Abstract
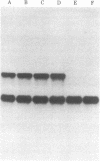
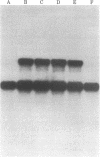
The ultimate product of yeast mating-type interconversion is a stable a/alpha diploid cell. A haploid cell carrying the HO gene gives rise to a diploid cell in a two-step process: first, the cell switches mating type as a result of genetic rearrangement (cassette substitution) catalyzed by HO; then, cells of opposite type mate to form a/alpha diploids. Mating-type interconversion does not occur in a/alpha diploids despite the presence of the HO gene. We have identified a plasmid carrying the HO gene by screening a yeast clone bank (constructed in vector YEp13) for plasmids that allow mating-type switching by ho cells. The yeast segment responsible for mating-type interconversion integrates by homology at the ho locus, thus confirming that it carries HO. Using the HO gene as a probe, we find that strains with an active mating-type interconversion system produce HO RNA, whereas a/alpha HO/HO cells do not and that this inhibition requires products of both the MATa1 and MATa2 genes. Thus, mating-type interconversion does not occur in a/alpha HO/HO cells because the HO gene product is not synthesized. These results demonstrate the following: (i) The mating-type locus, proposed on genetic grounds to be a regulatory locus, controls expression of an unlinked gene (HO) at the level of RNA production. (ii) The HO gene is under negative feedback control: its expression is inhibited after successful completion of diploidization (formation of a/alpha diploids).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Campbell A. Some general questions about movable elements and their implications. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):1–9. doi: 10.1101/sqb.1981.045.01.003. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Elder R. T., St John T. P., Stinchcomb D. T., Davis R. W., Scherer S., Davis R. W. Studies on the transposable element Ty1 of yeast. I. RNA homologous to Ty1. II. Recombination and expression of Ty1 and adjacent sequences. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):581–591. doi: 10.1101/sqb.1981.045.01.075. [DOI] [PubMed] [Google Scholar]
- Errede B., Cardillo T. S., Sherman F., Dubois E., Deschamps J., Wiame J. M. Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes. Cell. 1980 Nov;22(2 Pt 2):427–436. doi: 10.1016/0092-8674(80)90353-0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I., Hagen D. The lysis-lysogeny decision of phage lambda: explicit programming and responsiveness. Annu Rev Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types I. Direct Observations of the Action of the Homothallism (HO) Gene. Genetics. 1976 Jun;83(2):245–258. doi: 10.1093/genetics/83.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types II. Restoration of Mating Ability to Sterile Mutants in Homothallic and Heterothallic Strains. Genetics. 1977 Mar;85(3):373–393. doi: 10.1093/genetics/85.3.373b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassir Y., Simchen G. Regulation of mating and meiosis in yeast by the mating-type region. Genetics. 1976 Feb;82(2):187–206. doi: 10.1093/genetics/82.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S., Radin D. N. Switching of a mating-type a mutant allele in budding yeast Saccharomyces cerevisiae. Genetics. 1979 Jul;92(3):759–776. doi: 10.1093/genetics/92.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Hicks J. B., Strathern J. N. Irregular transpositions of mating-type genes in yeast. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):983–990. doi: 10.1101/sqb.1981.045.01.114. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Broach J. R., Hicks J. B. Regulation of transcription in expressed and unexpressed mating type cassettes of yeast. Nature. 1981 Jan 22;289(5795):239–244. doi: 10.1038/289239a0. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Hicks J. B. A position-effect control for gene transposition: state of expression of yeast mating-type genes affects their ability to switch. Cell. 1981 Aug;25(2):517–524. doi: 10.1016/0092-8674(81)90070-2. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Mackay V., Manney T. R. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. II. Genetic analysis of nonmating mutants. Genetics. 1974 Feb;76(2):273–288. doi: 10.1093/genetics/76.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae. Microbiol Rev. 1980 Dec;44(4):519–571. doi: 10.1128/mr.44.4.519-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Reed S. I. Isolation of genes by complementation in yeast: molecular cloning of a cell-cycle gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2119–2123. doi: 10.1073/pnas.77.4.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K., Hall B. D., Astell C., Smith M. A position effect in the control of transcription at yeast mating type loci. Nature. 1981 Jan 22;289(5795):244–250. doi: 10.1038/289244a0. [DOI] [PubMed] [Google Scholar]
- Rine J., Jensen R., Hagen D., Blair L., Herskowitz I. Pattern of switching and fate of the replaced cassette in yeast mating-type interconversion. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):951–960. doi: 10.1101/sqb.1981.045.01.112. [DOI] [PubMed] [Google Scholar]
- Rine J., Sprague G. F., Jr, Herskowitz I. rme1 Mutation of Saccharomyces cerevisiae: map position and bypass of mating type locus control of sporulation. Mol Cell Biol. 1981 Oct;1(10):958–960. doi: 10.1128/mcb.1.10.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Jensen R., Herskowitz I. Control of yeast cell type by the mating type locus: positive regulation of the alpha-specific STE3 gene by the MAT alpha 1 product. Cell. 1983 Feb;32(2):409–415. doi: 10.1016/0092-8674(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Rine J., Herskowitz I. Control of yeast cell type by the mating type locus. II. Genetic interactions between MAT alpha and unlinked alpha-specific STE genes. J Mol Biol. 1981 Dec 5;153(2):323–335. doi: 10.1016/0022-2836(81)90281-3. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Blair L. C., Herskowitz I. Healing of mat mutations and control of mating type interconversion by the mating type locus in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3425–3429. doi: 10.1073/pnas.76.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Herskowitz I. Asymmetry and directionality in production of new cell types during clonal growth: the switching pattern of homothallic yeast. Cell. 1979 Jun;17(2):371–381. doi: 10.1016/0092-8674(79)90163-6. [DOI] [PubMed] [Google Scholar]
- Strathern J., Hicks J., Herskowitz I. Control of cell type in yeast by the mating type locus. The alpha 1-alpha 2 hypothesis. J Mol Biol. 1981 Apr 15;147(3):357–372. doi: 10.1016/0022-2836(81)90488-5. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V. M., Young E. T., Ciriacy M. Transposable elements associated with constitutive expression of yeast alcohol dehydrogenase II. Cell. 1981 Feb;23(2):605–614. doi: 10.1016/0092-8674(81)90156-2. [DOI] [PubMed] [Google Scholar]