Abstract
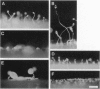
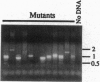
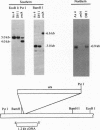
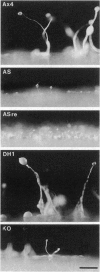
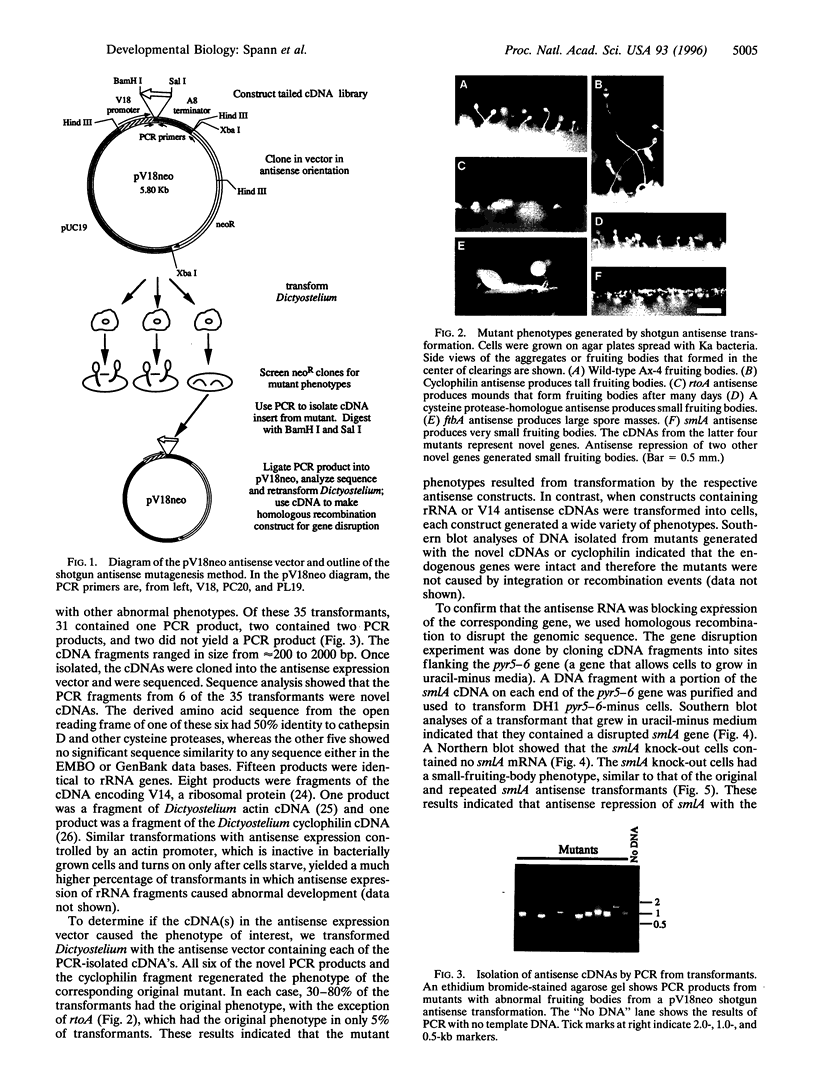
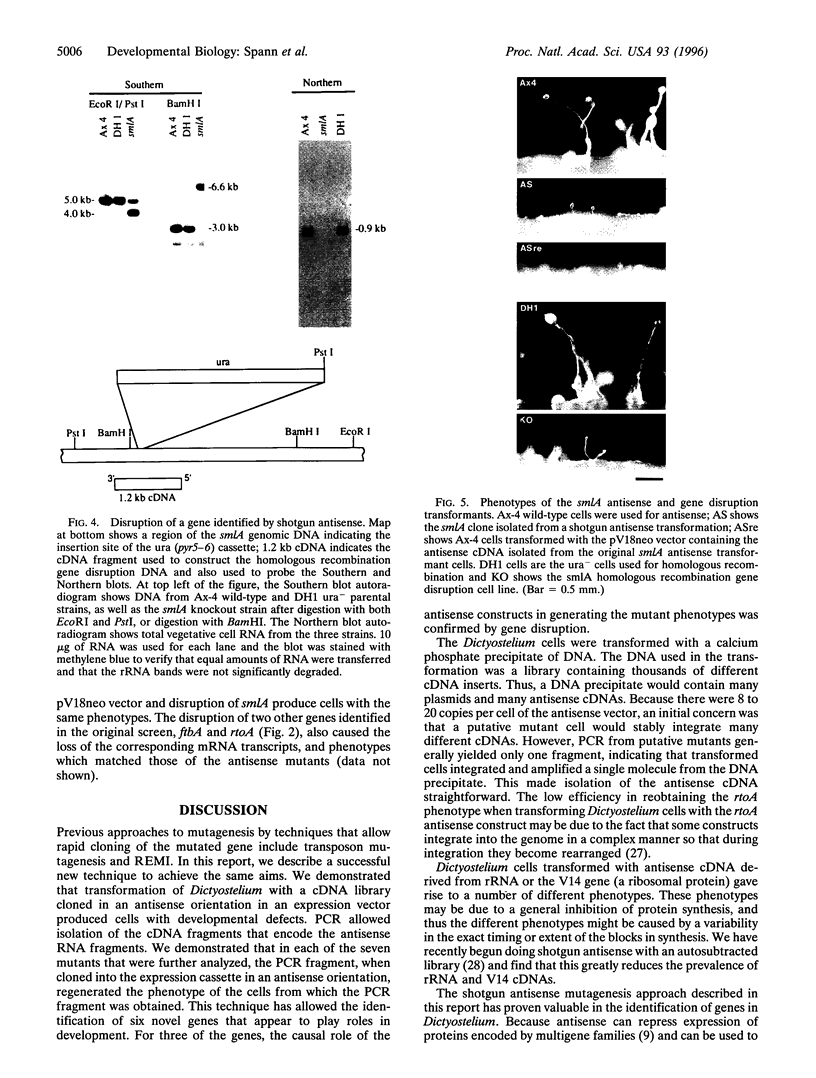
We have developed a mutagenesis technique that uses antisense cDNA to identify genes required for development in Dictyostelium discoideum. We transformed Dictyostelium cells with a cDNA library made from the mRNA of vegetative and developing cells. The cDNA was cloned in an antisense orientation immediately downstream of a vegetative promoter, so that in transformed cells the promoter will drive the synthesis of an antisense RNA transcript. We find that individual transformants typically contain one or occasionally two antisense cDNAs. Using this mutagenesis technique, we have generated mutants that fail to aggregate, aggregate but fail to form fruiting bodies, or aggregate but form abnormal fruiting bodies. The individual cDNA molecules from the mutants were identified and cloned using PCR. Initial sequence analysis of the PCR products from 35 mutants has identified six novel Dictyostelium genes, each from a transformant with one antisense cDNA. When the PCR-isolated antisense cDNAs were ligated into the antisense vector and the resulting constructs transformed into cells, the phenotypes of the transformed cells matched those of the original mutants from which each cDNA was obtained. We made homologous recombinant gene disruption transformants for three of the novel genes, in each case generating mutants with phenotypes indistinguishable from those of the original antisense transformants. Shotgun antisense thus is a rapid way to identify genes in Dictyostelium and possibly other organisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barisic K., Mollner S., Noegel A. A., Gerisch G., Segall J. E. cDNA sequence of cyclophilin from Dictyostelium discoideum. Dev Genet. 1991;12(1-2):50–53. doi: 10.1002/dvg.1020120110. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Early A. E., Williams J. G. Two vectors which facilitate gene manipulation and a simplified transformation procedure for Dictyostelium discoideum. Gene. 1987;59(1):99–106. doi: 10.1016/0378-1119(87)90270-8. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Silan C., Ward T. E., Howard P., Metz B. A., Nellen W., Jacobson A. Extrachromosomal replication of shuttle vectors in Dictyostelium discoideum. Mol Cell Biol. 1985 Nov;5(11):3241–3250. doi: 10.1128/mcb.5.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Yuen I. S., Taphouse C. R., Gomer R. H. A density-sensing factor controls development in Dictyostelium. Genes Dev. 1992 Mar;6(3):390–400. doi: 10.1101/gad.6.3.390. [DOI] [PubMed] [Google Scholar]
- Ken R., Singleton C. K. Redundant regulatory elements account for the developmental control of a ribosomal protein gene of Dictyostelium discoideum. Differentiation. 1994 Jan;55(2):97–103. doi: 10.1046/j.1432-0436.1994.5520097.x. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Jung J., Matthews L. Quantification of transformation efficiency using a new method for clonal growth and selection of axenic Dictyostelium cells. Dev Genet. 1990;11(5-6):403–409. doi: 10.1002/dvg.1020110513. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kuspa A., Loomis W. F. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiting B., Noegel A. Construction of an extrachromosomally replicating transformation vector for Dictyostelium discoideum. Plasmid. 1988 Nov;20(3):241–248. doi: 10.1016/0147-619x(88)90030-3. [DOI] [PubMed] [Google Scholar]
- Liu T., Williams J. G., Clarke M. Inducible expression of calmodulin antisense RNA in Dictyostelium cells inhibits the completion of cytokinesis. Mol Biol Cell. 1992 Dec;3(12):1403–1413. doi: 10.1091/mbc.3.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniak M., Nellen W. pISAR, a tool for cloning genomic sequences adjacent to the site of vector integration. Nucleic Acids Res. 1989 Jun 26;17(12):4894–4894. doi: 10.1093/nar/17.12.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M., Firtel R. A. Actin multigene family of Dictyostelium. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):495–505. doi: 10.1101/sqb.1982.046.01.046. [DOI] [PubMed] [Google Scholar]
- McPherson C. E., Singleton C. K. V4, a gene required for the transition from growth to development in Dictyostelium discoideum. Dev Biol. 1992 Apr;150(2):231–242. doi: 10.1016/0012-1606(92)90238-c. [DOI] [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanjali S. R., Parimoo S., Weissman S. M. Construction of a uniform-abundance (normalized) cDNA library. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1943–1947. doi: 10.1073/pnas.88.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D. L., Loomis W. F. Disruption of the sporulation-specific gene spiA in Dictyostelium discoideum leads to spore instability. Genes Dev. 1992 Jun;6(6):1058–1070. doi: 10.1101/gad.6.6.1058. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Manning S. S., Ken R. Primary structure and regulation of vegetative specific genes of Dictyostelium discoideum. Nucleic Acids Res. 1989 Dec 11;17(23):9679–9692. doi: 10.1093/nar/17.23.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K. Nucleotide sequence of V1, a ribosomal protein gene from Dictyostelium discoideum. Nucleic Acids Res. 1989 Oct 11;17(19):7989–7989. doi: 10.1093/nar/17.19.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. J., Devreotes P. N. Gene targeting of the aggregation stage cAMP receptor cAR1 in Dictyostelium. Genes Dev. 1991 Apr;5(4):572–582. doi: 10.1101/gad.5.4.572. [DOI] [PubMed] [Google Scholar]
- Sun T. J., Van Haastert P. J., Devreotes P. N. Surface cAMP receptors mediate multiple responses during development in Dictyostelium: evidenced by antisense mutagenesis. J Cell Biol. 1990 May;110(5):1549–1554. doi: 10.1083/jcb.110.5.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- Wilczynska Z., Fisher P. R. Analysis of a complex plasmid insertion in a phototaxis-deficient transformant of Dictyostelium discoideum selected on a Micrococcus luteus lawn. Plasmid. 1994 Sep;32(2):182–194. doi: 10.1006/plas.1994.1054. [DOI] [PubMed] [Google Scholar]
- Witke W., Nellen W., Noegel A. Homologous recombination in the Dictyostelium alpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 1987 Dec 20;6(13):4143–4148. doi: 10.1002/j.1460-2075.1987.tb02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]