, China). The use of these materials is approved by the Institutions with the permission of the patients and volunteers. Totally, 5 patients with CML (2 imatinib-resistant: #2 and #4) and 3 volunteers were recruited. Mononuclear cells were isolated by Ficoll-Paque and cultured in RPMI-1640 medium with 15% FCS as described previously (15).
Western blot analysis
Whole-cell lysates were prepared in radioimmunoprecipitation assay buffer (1 × PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS; ref. 15) supplemented with 10 mmol/L β-glycerophosphate, 1 mmol/L sodium orthovanadate, 10 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 1 × Roche Protease Inhibitor Cocktail (Roche). To detect the level of cytochrome C and AIF, the cytosolic fraction was prepared with a digitonin extraction buffer (10 mmol/L PIPES, 0.015% digitonin, 300 mmol/L sucrose, 100 mmol/L NaCl, 3 mmol/L MgCl2, 5 mmol/L EDTA, and 1 mmol/L PMSF) as described previously (15). Western blotting was performed as we previously described (15, 28).
Cell viability assay
MTS assay (CellTiter 96 Aqueous One Solution reagent, Promega) was used to measure cell viability (29). Briefly, 2 × 105/mL cells in 100 µL were treated with gambogic acid for 48 or 72 hours. Four hours before culture termination, 20 µL MTS was added to the wells. The absorbance density was read on a 96-well plate reader at wavelength 490 nm. The combinational treatment was done in serial fixed-ratio dilutions of the two-drug mixtures. The effects of combinations were estimated using the CalcuSyn software, as described elsewhere (30). The combination index (CI) was the ratio of the combination dose to the sum of the single-agent doses at an isoeffective level. Therefore, CI < 1 indicates synergy; CI > 1, antagonism; and CI = 1, additive.
Colony formation assay
KBM5, KBM5-T315I, and K562 cells pre-exposed to gambogic acid for 12 or 24 hours were suspended in 30% agarose supplemented with 20% FCS and 50% IMDM (for KBM5 and KBM5-T315I cells) or RPMI-1640 medium (for K562 cells) in a 24-well plate and then cultured in an atmosphere of 5% CO2 for 4 to 5 days. The colonies more than 60 µm were counted under the light microscope. The experiments were done in triplicate.
Cell death assay
A total of 1.0% PI was added to the culture medium to monitor temporal changes in the incidence of cell death in the live culture condition. The PI-positive cells were imaged with fluorescence microscope equipped with a digital camera (Axio Observer Z1, Zeiss; ref. 29). Apoptosis was determined by flow cytometry using Annexin V-fluoroisothio-cyanate (FITC)/PI double staining (15). Cells were collected, washed with binding buffer (Sigma-Aldrich), and then incubated in working solution (100 µL binding buffer with 0.3 µL Annexin V-FITC and PI) for 15 minutes in dark.
Measurement of mitochondrial membrane integrity
The mitochondrial membrane potential of gambogic acid–treated and untreated cells were assayed by using rhodamine-123 (Sigma-Aldrich) staining. Cells were treated with gambogic acid for 12 hours and stained with 1 µmol/L of rhodamine-123 for 1 hour at 37°C. After the staining, the cells were washed and harvested for either flow cytometry analysis or imaging with an inverted fluorescence microscope.
Chymotrypsin-like peptidase activity assay
About 4,000 cells were treated with gambogic acid for 6 hours. The treated cells were then incubated with the Glo Cell-Based Assay Reagent (Promega Bioscience) for 10 minutes (29). The proteasomal chymotrypsin-like (CT-like) activity was detected as the relative light unit generated from the cleaved substrate. Luminescence generated from each reaction was detected with luminescence microplate reader (Varioskan Flash 3001; Thermo Scientific).
RNA isolation and real-time quantitative PCR
Total RNA was extracted from 5 × 106 cells by use of TRIzol reagent (Invitrogen). The first-strand cDNA was synthesized from 1 µL of total RNA with the use of the RNA PCR Kit (AMV) Ver.3.0 (TaKaRa) and random primers. Then, 50 ng of total cDNA was used for real-time PCR with the SYBR Premix Ex TaqII Kit (TaKaRa). The relative gene expression was analyzed by the Comparative Ct method using 18s ribosomal RNA as endogenous control. The primers for real-time PCR are as follows: Bcr-Abl forward, 5′-AAG CGC AAC AAG CCC ACT GTC TAT-3′; reverse, 5′-CTT CGT CTG AGA TAC TGG ATT CCT-3′. 18s forward, 5′-AAA CGG CTA CCA CAT CCA AG-3′; reverse, 5′-CCT CCA ATG GAT CCT CGT TA-3′.
Nude mouse xenograft model
Nude Balb/c mice were bred at the animal facility of Guangzhou Medical College. The mice were housed in barrier facilities with a 12-hour light/dark cycle, with food and water available ad libitum. A total of 3 × 107 of KBM5 or KBM5-T315I cells were inoculated subcutaneously on the flanks of 5-week-old male nude mice. After 72 hours of inoculation, mice were treated with vehicle (10% DMSO, 30% cremophor and 60% NaCl), gambogic acid (3 mg/kg/2 days, i.p.), imatinib (50 mg/kg/d, i.g.) and the combination of gambogic acid and imatinib for a total of 17 days. Tumors were measured and tumor volumes were calculated by the following formula: a2 × b × 0.4, where a is the smallest diameter and b is the diameter perpendicular to a. Tumor xenografts were removed, weighed, stored, and fixed. All animal studies were conducted with the approval of the Institutional Animal Care and Use Committee of Guangzhou Medical College.
Statistical analysis
All experiments were performed at least thrice, and the results were expressed as mean ± SD where applicable. GraphPad Prism 4.0 software (GraphPad Software) was used for statistical analysis. Student t test was used to compare the differences between variables. P value of < 0.05 was considered statistically significant.
Results
Gambogic acid inhibits proliferation and colony formation of both Bcr-Abl wild-type and Bcr-Abl-T315I cells
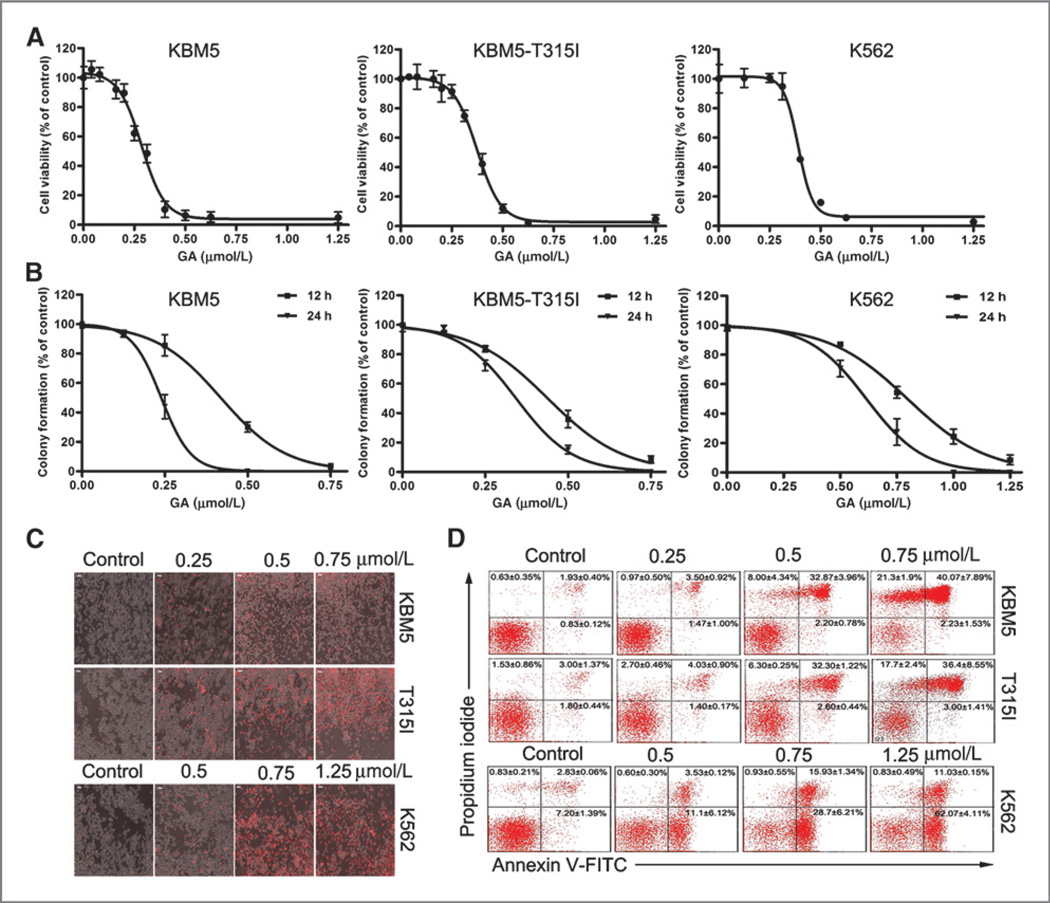
KBM5 (Bcr-Abl wild-type) cells are sensitive, whereas KBM5-T315I (Bcr-Abl-T315I) cells are resistant to imatinib treatment (Supplementary Fig. S2; ref. 15). To investigate the effect of gambogic acid on growth of CML cells, cultured KBM5, KBM5-T315I, and K562 cells were treated with gambogic acid at various concentrations for 48 hours and cell viability was detected by MTS assay. As shown in Fig. 1A, gambogic acid decreased the cell viability in KBM5, KBM5-T315I, and K562 lines in a dose-dependent manner, with IC50 values of 0.32, 0.35, and 0.40 µmol/L, respectively.
Figure 1.
Gambogic acid (GA) induces cell apoptosis in CML cells. A, gambogic acid decreases cell viability of KBM5, KBM5-T315I, K562 cells. KBM5, KBM5-T315I, K562 cells were exposed to gambogic acid in various concentrations for 48 hours, and then were subjected to MTS assay. Graphs represent data from three repeats. Mean ± SD (n = 3). B, gambogic acid inhibits the colony formation of KBM5, KBM5-T315I, and K562 cells. Cells were treated with increasing concentrations of gambogic acid for 12 or 24 hours, and then suspended in soft agar. The number of colonies >60 µm was counted after 5 days culture under light microscope. Mean ± SD (n = 3). C, gambogic acid induces cell death in KBM5, KBM5-T315I (T315I), and K562 cells. KBM5, KBM5-T315I, and K562 cells were treated with different doses of gambogic acid for 12 hours, then propidium iodide (PI) was added to the culture medium and the PI-positive cells were recorded under an inverted fluorescence microscope (Axio Obsever Z1, Zeiss). Representative images were shown. D, gambogic acid induces apoptosis in KBM5, KBM5-T315I, and K562 cells. KBM5, KBM5-T315I, and K562 cells were treated with gambogic acid at the indicated doses for 12 hours and cell apoptosis was detected by Annexin V-FITC/PI double staining with flow cytometry (FACScan, Becton Dickinson). Mean ± SD (n = 3). Representative images were shown.
To test the long-term effect of gambogic acid on CML cells, we performed a colony formation assay in soft agar, which is one of the best in vitro indicators of malignant behavior. As shown in Fig. 1B, treatment of KBM5, KBM5-T315I, and K562 cells with gambogic acid at concentrations from 0.125 to 1.25 µmol/L for 12 or 24 hours resulted in fewer colonies than those of the control group after 4 to 5 days of culture. The IC50 values of gambogic acid in colony assay after 24 hours were 0.24 µmol/L (for KBM5 cells), 0.34 µmol/L (for KBM5-T315I cells), and 0.62 µmol/L (for K562 cells), respectively.
Gambogic acid induces apoptosis in both Bcr-Abl wild-type and Bcr-Abl-T315I cells
We next analyzed the capacity of gambogic acid to induce cell death in live Bcr-Abl wild-type and mutant cell lines in a dynamic experiment. KBM5, KBM5-T315I, and K562 cells were exposed to gambogic acid, followed by recording the PI-positive cells under a fluorescence microscope or by flow cytometry with Annexin V/PI staining. A dose-dependent cell death was observed. The 12-hour time point images were shown in Fig. 1C and D.
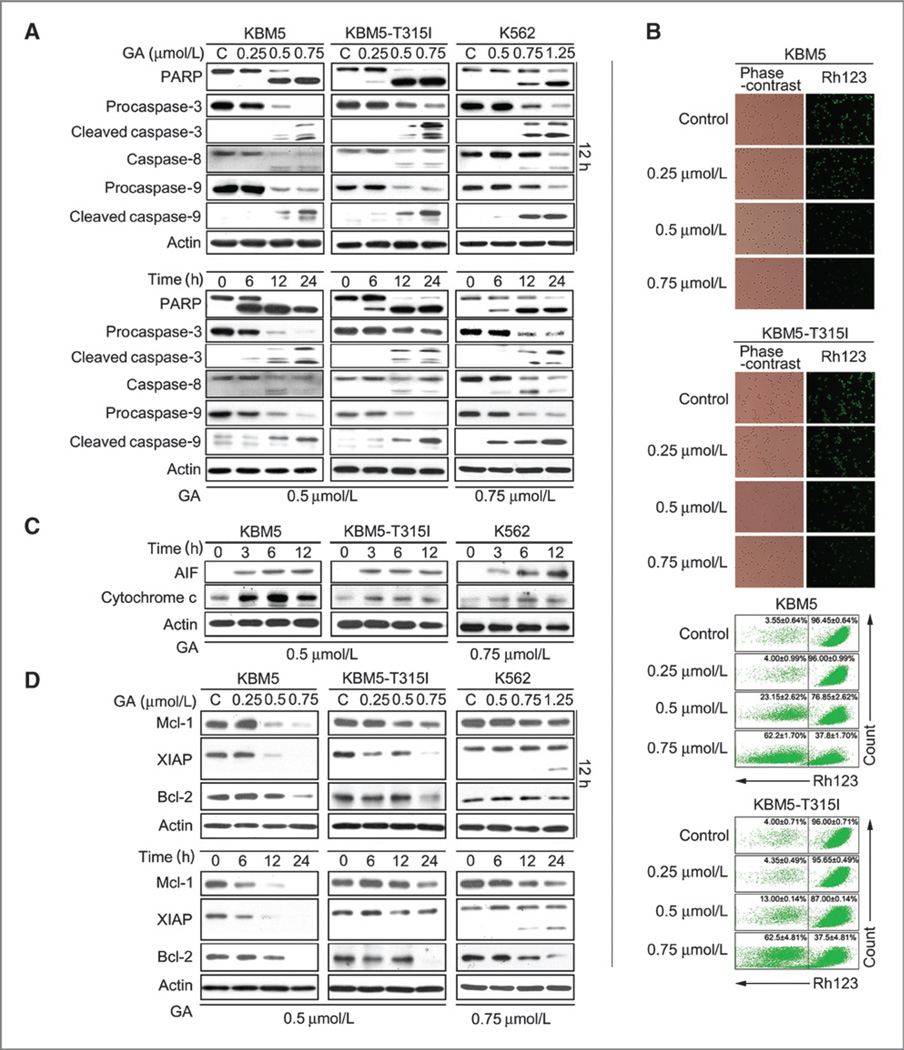
Gambogic acid induces caspase activation in CML cells
KBM5, KBM5-T315I, and K562 cells were then exposed to gambogic acid, followed by measurement of apoptosis. Western blot analysis showed that gambogic acid induced the cleavage of PARP (an indicator of apoptosis) in both dose- and time-dependent manner in these three CML cell lines. Consistently, the levels of the precursor forms of caspases-3, -8, and -9 were decreased and that of the active forms of caspases-3, -8, and -9 were increased after gambogic acid treatment, matching the pattern of PARP cleavage, demonstrating that gambogic acid triggers CML cell apoptosis via caspase activation (Fig. 2A).
Figure 2.
Gambogic acid (GA)-induced apoptosis is associated with caspase activation and decreased expression of anti-apoptotic proteins in CML cells. A, gambogic acid induces cleavage of PARP and caspases-3, -8, -9 in KBM5, KBM5-T315I, and K562 cells. Cells were dose- and time-dependently treated with gambogic acid, PARP, and caspase-3, -8, -9 cleavage were analyzed by Western blots. Actin was used as a loading control. C, control. B, gambogic acid induces downregulation of mitochondrial membrane potential in KBM5 and KBM5-T315I cells. Cells were treated with 0.25, 0.5, and 0.75 µmol/L gambogic acid for 12 hours, mitochondrial membrane potential were detected by rhodamine-123 staining either with inverted fluorescence microscope (top) or flow cytometry (bottom), Mean ± SD (n = 3). C, gambogic acid induces AIF and cytochrome C release. KBM5, KBM5-T315I, and K562 cells were exposed to gambogic acid for 3, 6, and 12 hours, then cell cytoplasm were extracted by digitonin buffer and the released AIF and cytochrome C were detected by Western blot analysis. D, gambogic acid decreases the expression of antiapoptotic proteins in KBM5, KBM5-T315I, and K562 cells. Cells were dose- and time-dependently treated with gambogic acid. The antiapoptotic proteins Mcl-1, XIAP, and Bcl-2 were analyzed by Western blot analysis.
It is well known that mitochondria are the regulating center of apoptosis. Release of cytochrome C and AIF from mitochondrial to the cytoplasm has been recognized as an indicator of the early stage of apoptosis (31). As shown in Fig. 2B, the integrity of mitochondrial membranes was decreased in both KBM5 and KBM5-T315I cells after gambogic acid treatment, and elevated levels of release of cytochrome C and AIF were detected in a time-dependent manner in these cells (Fig. 2C).
To further investigate the mechanism of gambogic acid–induced apoptosis, the effect of gambogic acid on the expression of other apoptosis-related proteins was measured. As displayed in Fig. 2D, gambogic acid induced marked decline of anti-apoptotic proteins Mcl-1, XIAP and Bcl-2 in KBM5, KBM5-T315I, and K562 cells.
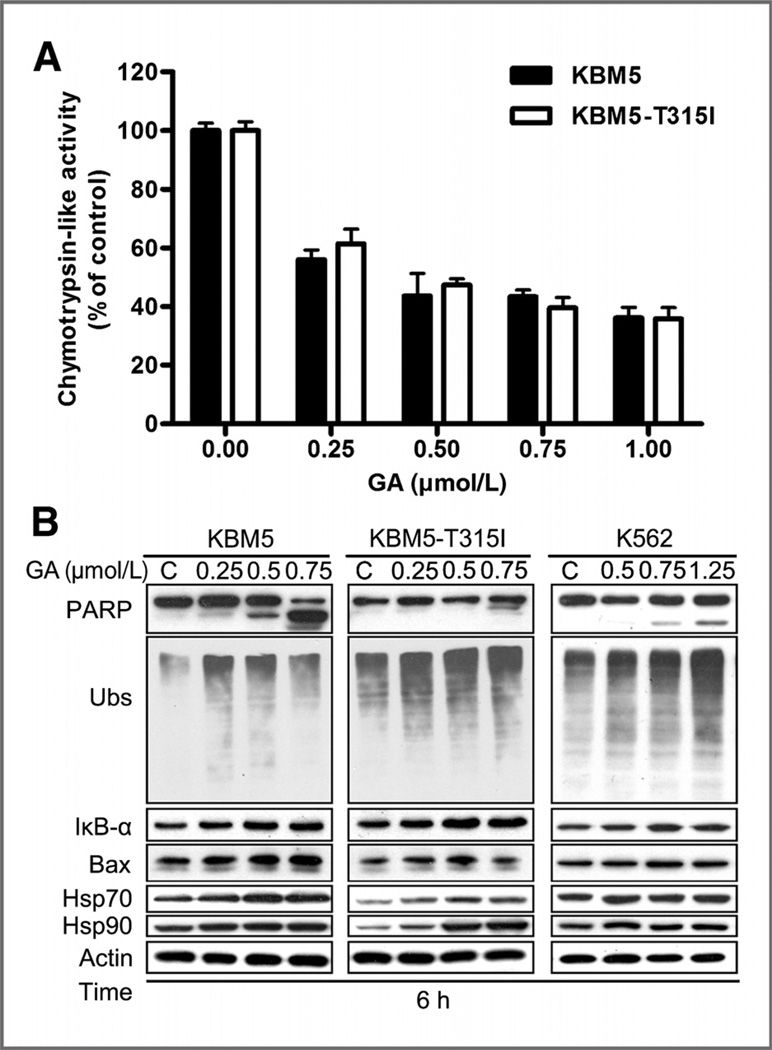
Gambogic acid inhibits the proteasome function in CML cells
To test the direct effect of the gambogic acid on proteasome function, we first examined the proteasome peptidase activities in cultured CML cells. It was found that gambogic acid dose dependently inhibited the CT-like activities in KBM5 and KBM5-T315I cells (Fig. 3A). Furthermore, gambogic acid induced accumulation of ubiquitinated proteins (Ubs) and proteasome substrate proteins, IκB-α, Bax, Hsp70, and Hsp90, in KBM5, KBM5-T315I, and K562 cells (Fig. 3B). These results confirmed that gambogic acid at a lower concentration significantly inhibited ubiquitin-proteasome function in these CML cells, associated with induced cytotoxicity (Fig. 1).
Figure 3.
Gambogic acid (GA) inhibits proteasome function in KBM5, KBM5-T315I, and K562 cells. A, gambogic acid inhibits proteasome peptidase activities in KBM5 and KBM5-T315I cells. The cells were treated with gambogic acid at 37°C for 6 hours, followed by detecting CT-like activity with a cell-based assay reagent. Mean ± SD (n = 3). B, gambogic acid accumulates proteasome substrate proteins in KBM5, KBM5-T315I and K562 cells. Cells were treated with various doses of gambogic acid for 6 hours. The protein levels of PARP, ubiquitinated proteins (Ubs), IκB-α, Bax, Hsp70, and Hsp90 were detected by Western blot analysis. Actin was used as a loading control. C, control.
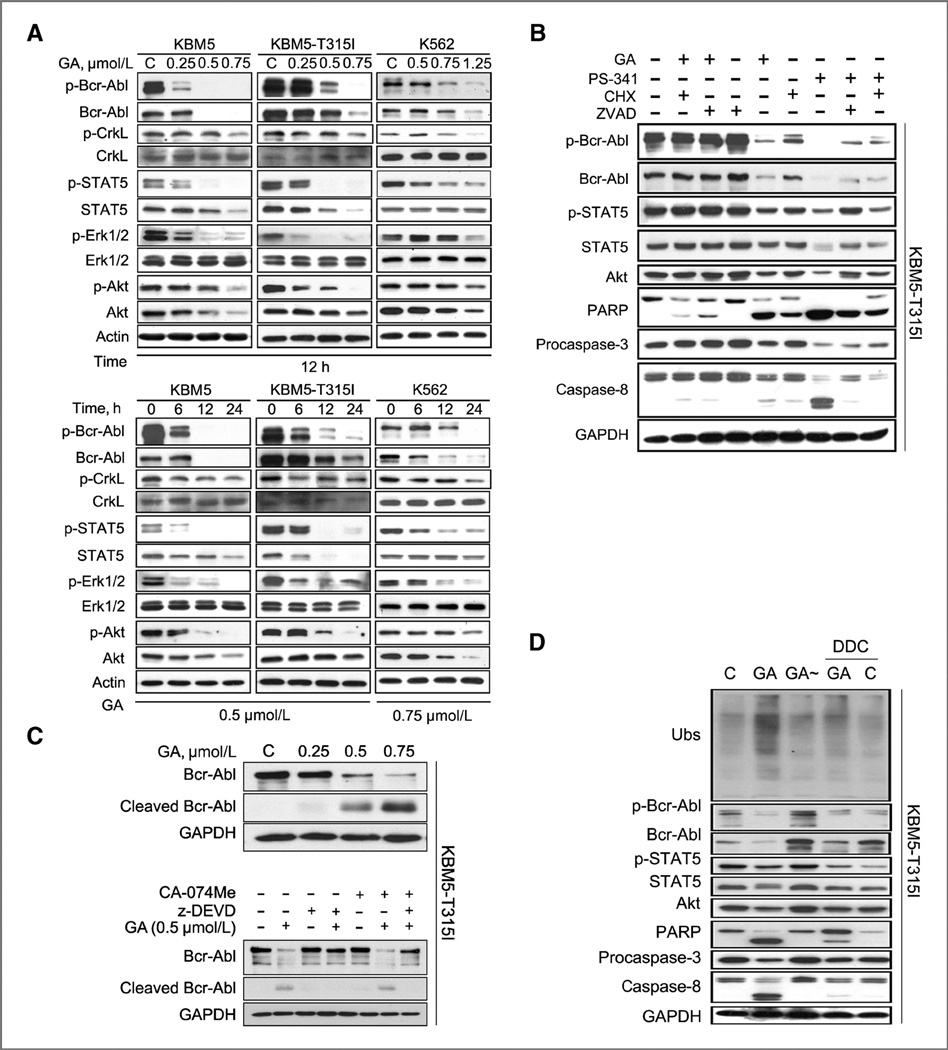
Gambogic acid downregulates Bcr-Abl protein and inhibits its downstream signaling
We also found that gambogic acid downregulated the total and phosphorylation levels of Bcr-Abl protein in KBM5, KBM5-T315I, and K562 cells in both dose- and time-dependent manners (Fig. 4A). Further results dis-played that gambogic acid treatment also inhibited the expression of Bcr-Abl downstream target proteins. The phosphorylation of CrkL, STAT5, ERK1/2, and Akt was also significantly decreased in a dose- and time-dependent manner with less dramatic changes in total expression of ERK1/2 and CrkL; even though there are decreases in total Akt and STAT5 proteins, they occur later than the changes of their phosphorylation forms (Fig. 4A) but gambogic acid did not dramatically affect Bcr-Abl mRNA expression (Supplementary Fig. S3), indicating that gambogic acid-mediated Bcr-Abl downregulation is not at a transcription level. Taken together, our data demonstrated that gambogic acid inhibits expression of Bcr-Abl protein and consequently the downstream signaling pathway of Bcr-Abl.
Figure 4.
Gambogic acid (GA)-mediated proteasome inhibition and caspase activation are required for the downregulation of Bcr-Abl and its downstream signaling proteins. A, gambogic acid decreases the protein levels of Bcr-Abl and its downstream targets. KBM5, KBM5-T315I, and K562 cells were treated with gambogic acid as indicated both dose- and time-dependently, cell lysates were analyzed by Western blot analysis with antibodies against either the Bcr-Abl or its downstream targets. C, control. B, gambogic acid decreases Bcr-Abl and the downstream signaling proteins in a caspase-dependent manner. KBM5-T315I cells were treated with 0.5 µmol/L gambogic acid for 12 hours in the absence and presence of cycloheximide (CHX; 5 µg/mL) or 20 µmol/L pan-caspase inhibitor z-VAD-fmk. Fifty nmol/L proteasome inhibitor PS341 was used as a positive control. The total and phosphorylated Bcr-Abl and its downstream proteins were analyzed by Western blot analysis. Representative images were shown. C, caspase activation is required for Bcr-Abl cleavage. KBM5-T315I cells were treated with gambogic acid with/without caspase inhibitor z-DEVD (20 µmol/L) or cathepsin B inhibitor CA-074Me (7.5 µmol/L) for 12 hours. Bcr-Abl and its cleaved form were detected by Western blot analysis. D, gambogic acid-mediated proteasome inhibition is required for caspase activation, down-regulation of Bcr-Abl and its downstream target proteins. KBM5-T315I cells were treated with 0.5 µmol/L gambogic acid or gambogic acid+DDC (CYP2E1 inhibitor, 50 µmol/L) for 12 hours and a reduced form of gambogic acid (GA~) was used as a negative control. Cell lysates were analyzed by Western blots by using antibodies against Ubs, Bcr-Abl, and its downstream targets, PARP, caspases-3, and -8. GAPDH was used as a loading control.
Caspase-3 activation is required for the downregulation of Bcr-Abl protein and its signaling
To gain insight into the mechanism of gambogic acid–induced Bcr-Abl decline, we studied its dependence on the caspase activation. We found that pan-caspase inhibitor z-VAD could inhibit gambogic acid-mediated decrease in Bcr-Abl protein and its downstream proteins (Fig. 4B), and treatment with cycloheximide, a protein synthesis inhibitor, partially restored the level of Bcr-Abl and downstream target proteins. Similar results were obtained when bortezomib was used in the place of gambogic acid in the presence of z-VAD or cycloheximide (Fig. 4B). Also, gambogic acid–induced Bcr-Abl decrease or cleavage was inhibited by a caspase-3 inhibitor z-DEVD but not by the cathepsin B inhibitor CA-074Me, indicating the involvement of caspase-3 but not lysosome (Fig. 4C). These results demonstrated that gambogic acid- and bortezomib-induced caspase activation is required for the downregulation of Bcr-Abl and its downstream events.
Gambogic acid-mediated proteasome inhibition is required for caspase activation and Bcr-Abl downregulation
Next, we investigated whether gambogic acid-mediated proteasome inhibition is responsible for caspase-mediated Bcr-Abl downregulation. We reported previously that a double bond between carbon 9 (C9) and carbon 10 (C10) in gambogic acid structure is the major chemical structure required for gambogic acid–induced proteasome inhibition (24). In the current study, a C9–C10-disrupted gambogic acid (GA~) was used to compare with gambogic acid. As shown in Fig. 4D, GA~ (0.5 µmol/L) lost its ability to inhibit proteasome activity, caspase activation, apoptosis, and downregulation of Bcr-Abl and its down-stream proteins, compared with gambogic acid treatment in KBM5-T315I cells. Since gambogic acid was metabolized to an active proteasome inhibitor by cellular CYP2E1, next we compared gambogic acid’s effects with or without a CYP2E1 inhibitor DDC. As shown in Fig. 4D, DDC mostly restored the PARP cleavage, caspase activation, downregulation of Bcr-Abl-related proteins, and the proteasome inhibition. These results showed that gambogic acid-mediated proteasome inhibition is responsible for gambogic acid–induced caspase activation and Bcr-Abl downregulation.
Ex vivo effect of gambogic acid on primary monocytes from patients with CML
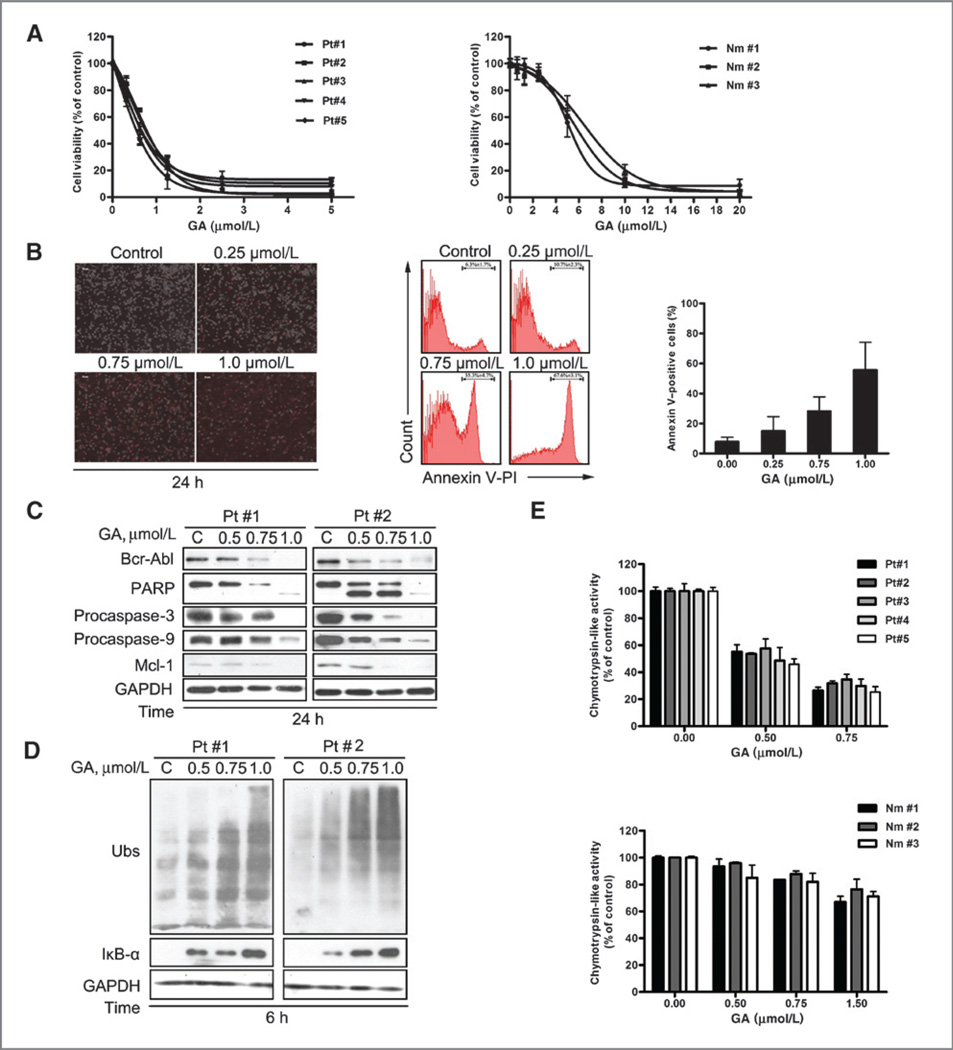
The above results showed that gambogic acid and gambogic acid-mediated proteasome inhibition are effective in both imatinib-sensitive and -resistant CML cells. We next evaluated the ex vivo antineoplastic effect of gambogic acid on bone marrow mononuclear cells from 5 patients with CML (2 of them were imatinib-resistant). Peripheral mononuclear cells from three healthy volunteers were used as controls. As shown in Fig. 5A, gambogic acid treatment decreased the cell viability of primary monocytes from patients with CML with IC50 values 0.58–0.82 µmol/L (Fig. 5A, left), whereas in normal controls IC50 values of more than 5.0 µmol/L were obtained (Fig. 5A, right). Furthermore, treatment of gambogic acid at doses of 0.25 to 1.0 µmol/L for 24 hours resulted in significant apoptosis in all the monocytes from 5 CML patients as detected by either PI staining (Fig. 5B, left) or Annexin V/PI double staining (Fig. 5B, middle and right). Treatment with 1.0 µmol/L gambogic acid for 24 hours led to almost 60% apoptosis. Gambogic acid treatment also significantly reduced the levels of Bcr-Abl, procaspases-3 and -9, Mcl-1, induced PARP cleavage (Fig. 5C), caused accumulation of ubiquitinated proteins and IκB-α in the monocytes from patients with CML (Fig. 5D). Importantly, gambogic acid markedly inhibited the proteasome peptidase activity in bone marrow mononuclear cells from patients with CML (Fig. 5E, top), whereas only slightly inhibited the activity in normal monocytes from healthy volunteers (Fig. 5E, bottom). These results are consistent with the in vitro inhibitory effect of gambogic acid on KBM5 and KBM5-T315I cells, suggesting the potential use of gambogic acid for treatment of patients with CML.
Figure 5.
Gambogic acid (GA) induces more cytotoxicity and proteasome inhibition in cancer cells from patients with CML than in human peripheral mononuclear cells. A, gambogic acid is more cytotoxic to human cancer cells than to human normal peripheral mononuclear cells. Cancer cells from 5 patients with CML (left) and peripheral blood mononuclear cells from 3 healthy volunteers (right) were treated with gambogic acid at the indicated doses for 48 hours and the cell viability was detected by MTS assay. Mean ± SD (n = 3). B, gambogic acid induces cell death of cancer cells either from imatinib (IM)-sensitive or -resistant patients with CML. Cancer cells from 5 patients with CML were isolated and incubated with gambogic acid for 24 hours, followed by detecting the PI-positive cells under an inverted microscope (left), or staining with Annexin V/PI and cell apoptosis was analyzed by flow cytometry (middle). Cell death data by flow cytometry were summarized (right), Mean ± SD (n = 5). C, gambogic acid induces cleavage of PARP, decreased pro-caspases-3 or -9 and downregulates Bcr-Abl and Mcl-1 protein in human cancer cells. Cells from patients with CML were treated with gambogic acid as indicated, and the indicated proteins were detected by Western blot analysis (#1, imatinib-sensitive patient; #2, imatinib-resistant patient). D, gambogic acid accumulates ubiquitinated proteins and IκB-α in CML cancer cells. Cancer cells from 2 patients with CML were treated with gambogic acid for 6 hours. The indicated proteins were analyzed by using Western blot analysis. E, gambogic acid more strongly inhibits CT-like activity in cancer cells than in normal mononuclear cells. Both cancer cells (top) or normal mononuclear cells (bottom) were treated with gambogic acid as indicated for 6 hours, CT-like activity was detected by cell-based CT-like assay reagent. Mean ± SD (top, n = 5; bottom, n = 3).
Gambogic acid inhibits the growth of Bcr-Abl wild-type and -T315I mutant xenografts grown in nude mice
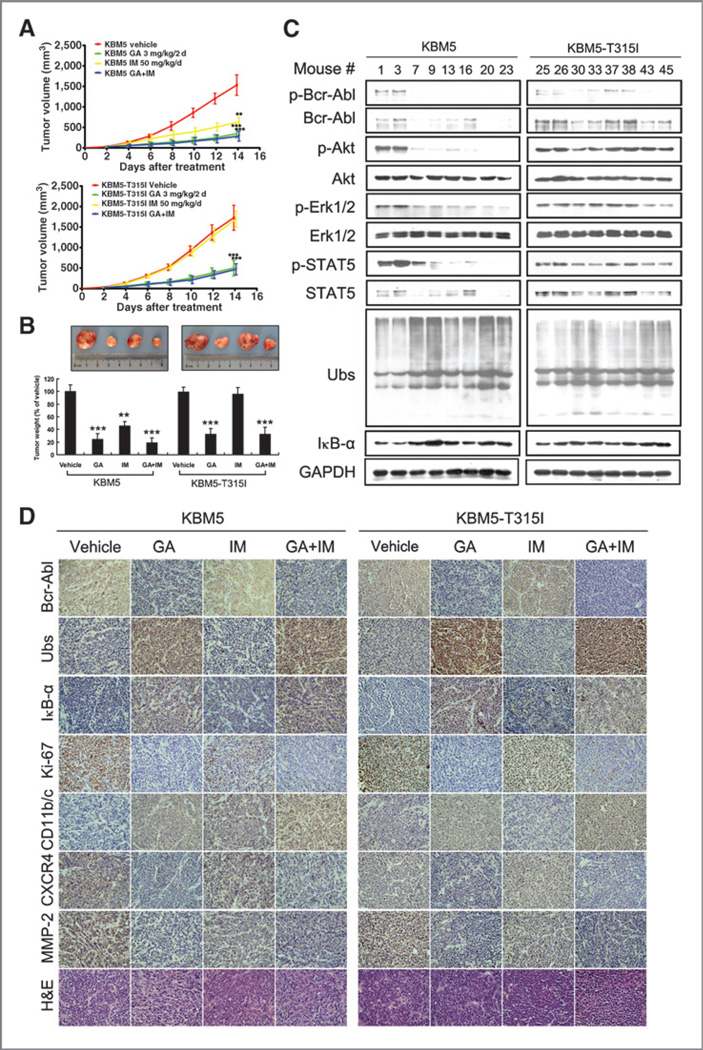
We next evaluate the effects of gambogic acid plus imatinib in vitro and in vivo using a nude mouse xenograft model. As shown in Supplementary Fig. S4, gambogic acid and imatinib each alone induced phosphorylated-Bcr-Abl downregulation and PARP cleavage, whereas the combination of gambogic acid and imatinib did not enhance these changes in KBM5 cells; in KBM5-T315I cells, only gambogic acid but not imatinib induced phosphorylated-Bcr-Abl downregulation and PARP cleavage (Supplementary Fig. S4). In the in vivo model, KBM5 and KBM5-T315I cells were inoculated subcutaneously in nude mice. Mice were then treated by intraperitoneal injection with vehicle, gambogic acid (3 mg/kg/2d), imatinib (50 mg/kg/d), or the combination of gambogic acid and imatinib for 14 days. It was found that gambogic acid treatment significantly inhibited the growth of both Bcr-Abl wild-type and Bcr-Abl-T315I–mutant xenografts; the weights of tumors were significantly reduced in the gambogic acid–treated group compared with the vehicle-treated group and no obvious synergistic effects of gambogic acid and imatinib were observed (Fig. 6A and B), consistent with the in vitro results (Supplementary Fig. S5), while body weight remained relatively stable in each group (data not shown). Protein levels, including Bcr-Abl and its downstream targets Akt, Erk1/2, and STAT5, were significantly decreased in the gambogic acid–treated tumors (Fig. 6C and D) whereas proteasome target protein IκB-α and the ubiquitinated proteins were highly accumulated in gambogic acid–treated tumors versus the control (Fig. 6C and D), indicating that gambogic acid inhibits proteasome function in both imatinib-sensitive and imatinib-resistant xenografts. Some of the protein biomarkers related to proliferation, differentiation, adhesion, and migration, such as Ki67, CD11b/c, CXCR4, and MMP2, were also detected (Fig. 6D). It was found that gambogic acid could downregulate the expression of Ki67, CXCR4, and MMP2 proteins and upregulate CD11b/c protein level in both KBM5 and KBM5-T315I models; imatinib could also downregulate Ki67 and upregulate CD11b/c level in the KBM5 but not in the KBM5-T315I model. No obvious synergistic effects of gambogic acid and imatinib on these proteins were observed. Together, the results demonstrated that gambogic acid inhibited both xenografted Bcr-Abl wild-type and Bcr-Abl-T315I harboring cells in vivo.
Figure 6.
Gambogic acid (GA) inhibits tumor growth, proteasome function and Bcr-Abl expression in nude BALB/c mice bearing wild-type and T315I-mutant Bcr-Abl xenograft. Nude mice bearing wild-type and T315I–mutant Bcr-Abl xenograft tumors were treated with vehicle, gambogic acid alone (3 mg/kg/2d), imatinib (IM) alone (50 mg/kg/d), or a combination of gambogic acid and imatinib for 4 to 17 days after inoculation of KBM5 and KBM5-T315I cells. A, tumor growth curves were recorded every 2 days in two sets of experiments. Mean ± SD (n = 6). **, P < 0.01; ***, P < 0.001, versus control group. B, on day 17 after inoculation, the mice were sacrificed, and the tumor tissues were weighed, imaged, and summarized. **, P < 0.01; ***, P < 0.001, versus control group. C and D, Bcr-Abl pathway and proteasome substrates related proteins in tumor tissues were detected by Western blot analysis (KBM5 control group: 1, 3; gambogic acid–treated group: 7, 9; imatinib-treated group: 13, 16; combination group: 20, 23; KBM5- T315I control group: 25, 26; gambogic acid–treated group: 30, 33 imatinib-treated group: 37, 38; combination group: 43, 45), Bcr-Abl, Ubs, IκB-α, Ki-67, CD11b/c, CXCR4, and MMP-2 were tested by immunohistologic analysis. All the immunostaining and Western blot analyses were repeated in three mouse tumor tissues and the most typical images were shown.
Discussion
Among several mechanisms proposed to explain the resistance to imatinib, amplification and mutation of Bcr-Abl is believed to be the predominant. To overcome acquired resistance to imatinib, several groups have developed new second-generation ATP-competitive Abl kinase inhibitors, such as AMN107, dasatinib, INNO-406 and PD166326, which have stronger affinities for the ATP-binding site of Bcr-Abl kinase than imatinib does, and thus are effective for imatinib-resistant patients to some extent (32–36). Although these novel inhibitors can effectively inhibit the phosphorylation of the mutated Bcr-Abl (E255K, M351T), they had little effect on Bcr-Abl-T315I. In addition, Bcr-Abl cells are relatively resistant to apoptosis induced by conventional cytotoxic agents (37, 38). All of these studies find that, CML, being highly dependent on presence of Bcr-Abl, is one of the typical models of oncogene addiction. Bcr-Abl downregulation is the common effect and is likely the major strategy inducing apoptosis in Bcr-Abl–expressing cells.
Gambogic acid, the major active component in the traditional Chinese medicine gamboge, has antitumor activities in a broad range of human cancer cells, including K562 cells expressing a wild-type Bcr-Abl gene (39). In this study, we reported that gambogic acid is highly effective in overcoming imatinib-resistant cancer cells in vitro, ex vivo, and in vivo. In the in vitro study, gambogic acid dose- and time-dependently decreased cell viability, colony formation, and induced cell death in both imatinib-sensitive and -resistant cell lines; in the mononuclear cancer cells from either imatinib-sensitive or -resistant patients with CML, gambogic acid also had the same effects as in cultured cell lines while it affected the normal peripheral monocytes to a much less extent; in the in vivo experiment, both imatinib-sensitive and -resistant xenografted tumors were all sensitive to gambogic acid treatment. We have reported that gambogic acid acts as a prodrug of a proteasome inhibitor and only gains proteasome-inhibitory function after being metabolized by, for example, intracellular CYP2E1 (24). As expression of CYP2E1 gene is high in tumor tissues but low in some of the normal tissues, gambogic acid could therefore produce tumor tissue-specific proteasome inhibition and tumor-specific toxicity, which might have clinical significance for designing novel strategies for cancer treatment (24). Our current results have clearly demonstrated that gambogic acid could efficiently induce cytotoxicity in imatinib-resistant CML cells. To our knowledge, this is the first report to show that gambogic acid is effective in vitro and in vivo against CML cells, including those with the T315I mutation.
Proteasome inhibitors have been reported to be effective in overcoming imatinib-resistant cancer cells via either inhibiting Bcr-Abl expression (40), but the mechanism is far from being understood. Recently, we have reported that gambogic acid is a tissue-specific proteasome inhibitor, with potency comparable with bortezomib but much less toxicity (24). In the current study, we discovered a novel pathway that gambogic acid-mediated proteasome inhibition and caspase activation is responsible for the downregulation of Bcr-Abl protein, resulting in overcoming imatinib resistance. Like in other cancer cells (24), gambogic acid induced typical proteasome inhibition not only in wild-type Bcr-Abl or T315I-Bcr-Abl cell lines but also in mononuclear cancer cells derived from CML patients, including imatinib-resistant or -sensitive in vitro. It was confirmed that gambogic acid also inhibited proteasome function in the xenografted tumor model bearing wild-type Bcr-Abl or T315I-Bcr-Abl genes in vivo. We have reported that proteasome inhibition-induced Bax accumulation plays an important role in proteasome inhibition-mediated caspase activation and cell apoptosis (41). We also found that gambogic acid dose- and time-dependently induced Bax accumulation while anti-apoptotic proteins such as Bcl-2, XIAP, and Mcl-1 were dramatically decreased. The imbalance between apoptotic versus antiapoptotic factors would lead to the decrease of mitochondrial membrane integrity, thus inducing the release of cytochrome C and AIF. The released apoptotic factors either directly or by forming caspase-9 complex, induced caspase activation. These processes have been confirmed in our study. After caspase activation, on one hand it induced PARP cleavage and then apoptosis; on the other hand, it directly cleaved Bcr-Abl protein, which has been confirmed by us (Fig. 4C) and other authors (42). It has been reported that lysosome cathepsin B also contributed to Bcr-Abl cleavage (43), but in this study cathepsin B inhibitor did not affect gambogic acid-mediated Bcr-Abl cleavage (Fig. 4C). It has been further confirmed that gambogic acid-mediated proteasome inhibition is responsible for the above processes by using a C9–C10-disrupted gambogic acid. The C9–C10 double bond is responsible for gambogic acid–induced proteasome inhibition (24), and consistently the lost function of proteasome inhibition recovered all the changes of caspase activation, apoptosis induction, and Bcr-Abl downregulation (Fig. 4D).
Bcr-Abl is a constitutively active tyrosine kinase that phosphorylates several substrates, including Ras and Raf, and activates multiple signal transduction pathways. The downstream targets of STAT3 and STAT5 responsible for enhanced survival of Bcr-Abl cells are involved the transcription of Mcl-1, survivin, or Bcl-2 (44, 45). Treatment with gambogic acid resulted in downregulation of Mcl-1, Bcl-2, and XIAP (Fig. 2D). Even though gambogic acid may impact multiple molecules, but downregulation of Bcr-Abl is at least one of the major factors to induce apoptosis in CML cells. Taken together, both caspase-induced apoptosis and Bcr-Abl cleavage should contribute to gambogic acid–induced cytotoxicity.
In the current study, a solid tumor model was used. The synergistic effect of gambogic acid and imatinib was not observed either in vitro or in vivo, implying that gambogic acid alone could be an effective agent in Bcr-Abl-sensitive or -resistant CML. Considering the occurrence happened in leukemia, a nonsolid tumor model (46) is better than the solid-tumor model even though the solid tumor model has also been used in some previous studies (15). We have also further investigated changes in some proteins related to proliferation (Ki67; 47), differentiation (CD11b/c; ref. 48), adhesion, migration, and metastasis (CXCR4 and MMP2; ref. 49). These results could provide an explanation on the potential effects in CML to some extent.
The prognosis of imatinib-resistant CML is poor (13), and therefore, it is urgent to search for novel agents to overcome imatinib-resistance in clinical treatments. On the basis of present reports, most of the studies are mainly involved in finding tyrosine kinase inhibitors to either downregulate Bcr-Abl transcription or inhibit tyrosine kinase activity (32–34). Here, we propose an alternative strategy to enhance proteasome inhibition-induced Bcr-Abl downregulation by activating the caspase system. Caspase-dependent Bcr-Abl cleavage is effective both in the KBM5-T315I cells and in the cancer cells from imatinib-resistant patients with CML, indicating that this strategy has a great promise in overcoming imatinib resistance.
Supplementary Material
Translational Relevance.
Gambogic acid is a small molecule extracted from the traditional Chinese medicine gamboges, which has been used for hundreds of years in China. Gambogic acid has been approved by the Chinese Food and Drug Admin-istration for phase II clinical trial in solid tumor therapy. CML is characterized by the constitutive activation of Bcr-Abl tyrosine kinase. Bcr-Abl-T315I is the predominant mutation that causes resistance to imatinib.
The results showed that (i) gambogic acid induced apoptosis and cell proliferation inhibition in chronic myelogenous leukemia (CML) cells, including the cells harboring Bcr-Abl-T315I mutation and primary mono-nuclear cells from patients with CML resistant to imatinib; (ii) gambogic acid inhibited the growth of imatinib-resistant Bcr-Abl-T315I xenografts in nude mice; and (iii) gambogic acid–induced proteasome inhibition and caspase activation are required for gambogic acid–induced Bcr-Abl downregulation and cell apoptosis. We here propose an alternative strategy to overcome imatinib resistance by enhancing Bcr-Abl downregulation, which should have great clinical significance in imatinib-resistant cancer therapy.
Acknowledgments
Grant Support
This work was supported by the National High Technology Research and Development Program of China (2006AA02Z4B5), NSFC (81272451/H1609, 81070033/H0108), Key Project (10A057S) from Guangzhou Education Commission (to J. Liu); NSFC (81100378, 81272556/H1612), Projects (2012J4100014, B2012159) from the Foundation of GZ-STB and GD-NSF (to X. Shi); partially supported by Projects (S2011040000131, 2012J2200034) from GZ-STB and GD-NSF (to S. Liao).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: X. Shi, X. Chen, C. Zhao, S. Liao, J. Liu
Development of methodology: X. Shi, C. Zhao, S. Liao
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): X. Lan, S. Liu, S. Liao, S. Wang, L. Xu, J. Liu
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): X. Li, C. Zhao, S. Liu, N. Liu, S. Liao, W. Song, X. Wang, Q.P. Dou, J. Liu
Writing, review, and/or revision of the manuscript: X. Shi, S. Liao, Q.P. Dou, J. Liu
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S. Liao
Study supervision: S. Liao, J. Liu
Performed the research: H. Huang
References
- 1.Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441–453. doi: 10.1038/nrc2147. [DOI] [PubMed] [Google Scholar]
- 2.Schiffer CA, Hehlmann R, Larson R. Perspectives on the treatment of chronic phase and advanced phase CML and Philadelphia chromosome positive ALL(1) Leukemia. 2003;17:691–699. doi: 10.1038/sj.leu.2402879. [DOI] [PubMed] [Google Scholar]
- 3.Danial NN, Rothman P. JAK-STAT signaling activated by Abl oncogenes. Oncogene. 2000;19:2523–2531. doi: 10.1038/sj.onc.1203484. [DOI] [PubMed] [Google Scholar]
- 4.Gesbert F, Sellers WR, Signoretti S, Loda M, Griffin JD. BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway. J Biol Chem. 2000;275:39223–39230. doi: 10.1074/jbc.M007291200. [DOI] [PubMed] [Google Scholar]
- 5.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 6.Airiau K, Mahon FX, Josselin M, Jeanneteau M, Turcq B, Belloc F. ABT-737 increases tyrosine kinase inhibitor-induced apoptosis in chronic myeloid leukemia cells through XIAP downregulation and sensitizes CD34(+) CD38(−) population to imatinib. Exp Hematol. 2012;40:367–378. doi: 10.1016/j.exphem.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Amarante-Mendes GP, McGahon AJ, Nishioka WK, Afar DE, Witte ON, Green DR. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene. 1998;16:1383–1390. doi: 10.1038/sj.onc.1201664. [DOI] [PubMed] [Google Scholar]
- 8.Soliera AR, Mariani SA, Audia A, Lidonnici MR, Addya S, Ferrari-Amorotti G, et al. Gfi-1 inhibits proliferation and colony formation of p210BCR/ABL-expressing cells via transcriptional repression of STAT 5 Mcl-1. Leukemia. 2012;26:1555–1563. doi: 10.1038/leu.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Stuible M, Chalandon Y, Li A, Chan WY, Eisterer W, et al. Evidence for a positive role of SHIP in the BCR-ABL-mediated transformation of primitive murine hematopoietic cells and in human chronic myeloid leukemia. Blood. 2003;102:2976–2984. doi: 10.1182/blood-2003-05-1550. [DOI] [PubMed] [Google Scholar]
- 10.Thienelt CD, Green K, Bowles DW. New and established tyrosine kinase inhibitors for chronic myeloid leukemia. Drugs Today (Barc) 2012;48:601–613. doi: 10.1358/dot.2012.48.9.1869590. [DOI] [PubMed] [Google Scholar]
- 11.Gora-Tybor J, Robak T. Targeted drugs in chronic myeloid leukemia. Curr Med Chem. 2008;15:3036–3051. doi: 10.2174/092986708786848578. [DOI] [PubMed] [Google Scholar]
- 12.Druker BJ, Guilhot GF, O′Brien S, Larson N. Long-term benefits of imatinib (IM) for patients newly diagnosed with chronic myelogenous leukemia in chronic phase (CML-CP): the 5-year update from the IRIS study. Proc Am Soc Clin Oncol. 2006;24:338S. [Google Scholar]
- 13.Kantarjian HM, Talpaz M, Giles F, O'Brien S. Cortes J.New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance. Ann Intern Med. 2006;145:913–923. doi: 10.7326/0003-4819-145-12-200612190-00008. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP. Loss of response to imatinib: mechanisms and management. Hematology Am Soc Hematol Educ Program. 2005;1:183–187. doi: 10.1182/asheducation-2005.1.183. [DOI] [PubMed] [Google Scholar]
- 15.Shi X, Jin Y, Cheng C, Zhang H, Zou W, Zheng Q, et al. Triptolide inhibits Bcr-Abl transcription and induces apoptosis in STI571-resistant chronic myelogenous leukemia cells harboring T315I mutation. Clin Cancer Res. 2009;15:1686–1697. doi: 10.1158/1078-0432.CCR-08-2141. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl +human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007;109:4006–4015. doi: 10.1182/blood-2006-09-045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HZ, Kasibhatla S, Wang Y, Herich J, Guastella J, Tseng B, et al. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg Med Chem. 2004;12:309–317. doi: 10.1016/j.bmc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Guo QL, You QD, Zhao L, Gu HY, Yuan ST. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J Gastroenterol. 2005;11:3655–3659. doi: 10.3748/wjg.v11.i24.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo QL, Lin SS, You QD, Gu HY, Yu J, Zhao L, et al. Inhibition of human telomerase reverse transcriptase gene expression by gambogic acid in human hepatoma SMMC-7721 cells. Life Sci. 2006;78:1238–1245. doi: 10.1016/j.lfs.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 20.Chuah LO, Yeap SK, Ho WY, Beh BK, Alitheen NB. In vitro and in vivo toxicity of garcinia or hydroxycitric Acid: a review. Evid Based Complement Alternat Med. 2012;2012:197920. doi: 10.1155/2012/197920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez F, Babio N, Bullo M, Salas-Salvado J. Evaluation of the safety and efficacy of hydroxycitric acid or Garcinia cambogia extracts in humans. Crit Rev Food Sci Nutr. 2012;52:585–594. doi: 10.1080/10408398.2010.500551. [DOI] [PubMed] [Google Scholar]
- 22.Palempalli UD, Gandhi U, Kalantari P, Vunta H, Arner RJ, Narayan V, et al. Gambogic acid covalently modifies IkappaB kinase-beta subunit to mediate suppression of lipopolysaccharide-induced activation of NF-kappaB in macrophages. Biochem J. 2009;419:401–409. doi: 10.1042/BJ20081482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull. 2004;27:998–1003. doi: 10.1248/bpb.27.998. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Liu S, Huang H, Liu N, Zhao C, Liao S, et al. Gambogic acid is a tissue-specific proteasome inhibitor in vitro and in vivo. Cell Rep. 2013;3:211–222. doi: 10.1016/j.celrep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Heaney NB, Pellicano F, Zhang B, Crawford L, Chu S, Kazmi SM, et al. Bortezomib induces apoptosis in primitive chronic myeloid leukemia cells including LTC-IC and NOD/SCID repopulating cells. Blood. 2010;115:2241–2250. doi: 10.1182/blood-2008-06-164582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagani Z, Song K, Kutok JL, Dewar MR, Melet A, Santos T, et al. Proteasome inhibition causes regression of leukemia and abrogates BCR-ABL-induced evasion of apoptosis in part through regulation of forkhead tumor suppressors. Cancer Res. 2009;69:6546–6555. doi: 10.1158/0008-5472.CAN-09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dou QP, McGuire TF, Peng Y, An B. Proteasome inhibition leads to significant reduction of Bcr-Abl expression and subsequent induction of apoptosis in K562 human chronic myelogenous leukemia cells. J Pharmacol Exp Ther. 1999;289:781–790. [PubMed] [Google Scholar]
- 28.Xu F, Shi X, Cheng C, Lu Z, Lai Y, Pan J. Design, synthesis, and biological evaluation of novel water-soluble triptolide derivatives: antineoplastic activity against imatinib-resistant CML cells bearing T315I mutant Bcr-Abl. Bioorg Med Chem. 2010;18:1806–1815. doi: 10.1016/j.bmc.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Zhang X, Li S, Liu N, Lian W, McDowell E, et al. Physiological levels of ATP negatively regulate proteasome function. Cell Res. 2010;20:1372–1385. doi: 10.1038/cr.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Y, Lu Z, Cao K, Zhu Y, Chen Q, Zhu F, et al. The antitumor activity of homoharringtonine against human mast cells harboring the KIT D816V mutation. Mol Cancer Ther. 2010;9:211–223. doi: 10.1158/1535-7163.MCT-09-0468. [DOI] [PubMed] [Google Scholar]
- 31.Hisatomi T, Nakao S, Murakami Y, Noda K, Nakazawa T, Notomi S, et al. The regulatory roles of apoptosis-inducing factor in the formation and regression processes of ocular neovascularization. Am J Pathol. 2012;181:53–61. doi: 10.1016/j.ajpath.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 33.Kaur P, Feldhahn N, Zhang B, Trageser D, Muschen M, Pertz V, et al. Nilotinib treatment in mouse models of P190 Bcr/Abl lymphoblastic leukemia. Mol Cancer. 2007;6:67. doi: 10.1186/1476-4598-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morinaga K, Yamauchi T, Kimura S, Maekawa T, Ueda T. Overcoming imatinib resistance using Src inhibitor CGP76030, Abl inhibitor nilotinib and Abl/Lyn inhibitor INNO-406 in newly established K562 variants with BCR-ABL gene amplification. Int J Cancer. 2008;122:2621–2627. doi: 10.1002/ijc.23435. [DOI] [PubMed] [Google Scholar]
- 35.Grosso S, Puissant A, Dufies M, Colosetti P, Jacquel A, Lebrigand K, et al. Gene expression profiling of imatinib and PD166326-resistant CML cell lines identifies Fyn as a gene associated with resistance to BCR-ABL inhibitors. Mol Cancer Ther. 2009;8:1924–1933. doi: 10.1158/1535-7163.MCT-09-0168. [DOI] [PubMed] [Google Scholar]
- 36.Beyazit Y, Kekilli M, Haznedaroglu IC. Second-generation BCR-ABL kinase inhibitors in CML. N Engl J Med. 2010;363:1673. doi: 10.1056/NEJMc1007927. [DOI] [PubMed] [Google Scholar]
- 37.Bedi A, Barber JP, Bedi GC, el-Deiry WS, Sidransky D, Vala MS, et al. BCR-ABL-mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: a mechanism of resistance to multiple anticancer agents. Blood. 1995;86:1148–1158. [PubMed] [Google Scholar]
- 38.Bueno-da-Silva AE, Brumatti G, Russo FO, Green DR, Amarante-Mendes GP. Bcr-Abl-mediated resistance to apoptosis is independent of constant tyrosine-kinase activity. Cell Death Differ. 2003;10:592–598. doi: 10.1038/sj.cdd.4401210. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Chen Y, Zeng LL, Shu WX, Zhao F, Wen L, et al. Gambogic acid induces G0/G1 arrest and apoptosis involving inhibition of SRC-3 and inactivation of Akt pathway in K562 leukemia cells. Toxicology. 2009;262:98–105. doi: 10.1016/j.tox.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 40.Gatto S, Scappini B, Pham L, Onida F, Milella M, Ball G, et al. The proteasome inhibitor PS-341 inhibits growth and induces apoptosis in Bcr/Abl-positive cell lines sensitive and resistant to imatinib mesylate. Haematologica. 2003;88:853–863. [PubMed] [Google Scholar]
- 41.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci U S A. 2000;97:3850–3855. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Bacco AM, Cotter TG. p53 expression in K562 cells is associated with caspase-mediated cleavage of c-ABL and BCR-ABL protein kinases. Br J Haematol. 2002;117:588–597. doi: 10.1046/j.1365-2141.2002.03468.x. [DOI] [PubMed] [Google Scholar]
- 43.Puissant A, Colosetti P, Robert G, Cassuto JP, Raynaud S, Auberger P. Cathepsin B release after imatinib-mediated lysosomal membrane permeabilization triggers BCR-ABL cleavage and elimination of chronic myelogenous leukemia cells. Leukemia. 2010;24:115–124. doi: 10.1038/leu.2009.233. [DOI] [PubMed] [Google Scholar]
- 44.Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warsch W, Kollmann K, Eckelhart E, Fajmann S, Cerny-Reiterer S, Holbl A, et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood. 2011;117:3409–3420. doi: 10.1182/blood-2009-10-248211. [DOI] [PubMed] [Google Scholar]
- 46.Ilaria RL., Jr Animal models of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:525–543. doi: 10.1016/j.hoc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Cai X, Shen YL, Zhu Q, Jia PM, Yu Y, Zhou L, et al. Arsenic trioxide-induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia. Leukemia. 2000;14:262–270. doi: 10.1038/sj.leu.2401650. [DOI] [PubMed] [Google Scholar]
- 49.Li JK, Yu L, Shen Y, Zhou LS, Wang YC, Zhang JH. Inhibition of CXCR4 activity with AMD3100 decreases invasion of human colorectal cancer cells in vitro. World J Gastroenterol. 2008;14:2308–2313. doi: 10.3748/wjg.14.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.