Abstract
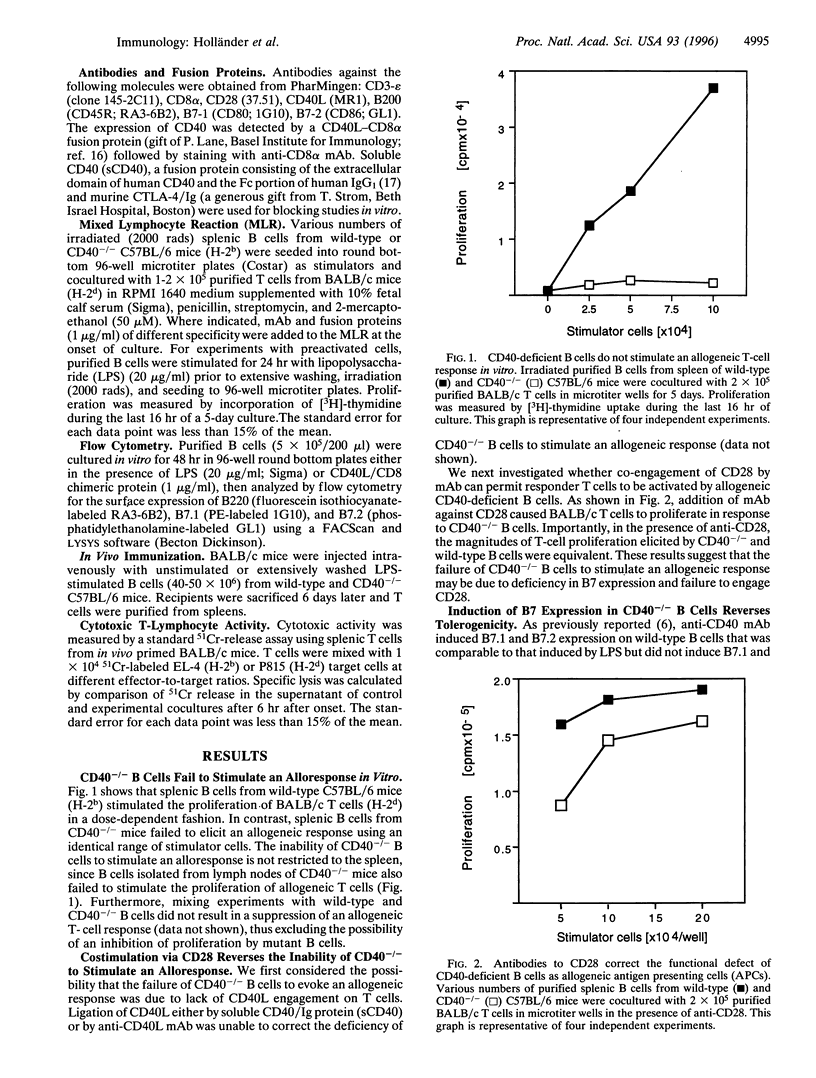
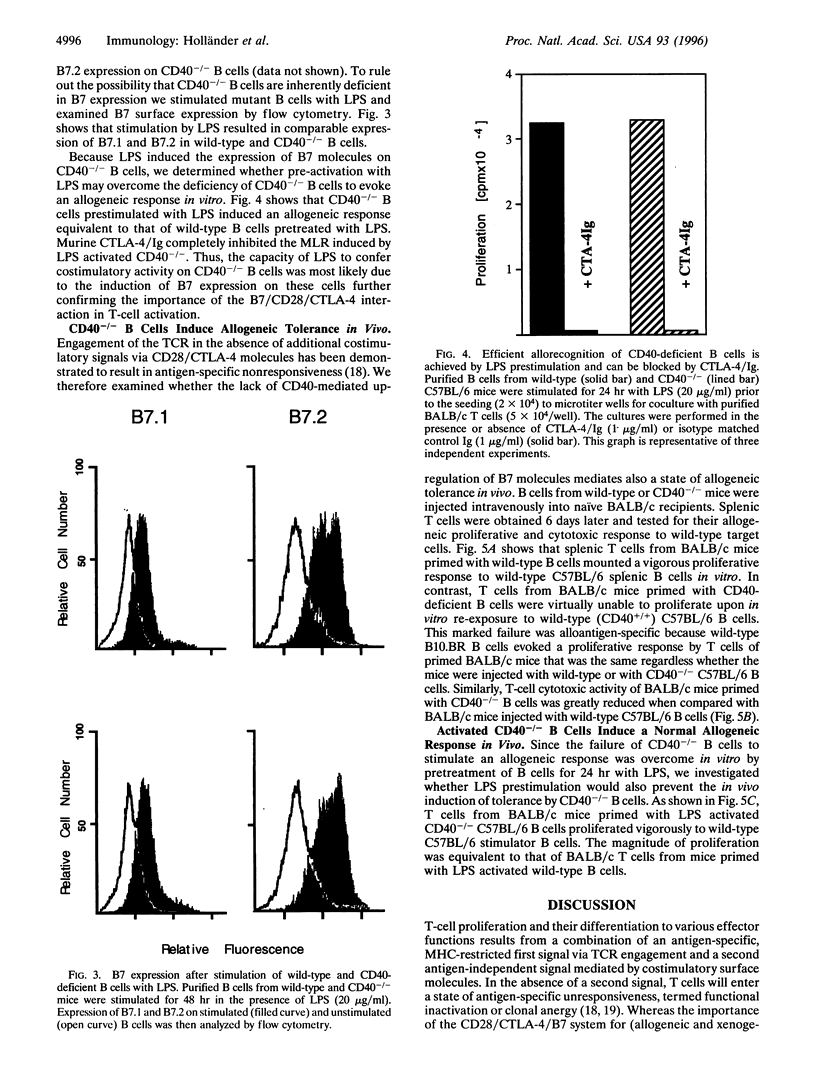
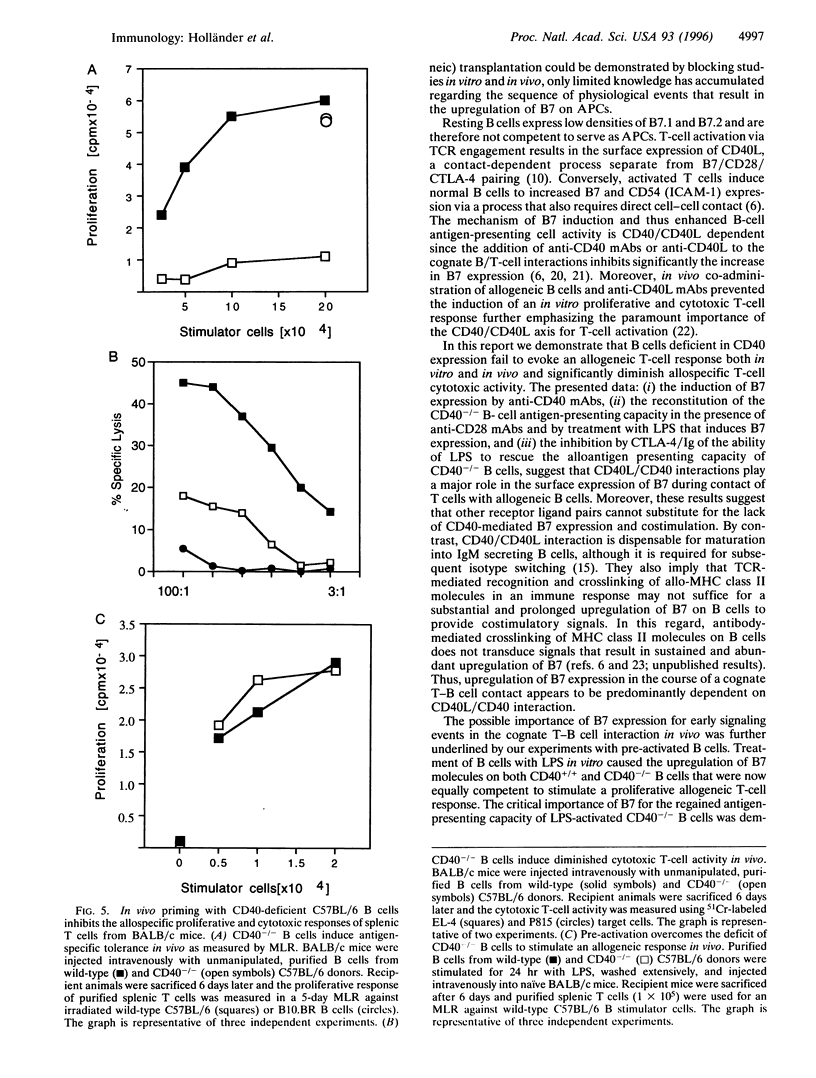
Interaction between CD40 on B cells and CD40 ligand molecules on T cells is pivotal for the generation of a thymus-dependent antibody response. Here we show that B cells deficient in CD40 expression are unable to elicit the proliferation of allogeneic T cells in vitro. More importantly, mice immunized with CD40-/- B cells become tolerant to allogeneic major histocompatibility complex (MHC) antigens as measured by a mixed lymphocyte reaction and cytotoxic T-cell assay. The failure of CD40-/- B cells to serve as antigen presenting cells in vitro was corrected by the addition of anti-CD28 mAb. Moreover, lipopolysaccharide stimulation, which upregulates B7 expression, reversed the inability of CD40-/- B cells to stimulate an alloresponse in vitro and abrogated the capacity of these B cells to induce tolerance in vivo. These results suggest that CD40 engagement by CD40 ligand expressed on antigen-activated T cells is critical for the upregulation of B7 molecules on antigen-presenting B cells that subsequently deliver the costimulatory signals necessary for T-cell proliferation and differentiation. Our experiments suggest a novel strategy for the induction of antigen-specific tolerance in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann J. E., Foy T. M., Aruffo A., Crassi K. M., Ledbetter J. A., Green W. R., Xu J. C., Shultz L. D., Roopesian D., Flavell R. A. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995 Jun;2(6):645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Castigli E., Alt F. W., Davidson L., Bottaro A., Mizoguchi E., Bhan A. K., Geha R. S. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V., Grosmaire L. S., Ledbetter J. A. Differential regulatory signals delivered by antibody binding to the CD28 (Tp44) molecule during the activation of human T lymphocytes. J Immunol. 1988 Mar 15;140(6):1753–1761. [PubMed] [Google Scholar]
- Durie F. H., Foy T. M., Masters S. R., Laman J. D., Noelle R. J. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. 1994 Sep;15(9):406–411. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy T. M., Durie F. H., Noelle R. J. The expansive role of CD40 and its ligand, gp39, in immunity. Semin Immunol. 1994 Oct;6(5):259–266. doi: 10.1006/smim.1994.1034. [DOI] [PubMed] [Google Scholar]
- Fuleihan R., Ramesh N., Loh R., Jabara H., Rosen R. S., Chatila T., Fu S. M., Stamenkovic I., Geha R. S. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hollenbaugh D., Grosmaire L. S., Kullas C. D., Chalupny N. J., Braesch-Andersen S., Noelle R. J., Stamenkovic I., Ledbetter J. A., Aruffo A. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992 Dec;11(12):4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara H. H., Fu S. M., Geha R. S., Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990 Dec 1;172(6):1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K. The ups and downs of T cell costimulation. Immunity. 1994 Sep;1(6):443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M. K., Mohler K. M., Shanebeck K. D., Baum P. R., Picha K. S., Otten-Evans C. A., Janeway C. A., Jr, Grabstein K. H. Induction of B cell costimulatory function by recombinant murine CD40 ligand. Eur J Immunol. 1994 Jan;24(1):116–123. doi: 10.1002/eji.1830240118. [DOI] [PubMed] [Google Scholar]
- Koulova L., Clark E. A., Shu G., Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991 Mar 1;173(3):759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Weiss A., Crabtree G., Davis M. M., Parham P. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med. 1990 Feb 22;322(8):510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- Lane P., Brocker T., Hubele S., Padovan E., Lanzavecchia A., McConnell F. Soluble CD40 ligand can replace the normal T cell-derived CD40 ligand signal to B cells in T cell-dependent activation. J Exp Med. 1993 Apr 1;177(4):1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jones B., Brady W., Janeway C. A., Jr, Linsley P. S., Linley P. S. Co-stimulation of murine CD4 T cell growth: cooperation between B7 and heat-stable antigen. Eur J Immunol. 1992 Nov;22(11):2855–2859. doi: 10.1002/eji.1830221115. [DOI] [PubMed] [Google Scholar]
- Ranheim E. A., Kipps T. J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993 Apr 1;177(4):925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh M. H., Watschinger B., Carpenter C. B. Mechanisms of T cell recognition of alloantigen. The role of peptides. Transplantation. 1994 May 15;57(9):1295–1302. doi: 10.1097/00007890-199405150-00001. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Armitage R. J., Strockbine L., Clifford K. N., Macduff B. M., Sato T. A., Maliszewski C. R., Fanslow W. C. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992 Dec 1;176(6):1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Hawrylowicz C. M., Unanue E. R. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8181–8185. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]


