Abstract
Background
Ambient particles are associated with cardiovascular events, and recently with total plasma homocysteine. High total plasma homocysteine is a risk for human health. However, the biological mechanisms are not fully understood. One of putative pathways is through oxidative stress. We aimed to examine whether associations of PM2.5 and black carbon with homocysteine were modified by genotypes including HFE H63D, C282Y, CAT (rs480575, rs1001179, rs2284367 and rs2300181), NQO1 (rs1800566), GSTP1 I105V, GSTM1, GSTT1(deletion vs non-deletion) and HMOX-1 (any short vs both long). We attempted to replicate identified genes in an analysis of heart rate variability, and in other outcomes reported in the literature.
Methods
Study subjects were 1000 white non-Hispanic men in the Boston area, participating in a cohort study of aging. PM2.5, black carbon, total plasma homocysteine and other covariates were measured at several points in time between 1995 and 2006. We fit mixed models to examine effect modification of genes on associations of pollution with total plasma homocysteine.
Results
Interquartile range (IQR) increases in PM2.5 and black carbon (7-day moving averages) were associated with 1.5% (95% confidence interval = 0.2% to 2.8%) and 2.2% (0.6% to 3.9%) increases in total plasma homocysteine, respectively. GSTT1 and HFE C282Y modified effects of black carbon on total plasma homocysteine, and HFE C282Y and CAT (rs2300181) modified effects of PM2.5 on homocysteine. Several genotypes marginally modified effects of PM2.5 and black carbon on various endpoints. All genes with significant interactions with particulate air pollution had modest main effects on total plasma homocysteine.
Conclusions
Effects of PM2.5 and black carbon on various endpoints appeared to be mediated by genes related to oxidative stress pathways.
Exposure to ambient particulate matter (PM) is consistently associated with cardiovascular diseases.1– 7 However, the mechanisms by which PM exerts these effects are not fully understood. Putative biologic mechanisms include direct effects on the myocardium, disturbances of the cardiac autonomic nervous system, and pulmonary and systematic oxidative stress and inflammatory responses that trigger endothelial dysfunction, atherosclerosis, and coagulation/thrombosis.8, 9 Understanding the relative roles of such potential pathways is a major goal of recent air pollution epidemiology. Although traditional epidemiologic studies have been viewed as purely correlative, the ability to integrate genetics in this analysis of susceptibility factors enables population-based research to provide valuable clues to the underlying mechanisms for the associations observed.
Several studies have found that exposure to PM increases global oxidative stress. 10–14 For example, Gurgueira et al.11 reported that oxidative stress in cardiac tissue increased after adult Sprague-Dawley rats were exposed to concentrated ambient particles. Kim et al. 12 reported that levels of urinary 8-hydrox-2′-deoxyguanosine (a biomarker of oxidative DNA damage and repair) increased in workers after occupational exposure to fine particulate matter.
Hyperhomocysteinemia has been conjectured to be a major and independent risk factor for venous thrombosis, atherosclerosis, myocardial infarction, and stroke. 15–19 The elevation of blood concentration of total homocysteine induces endothelial cell injury, primarily through oxidative stress.20 Elevated plasma homocysteine concentrations may repress vasodilator nitric oxide21 and spark the proliferation of vascular smooth muscle cells,22 which may, in turn, raise the risk of cardiovascular diseases. In addition, homocysteine is associated with increased proinflammatory markers, such as interleukin-6, fibrinogen and C-reactive protein.23, 24 Our previous study showed that plasma homocysteine was independently associated with traffic-related pollutant exposures, especially PM2.5 and black carbon, after adjusting for confounders. 7 This association may be a part of the mechanisms whereby particles reduce endothelial function. We also reported that those associations were altered by blood concentrations of folate, vitamin B6, and B12, which is related to homocysteine metabolism.7 However, the exact biologic mechanisms linking air pollution exposure to elevated plasma homocysteine are not yet established. In particular, it is unclear whether particles influence homocysteine by increasing oxidative stress.
Several studies have demonstrated that certain genetic polymorphisms related to oxidative stress modified the effects of PM on cardiovascular responses.25–27 However, very limited studies examined a set of genetic polymorphisms and none of the studies considered homocysteine as an intermediary biomarker of effects. For example, Park et al. 25 reported that associations between exposure to PM2.5 and heart rate variability were modified by polymorphisms of hemochromatosis genes (C282Y and H63D). Variant carriers have increased uptake of transition iron into cells. In the absence of this uptake, transition metals can catalyze the formation of reactive oxygen species via Fenton chemistry. Other studies have shown that associations between exposure to PM2.5 and heart rate variability were modified by polymorphisms of the glutathione-S-transferase M1 (GSTM1) gene 26 or Heme Oxygenase-1 (HMOX-1), 27 enzymes that reduce the impact of reactive oxygen species. Zeka et al. 28 reported that black carbon was associated with elevated C-reactive protein in subjects who were GSTM1 null, but not with the gene.
The main purpose of this study was to examine associations of PM2.5 and black carbon with total plasma homocysteine, an intermediate outcome from the Normative Aging Study, and to examine whether a broad set of gene polymorphisms related to oxidative stress modified these associations. To avoid excessive enthusiasm about candidate genes, we also tested genes that were seen to modify the association of air particles with total plasma homocysteine to see if they also modified associations with heart rate variability--overall, the predominantly parasympathetic component, or the predominantly sympathetically driven component. This study also briefly reported effect modifications using other endpoints, of which effect modifications were seen by these genes from other reports. The set of candidate genotypes in this study include HFE, 29, 30 NAD(P)-quinone oxidoreductase (NQO1), 31 catalase (CAT), 32 glutathione-S-transferase (GST) M1 (GSTM1), P1 (GSTP1, Ile105Val), T1 (GSTT1) 26, 33 and HMOX-1 (GT repeated numbers: < 25 (short) and ≥ 25 (long)). 27
Methods
Study population
The Normative Aging Study is a longitudinal aging study established by the Veterans Administration (VA) in 1961 when 2,280 men from the greater Boston area, free of known chronic medical conditions, were enrolled. 34 Participants underwent detailed examination every 3 to 5 years, including routine physical examination, laboratory tests, collection of medical history, social status information, and administration of questionnaires on smoking history, food intake and other factors that may influence health. Between January 1995 and December 2006, all 1035 participants still appearing for examination were evaluated for homocysteine, gene polymorphisms and other covariates once or more times; 1000 (96.6%) of these men were non-Hispanic white. Due to the racial domination, we restricted all analyses to the non-Hispanic white participants, having a total of 2414 observations (271 with one visit, 232 two visits, 314 three visits, 178 four visits, and 5 five visits).
Plasma analysis of B vitamins and homocysteine
Fasting plasma samples were drawn at the VA field site and stored at −80 °C. Folate and total plasma homocysteine, vitamins B6 and B12 in fasting plasma were measured at the USDA Human Nutrition Research Center on Aging at Tufts University. Folate and vitamin B12 were assessed by radioassay using a commercial kit from Bio-Rad (Hercules, CA); vitamin B6 (as pyridoxal-5-phosphate) by an enzymatic method using tyrosine decarboxylase; and total plasma homocysteine using high-performance liquid chromatography with fluorescence detection. Further details are described elsewhere. 7, 35
Measurement of Heart Rate Variability
Heart rate variability was measured at rest during normal breathing for seven minutes using a two-channel (five-lead) ECG monitor (Model: Trillium 3000; Forest Medical, East Syracuse, NY) while the man was seated. Standard deviation of normal-to-normal intervals, high frequency (0.15 to 0.4 Hz), and low frequency (0.04 to 0.15 Hz) was computed with a Fast Fourier transform using software (Trillium 3000 PC Companion Software; Forest Medical) complying with established guidelines (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996).
Air pollution and Weather Data
PM2.5 and black carbon were measured at a stationary monitoring site 1 km from the examination site, with a tapered-element oscillating microbalance (model 1400A, Rupprecht & Pataschnick Co, East Greenbush, NY), aethalometer (Magee Scientific, Berkeley, Calif), respectively. The moving averages of PM2.5 and black carbon up to seven days before the visit were used as the exposure index because our preliminary analysis indicated that this exposure period was consistently associated with total plasma homocysteine.7 To adjust for outdoor weather, we used apparent temperature as an index, defined as a person’s perceived air temperature, given the humidity. 36
Genotypes
We selected a set of single nucleotide polymorphisms (SNPs) related to oxidative stress based on the gene functions and data available in this study: HFE H63D (rs1799945), HFE C282Y (rs1800562), CAT (rs480575, rs1001179, rs2284367 and rs2300181), GSTM1, GSTT1, GSTP1 I105V (rs1695), NQO1 (rs1800566) and HMOX-1. Previous studies have shown that variations of HFE C282Y, HFE H63D, HMOX-1 and GSTM1 genes modify associations between heart rate variability and PM2.5 or black carbon. 25–27 Glutathione pathways play a key role in cellular defenses against reactive oxygen species.33 GSTP1, GSTT1 and GSTM1 are members of the glutathione-S-transferase family involved in the mechanism of reactive oxygen species and xenobiotic components. 37 Deletions of GSTM1 and GSTT1 are prevalent, and the absence of their proteins has been associated with health outcomes. 26, 37 Catalase helps to maintain oxidative balance by converting hydrogen peroxide, a powerful reactive oxygen species, into water and molecular oxygen. 32 Polymorphisms of CAT genes have been shown to affect the transcriptional activity. 38 Similarly, NQO1 is also involved in reactive oxygen species mechanism. 39 We categorized HMOX-1 into two levels (any short and both long) based on repeated number of microsatellite (GTn) because previous studies have shown that high GT repeats at 5′-flanking region may reduce HMOX-1 inducibility by reactive oxygen species and has been associated with increased risk of cardiovascular diseases. 40, 41 Consequently, persons with a high number of GT repeats may be more susceptible to the effects of airborne particles. 27
Multiplex polymerase chain reaction assays were designed using Sequenom SpectroDESIGNER software (Sequenom Inc, San Diego, Calif) by inputting sequence containing the SNP site and 100 bp of flanking sequence on either side of the SNP. Assays were genotyped using the Sequenom MassArray MALDI-TOF mass spectrometer (Sequonom, CA, USA) with semiautomated primer design (SpectroDESIGNER, Sequenom) and implementation of the very short extension method. 42 Assays that failed to multiplex were genotyped using the TaqMan 5′ exonuclease [Applied Biosystems (ABI), Foster City, CA, USA] with primers from ABI using radioactive labeled probes detected using ABI PRISM 7900 Sequence Detector System. 43
Statistical analyses
The dataset had repeated measures over time, and the dependent variable might relate nonlinearly to covariates. We therefore used generalized additive mixed models (GAMM in R (version 2.7.2)) to incorporate random subject intercepts and the possibility that total plasma homocysteine is nonlinearly related to some continuous predictors such as temperature, seasonality, age and plasma vitamins. We used log-transformed total plasma homocysteine to improve normality and stabilize variance. We identified a priori the following variables as important determinants of homocysteine: age, serum creatinine, body mass index (BMI), systolic blood pressure (SBP), smoking status (never, former, current), pack-years of cigarettes smoked, alcohol consumption (≥ 2 drinks/day; yes/no), and plasma folate, vitamins B6 and B12, according to previous studies.7, 44, 45 We controlled for age, serum creatinine, pack-years smoked, plasma folate, vitamins B6 and B12, apparent temperature, and time (years) as continous variables, using default thin-plate regression spline. The degrees of freedoms were automatically selected via generalized cross-validation. 46 We further adjusted for alcohol use and smoking status, and adjusted for BMI and SBP as linear predictors.7 We adjusted for seasonality (days of the year) using seven degrees of freedom (df) per year.47, 48 We adjusted for apparent temperature using averages of apparent temperature in the same period as pollutants within three-day lags and then using three-day averages of apparent temperature for other lags. We conducted sensitivity analyses using different degrees of freedom for days of the year.
We then examined effect modification by genotypes. We categorized each genotype into a dummy variable (i.e, yes/no, or wild/non-wild, or short/long types). We combined heterozygous and homozygous types into non-wild type because of the small number of homozygous non-wild types. We introduced an interaction term for a pollutant and a gene, including both main-effect terms. We then estimated effects of each pollutant on total plasma homocysteine across genotypes by linearly combining coefficients of the main effect and interaction effect of the pollutant.
Polymorphisms that were significant modifiers of the association between particles and total plasma homocysteine were then tested to see if they modified the effects of particles on heart rate variability.
Results
Table 1 describes the demographic and clinical characteristics of participants, total plasma homocysteine measures, pollutants, and some covariate measures. The average age at visit was 72 years (95% confidence interval (CI) = 58 years, 86 years). Means of average concentrations of PM2.5 and black carbon in the past week were 11.3μg/m3 and 1.0μg/m3, respectively. Interquartile ranges (IQR) for weekly averages of PM2.5 and black carbon were 4.56 μg/m3 and 0.49 μg/m3, respectively.
Table 1.
Descriptive statistics of the demographic, health and environmental variables of observations at visit at 2414
| Variable | Values a |
|---|---|
| Age (years) | 72.0 (7.2) |
| Body mass index (kg/m2) | 28.0 (4.0) |
| Systolic blood pressure (mmHg) | 132.5 (17.5) |
| Fasting blood creatinine (mg/dl) | 1.07 (0.33) |
| Plasma folate (ng/L) | 15.2 (14.7) |
| Plasma pyridoxal-5-phosphate (nmol/L) | 105.7 (105.4) |
| Plasma vitamin B12 (pg/mL) | 505.4 (290.1) |
| Cumulative cigarette package years | 21.5 (26.7) |
| Average PM2.5 over 7 days (μg/m3) | 11.26 (3.98) |
| Average black carbon over 7 days (μg/m3) | 0.99 (0.39) |
| Average apparent temperature over 7 days (°C) | 11.8 (9.0) |
| Alcohol intake (≥ 2/day), % | 21 |
| Smoking status, % | |
| Never smoker | 29 |
| Current smoker | 4 |
| Former smoker | 66 |
Mean (SD) unless otherwise indicated.
Table 2 shows distributions of gene polymorphisms of CATs, NQO1, HFEs, GSTs and HMOX-1. Among 1000 participants, wild types were dominant for CATs, NQO1 and HFEs, but the situation varied for GST. 47% were classified as wild type for GSTP1. 79% and 43% of subjects were classified as non deletions for GSTT1 and GSTM1, respectively. Mean of the HMOX-1 GC repeated number was 26 (95% CI = 19.6 to 32.4) with median 24. Proportions for any-short and both-long HMOX-1 were approximately equal, using 24 repeated number of GC as the cutoff. We examined whether each genotype was independent within individuals, and found that CAT (rs480575), CAT (rs1001179) and HFE (rs1799945) departed significantly from Hardy-Weinberg equilibrium.
Table 2.
Genotype distribution of participants
| Polymorphism | Type | No. (%) |
|---|---|---|
| CAT (C/T) rs480575 b | Wild | 412 (51.2) |
| Heterozygous | 305 (37.9) | |
| Homozygous | 88 (10.9) | |
| CAT(A/G) rs1001179 b | Wild | 570 (65.4) |
| Heterozygous | 254 (29.2) | |
| Homozygous | 47 (5.4) | |
| CAT(G/A) rs2284367 | Wild | 481 (56.2) |
| Heterozygous | 315 (36.8) | |
| Homozygous | 60 (7.0) | |
| CAT (A/G) rs2300181 | Wild | 485 (55.2) |
| Heterozygous | 328 (37.3) | |
| Homozygous | 66 (7.5) | |
| NQO1 (T/C) rs1800566 | Wild | 597 (67.7) |
| Heterozygous | 254 (28.8) | |
| Homozygous | 31 (3.5) | |
| HFE (G/T) rs1799945 b | Wild | 696 (75.7) |
| Heterozygous | 199 (21.7) | |
| Homozygous | 24 (2.6) | |
| HFE (G/A) rs1800562 | Wild | 789 (86.0) |
| Heterozygous | 122 (13.3) | |
| Homozygous | 7 (0.8) | |
| GSTP1 (A/G) rs1695 | Wild Type | 421 (47.3) |
| Heterozygous | 384 (43.2) | |
| Homozygous | 85 (9.6) | |
| GSTT1 | Deletion | 141 (21.2) |
| Non deletion | 525 (78.8) | |
| GSTM1 | Deletion | 518 (56.6) |
| Non deletion | 397 (43.4) | |
| HMOX-1 | Both short | 83 (9.3) |
| Any short | 388 (43.4) | |
| Both long | 423 (47.3) |
The sum of the subjects in each genotype may not add up to the total number of subjects due to missing genotyping data. Missing genotyping is due to a variable number of samples for each locus for which genotyping was not successful.
significant departure from Hardy-Weinberg equilibrium (HWE) (p < 0.05).
We separately assessed associations using shorter averaging periods (previous day through 6-day moving average), adjusting for selected covariates. Results show that moving averages of pollutants over longer period exerted stronger effects on total plasma homocysteine (Table 3). IQR increases in 7-day moving averages of PM2.5 and black carbon were associated with 1.5 % (CI = 0.2 to 2.8%) and 2.2% (0.6% to 3.9%) increases in total plasma homocysteine, respectively.
Table 3.
Estimated percentage changes (log) in total homocysteine for an interquartile range increase in particulate air pollutants
| Lag model | PM2.5 | Black carbon | ||
|---|---|---|---|---|
|
| ||||
| IQR (μg/m3) | IQR Change; % (95% CI) a | IQR (μg/m3) | IQR Change; % (95% CI) a | |
| Current day | 7.48 | 0.89 (−0.18 to 1.96) | 0.71 | 0.68 (−0.46 to 1.81) |
| 1-Day previous | 7.07 | −0.02 (−1.08 to 1.04) | 0.71 | 0.43 (−0.67 to1.52) |
| 2-Day moving average | 6.64 | 0.79 (−0.37 to 1.95) | 0.64 | 0.78 (−0.43 to 1.98) |
| 3-Day moving average | 6.02 | 0.86 (−0.37 to 2.08) | 0.58 | 0.99 (−0.37 to 2.34) |
| 4-Day moving average | 5.40 | 1.26 (−0.01 to 2.53) | 0.54 | 1.85 (0.35 to 3.36) |
| 5-Day moving average | 4.98 | 1.41 (0.13 to 2.69) | 0.51 | 1.76 (0.20 to 3.31) |
| 6-Day moving average | 4.72 | 1.42 (0.13 to 2.70) | 0.51 | 1.79 (0.15 to 3.42) |
| 7-Day moving average | 4.56 | 1.52 (0.21 to2.82) | 0.49 | 2.22 (0.56 to 3.87) |
Adjusted for apparent temperature, age, serum creatinine, body mass index, systolic blood pressure, smoking status, pack-years of cigarettes smoked, alcohol consumption, plasma folate, vitamins B6 and B12, long-term trend and seasonality (using days of the year).
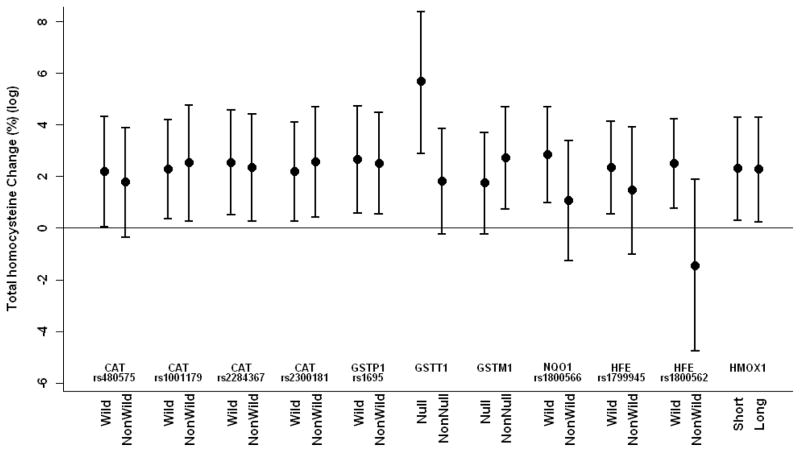
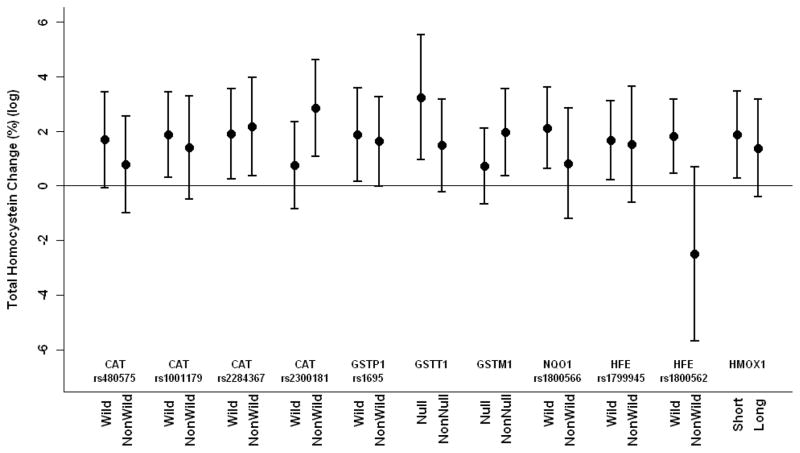
We separately estimated effect modifications of pollutants on total plasma homocysteine by individual gene polymorphisms using 7-day moving averages of PM2.5 and black carbon, because this exposure period showed the strongest associations with total plasma homocysteine. Associations of black carbon and PM2.5 with homocysteine were modified by genotypes. CAT (rs2300181) and HFE C282Y significantly modified associations between PM2.5 and total plasma homocysteine; GSTT1 and GSTM1 marginally significantly modified associations between PM2.5 and total plasma homocysteine; GSTT1 and HFE C282Y significantly modified associations between black carbon and total plasma homocysteine; and NQO1 marginally significantly modified associations between black carbon and total plasma homocysteine (Table 4 and Figures 1 and 2). An IQR increase of 7-day moving average of PM2.5 was associated with a 1.8% increase (95% CI = 0.5% to 3.2%) and 2.5% decrease (−5.7 to 0.7%) in total plasma homocysteine for wild and non-wild carriers of HFE C282Y, respectively. An IQR increase of 7-day moving average of black carbon was associated with a 2.5% increase (0.8% to 4.2%) and 1.4% decrease (−4.8 to 1.9%) in total plasma homocysteine (log) for wild and non-wild carriers of HFE C282Y, respectively. We also examined main effects of individual genes on homocysteine in the model. All genes with significant interactions had marginal significant main effects on total plasma homocysteine, while there were no signs of main effects for genes without significant interaction.
Table 4.
Adjusted percent change in homocysteine (log) associated with IQR increases of 7-day moving averages of PM2.5 and black carbon by gene polymorphisms
| Isoform | PM2.5 % change (95%CI) |
Black carbon % change (95% CI) |
|
|---|---|---|---|
| CAT (rs480575) | Wild | 1.68 (−0.08 – 3.44) | 2.19 (0.06 – 4.31) |
| Non-Wild | 0.78 (−0.99 – 2.56) | 1.76 (−0.36 – 3.88) | |
| CAT (rs1001179) | Wild | 1.87 (0.31 – 3.44) | 2.28 (0.36 – 4.19) |
| Non-Wild | 1.39 (−0.48 – 3.27) | 2.51 (0.27 – 4.75) | |
| CAT(rs2284367) | Wild | 1.9 (0.25 – 3.54) | 2.53 (0.5 – 4.56) |
| Non-Wild | 2.15 (0.36 – 3.95) | 2.34 (0.26 – 4.42) | |
| CAT (rs2300181) | Wild | 0.75 (−0.85 – 2.35) | 2.19 (0.27 – 4.11) |
| Non-Wild | 2.84 (1.06 – 4.62) | 2.56 (0.41 – 4.7) | |
| GSTP1 I105V(rs1695) | Wild | 1.87 (0.15 – 3.58) | 2.65 (0.59 – 4.71) |
| Non-Wild | 1.62 (−0.02 – 3.25) | 2.5 (0.54 – 4.46) | |
| GSTT1 | Deletion | 3.24 (0.97 – 5.51) | 5.63 (2.88 – 8.38) |
| Non-Deletion | 1.48 (−0.21 – 3.16) | 1.81 (−0.23 – 3.86) | |
| GSTM1 | Deletion | 0.72 (−0.66 – 2.1) | 1.74 (−0.22 – 3.7) |
| Non-Deletion | 1.96 (0.38 – 3.54) | 2.72 (0.74 – 4.69) | |
| NQO1 (rs1800566) | Wild | 2.12 (0.62 – 3.62) | 2.83 (0.97 – 4.69) |
| Non-Wild | 0.82 (−1.2 – 2.85) | 1.06 (−1.25 – 3.38) | |
| HFE (rs1799945) | Wild | 1.66 (0.21 – 3.11) | 2.34 (0.55 – 4.13) |
| Non-Wild | 1.51 (−0.62 – 3.64) | 1.45 (−1 – 3.9) | |
| HFE (rs1800562) | Wild | 1.81 (0.46 – 3.16) | 2.49 (0.76 – 4.22) |
| Non-Wild | −2.5 (−5.68 – 0.68) | −1.44 (−4.76 – 1.88) | |
| HMOX-1 | Any short | 1.86 (0.28 – 3.45) | 2.3 (0.3 – 4.29) |
| Both long | 1.38 (−0.41 – 3.17) | 2.26 (0.23 – 4.3) |
Adjusted for apparent temperature, age, serum creatinine, body mass index, systolic blood pressure, smoking status, pack-years of cigarettes smoked, alcohol consumption, plasma folate, vitamins B6 and B12, long-term trend and seasonality (using days of the year).
Figure 1.
Estimated percent change in homocysteine (log) (95% CI) associated with an IQR increase of 7-day moving average of black carbon by gene polymorphisms adjusting for apparent temperature, age, serum creatinine, body mass index, systolic blood pressure, smoking status, pack-years of cigarettes smoked, alcohol consumption, plasma folate, vitamins B6 and B12, and seasonal and long-term trends using days of the year.
Figure 2.
Estimated percent change in homocysteine (log) (95% CI) associated with an IQR increase of 7-day moving average of PM2.5 by gene polymorphisms adjusting for apparent temperature, age, serum creatinine, body mass indes, systolic blood pressure, smoking status, pack-years of cigarettes smoked, alcohol consumption, plasma folate, vitamins B6 and B12, and seasonal and long-term trends using days of the year.
Because several SNPs modified associations between particulate pollution and total plasma homocysteine, we conducted a sensitivity analysis, by combining the number of variants. We assigned 0 to the favorable variant, 1 to the heterozygous unfavorable variant and 2 to the homozygous unfavorable variant for CATs, NQO1, HFEs and GSTP1, and assigned 0 to non-deletion for GSTM1 and GSTT1 or any/both short for HMOX-1, and 1 to deletion for GSTM1 and GSTT1 or both long for HMOX-1; we then assess whether there was a trend with increasing number of variants in the pathway. We fit similar interaction models replacing single genes with scores adjusting for the same covariates. Effect modifications were observed for both PM2.5 and black carbon using accumulating continuous scores (P=0.094 and 0.087 for interaction terms of PM2.5 and black carbon, respectively). If the continuous scores are categorized into two levels by median, the significant effect modification was observed in PM2.5 (P =0.048 for interaction term) but not for black carbon (P = 0.22 for interaction term).
We then tested the genes that modified the effects of particles on total plasma homocysteine to see if they likewise modified the effects on the heart rate variability parameters. We examined whether GSTT1 modified associations between heart rate variability and PM2.5 or black carbon (adjusting for age, alcohol consumption, body mass index, smoking, season, etc., as done by Schwartz at el,26) and found marginal effect modification with low frequency and black carbon (P<0.2), but not for PM2.5 (Table 5). For HFE (rs1800562) we saw marginal effect modification for low frequency and for standard deviation of normal-to-normal intervals, again only for black carbon (Table 5).
Table 5.
Adjusted percent change in heart rate variability (log) associated with IQR increases of 7-day moving averages of PM2.5 and black carbon by gene polymorphisms
| Heart rate variability | Isoform | PM2.5 % change (95% CI) |
Black carbon % change (95% CI) |
|
|---|---|---|---|---|
| GSTT1 | High frequency | Deletion | 1.46 (−11.49 – 14.46) | −6.44 (−30.29 – 17.39) |
| High frequency | Non-Deletion | −3.74 (−11.54 – 4.01) | −1.33 (−16.01 – 13.35) | |
| HFE (rs1800562) | High frequency | Wild | −2.55 (−9.80 – 4.70) | −5.59 (−19.10 – 7.92) |
| High frequency | Non-Wild | 0.59 (−14.09 – 15.28) | 9.02 (−20.80 – 38.84) | |
| GSTT1 | Low frequency | Deletion | 0.36 (−10.58 – 11.31) | −13.61 (−33.88 – 6.67) |
| Low frequency | Non-Deletion | −1.32 (−7.89 – 5.29) | 0.95 (−11.53 – 13.44) | |
| HFE (rs1800562) | Low frequency | Wild | −1.23 (−7.34 – 4.88) | −6.02 (−17.48 – 5.44) |
| Low frequency | Non-Wild | 0.41 (−12.08 – 12.95) | 7.97 (−17.27 – 33.20) | |
| GSTT1 | Standard deviation of normal-to-normal intervals | Deletion | 1.23 (−4.38 – 6.84) | −3.83 (−14.4 – 6.740) |
| Standard deviation of normal-to-normal intervals | Non-Deletion | −1.73 (−5.11 – 1.64) | −2.94 (−9.37– 3.51) | |
| HFE (rs1800562) | Standard deviation of normal-to-normal intervals | Wild | −1.46 (−4.61 –1.69) | −5.35 (−11.26– 0.55) |
| Standard deviation of normal-to-normal intervals | Non-Wild | 1.41 (−5.15 – 7.93) | 4.48 (−8.54 – 17.50) |
Interquartile ranges (IQR) of 7-day moving averages of PM2.5 and BC were 4.56 and 0.49 μg/m3, respectively.
Discussion
The present study confirms our previous results—that PM2.5 and, more strongly, black carbon are associated with elevated plasma homocysteine. We found effect modification by a set of gene polymorphisms. We also confirmed the increasingly common finding of genes with only marginal main effects, but significant interactions with markers of air pollution. We believe this observation is important, particularly with the advent of higher density genotyping studies. A common strategy is to examine only genes with an observed main effect on the phenotype. This strategy seems likely to miss many important interactions. Our work suggests that this may be a common scenario, as the main effects for both genetic and environmental factors may simply represent unmeasured effect modification and therefore are weak estimates of independent effects. By correctly modeling the variance as a gene-environment interaction, sufficient power is gained to detect the underlying relationship.
This study found that associations of PM2.5 and black carbon with total plasma homocysteine were modified by polymorphisms of HFE C282Y and GSTT1. We have also shown that particles exerted stronger effects on total plasma homocysteine among subjects carrying the wild-type HFE-C282Y gene variant or carrying the deletion of GSTT1. In our replication analyses, these polymorphisms marginally modified the association of particles with heart rate variability parameters. These findings are consistent with our previous studies, as well as with others using different endpoints. For example, Park et al. 25 reported that this HFE variant modified the effects of particulate pollution on heart rate variability in a subset of our data. Schwartz et al 26 reported an association between PM2.5 and parasympathetic part among those without the GSTM1 allele, but not for those with the allele. Our colleagues49 have examined possible modification of the associations between PM2.5 and soluble vascular cell adhesion molecule-1 or intercellular adhesion molecule by polymorphisms of HFE and GSTM1; polymorphisms of HFE but not for GSTM1 marginally modified effects of PM2.5 on VCAM. Earlier, Zeka28 reported that GSTM1 modified the effects of particles on C-reactive protein. Hence there is a good consistency for modification by HFE, and broad consistency for modification by polymorphisms in Glutathione S Transferases, although somewhat weaker for specific GSTs.27, 28
Our study utilized a well-described biomarker of oxidative stress and cardiovascular health- plasma homocysteine levels. Homocysteine is made from methionine in a multi-step reaction via S-adenosyl methionine. Homocysteine not obtained from the diet is a normal temporary and chemically-reactive product measured in blood. A large body of evidence suggests that a high plasma homocysteine concentration is a powerful and independent risk factor for cardiovascular diseases. 15, 16, 18, 19 In normal situations, homocysteine can be recycled into methionine or permanently converted to cysteine via the transsulfuration pathway. Cysteine further joins syntheses of proteins or glutathione (an antioxidant) through complex enzymatic pathways. Biologically, cysteine and homocysteine metabolism is directly related to GST isozymes and indirectly to HFE and catalase metabolism, as iron is critical to the Fenton reaction that produces hydrogen peroxide, and catalase is critical to the detoxification of hydrogen peroxide.29 Therefore, it is possible that elevated homocysteine may be related to oxidative stress enzymes such as HFEs, CATs, NQO1 and GSTs. We believe these established metabolic processes lend strength to our studies, potentially describing biologic mechanisms whereby air pollution particles can damage cardiovascular health.
Glutathione is a tripeptide antioxidant, containing an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side chain. It plays a vital role in cellular defenses against reactive oxygen species. 33 GSTs are a family of enzymes involved in the metabolism of reactive oxygen species and xenobiotic compounds, expressed nearly ubiquitously in human tissues. Previous studies have shown that GSTM1 null variants modified effects of particles and ozone on heart rate variability or lung function.26, 31 This study observed that GSTT1 modified associations of black carbon and of PM2.5 with total plasma homocysteine, but with no clear modification by GSTM1. GSTT1 catalyzes the conjugation of glutathione to numerous potentially genotoxic compounds.50 Individuals with homozygous deletion of GSTM1 or GSTT1 have reduced GST activity and thus may be unable to eliminate toxins as efficiently. 50 Therefore, glutathione deletion and conjugation may be reduced among those who are null for the GSTT1 gene when exposed to particles. Differences in effect modification between M1 and T1 null variants may reflect tissue-specific expression difference, measurement error in genotyping, measurement error in exposure or phenotype, or chance.
This study also found that HFE C282Y modified associations of black carbon and PM2.5 with total plasma homocysteine, black carbon and PM2.5 appear to exert weaker effects on homocysteine among individuals with at least one copy of HFE C282Y mutation than for those with wild-type HFE C282Y. Such effect modification was not obvious for the HFE H63D allele. Variants in HFE are the major risk factors for adult-onset hemochromatosis. C282Y and H63D variants are described in details elsewhere, but are 2 primary functional variants responsible for this disease. 51 The penetrance of the C282Y variant is considerably higher than that of the H63D variant in hereditary hemochromatosis patients but is still less than 10% even for those homozygous for C282Y in common population. 30, 52 The HFE protein product binds to β2 microglobulin and is a regulatory factor that determines transferrin receptor affinity for iron-loaded transferrin. Binding by the transferrin receptor to HFE reduces affinity of the receptor for iron-loaded transferrin by 5- to 10-fold, thereby inhibiting iron transfer across cell membranes. 30 The C282Y variant alters the HFE protein structure, disrupting its transport to and presentation on the cell surface. The net result is increased transfer of iron across the cell membrane. The C282Y variant results in a greater loss of protein function than does H63D. 53
The Catalase gene is 34kb in length and split into 13 exons, encoding for a protein of 526 amino acids. 54 Catalase is the main regulator of hydrogen peroxide metabolism. 55 Hydrogen peroxide is a by-product of physiologic processes, and oxidase enzymes and is also produced as a part of defense mechanism against oxygen free radicals. The reactive superoxide anion is converted into less toxin hydrogen peroxide by superoxide dismutase. Catalase enzyme mutations may reduce the catalase activity 56 and potentially increase concentrations of hydrogen peroxide. Inherited catalase deficiency will result in acatalasemia (homozygous state) and hypocatalasemia (heterozygous). 57 It has been associated with increased plasma homocysteine concentrations. 32 In recent years, many studies have reported that polymorphism of CAT-262C>T is associated with modified catalase activity. 56, 58,–61 No functional CAT modification has been reported for the CAT polymorphism so far.
Experimental studies have shown that ambient particles toxicologically act via the oxidative stress pathway. 10, 62, 63 Ghio et al. 62 found that homozygous Belgrade rats functionally deficient in divalent metal transporter-1 (DMT1) display decreased metal transport from the lower respiratory tract and have stronger lung injury than control littermates, when exposed to oil fly ash containing iron. Belgrade rats cannot transport iron and other divalent metals across membranes via HFE gene-regulated processes. They also reported that healthy volunteers exposed to concentrated ambient air particles had increased concentrations of blood fibrinogen and induced mild pulmonary inflammation. 10 Tamagawa et al. 63 reported that the exposure of New Zealand rabbits to PM10 for 5 days and 4 weeks caused acute and chronic lung and systematic inflammation that were both associated with vascular endothelial dysfunction.
There are several limitations with this study. We used air pollution concentrations from a single monitoring site, which might lead to exposure misclassification. The extent of error depends on the spatial homogeneity of the exposure, as we compared exposures with temporality. A recent study compared ambient concentrations with personal exposures using monitoring measurement, and results showed high correlation between these two measurements for PM2.5 in the Boston area. 64 In contrast, black carbon concentrations were more spatially varied. Our previous analyses showed greater exposure errors for black carbon, although concentrations were highly correlated between 14 monitoring sites (coefficient 0.54 to 0.94). 7 Nevertheless, with non-differential misclassification, any potential bias would be expected toward the null. The Normative Aging Study consists of an aged population of mainly non-Hispanic white men. Therefore, the findings are not well generalizable to other populations. In addition, although the availability of a set of oxidative stress-related genes was beneficial for consistent assessment, this might to some degree introduce the problem, of multiple comparisons, thus increasing the uncertainty.
In conclusion, this study found that variations of oxidative stress-related genes modified effects of black carbon/PM2.5 on total plasma homocysteine, which is consistent with effects on heart rate variability and various other endpoints. Persons carrying the deletion of GSTT1 or wild types of HFE C282Y had higher plasma homocysteine than those carrying non-deletion or non-wild types of corresponding genes when they exposed to ambient particles. This suggests that effects of black carbon and PM2.5 on plasma homocysteine and other endpoints may be mediated by the oxidative stress pathway.
Acknowledgments
Financial Support:
Supported by the U.S. Environmental Protection Agency grants EPA R827353 and R832416; National Institute of Environmental Health Sciences (NIEHS) grants RO1-ES015172, ES014663, ES00002, PO1_ES008925, ES05257, P42-ES05947, and ES10798; and U.S. Department of Agriculture contract 53-3K06-5-10 and 53-1950-7-707. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
References
- 1.Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 1995;142:23–35. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz J. Air pollution and hospital admissions for heart disease in eight U.S. counties. Epidemiology. 1999;10:17–22. [PubMed] [Google Scholar]
- 3.Ren C, Tong S. Health effects of ambient air pollution – recent research development and contemporary methodological challenges. Environ Health. 2008;7:56. doi: 10.1186/1476-069X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laden F, Schwartz J, Speizer FE, et al. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard six cities study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baccarelli A, Martinelli I, Zanobetti A, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med. 2008;168:920–927. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SK, O’Neill MS, Vokonas PS, et al. Traffic-related particles are associated with elevated homocysteine – the VA Normative Aging Study. Am J Respir Crit Care Med. 2008;178:283–289. doi: 10.1164/rccm.200708-1286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 9.Ren C, Baccarelli A, Wilker E, et al. Lipid and endothelial related genes, ambient particulate matter, and heart rate variability -- the VA Normative Aging Study. J Epidemiol Community Health. 2009 doi: 10.1136/jech.2008.083295. online July 13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162:981–988. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- 11.Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JY, Mukherjee S, Ngo L, et al. Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulates. Environ Health Perspect. 2004;112:666–671. doi: 10.1289/ehp.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinzents PS, Møller P, Sørensen M, et al. Personal exposure to ultrafine particles and oxidative DNA damage. Environ Health Perspect. 2005;113:1485–1490. doi: 10.1289/ehp.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 15.Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infacrction in US physicians. JAMA. 1992;268:877–881. [PubMed] [Google Scholar]
- 16.Selhub J, Jacques PF, Bostom AG, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg M, Stehouwer CD, Bierdrager E, et al. Plasma homocysteine and severity of atherosclerosis in young patients with lower-limb atherosclerostic disease. Arterioslcer Thromb Vas Biol. 1996;16:165–171. doi: 10.1161/01.atv.16.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 19.Perry IJ, Refsum H, Morris RW, et al. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- 20.Chambers JC, McGregor A, Jean-Marie J, et al. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia – an effect reversible with vitamin C therapy. Circulation. 1999;99:1156–1160. doi: 10.1161/01.cir.99.9.1156. [DOI] [PubMed] [Google Scholar]
- 21.Stühlinger MC, Oka RK, Graf EE, et al. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 2003;108:933–938. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- 22.Buemi M, Marino D, Pasquale GD, et al. Effects of homocysteine on proliferation, necrosis, and apoptosis of vascular smooth muscle cells in culture and influence of folic acid. Thromb Res. 2001;104:207–213. doi: 10.1016/s0049-3848(01)00363-2. [DOI] [PubMed] [Google Scholar]
- 23.Gori AM, Corsi AM, Fedi S, et al. A proinflammatory state is associated with hyperhomocysteinenia in the elderly. Am J Clin Nutr. 2005;82:335–341. doi: 10.1093/ajcn.82.2.335. [DOI] [PubMed] [Google Scholar]
- 24.Evans RW, Shaten BJ, Hempel JD, et al. Homocyst(e)ine and risk of cardiovascular disease in the multiple risk factor intervention trial. Arterioscler Thromb Vasc Biol. 1997;17:1947–1953. doi: 10.1161/01.atv.17.10.1947. [DOI] [PubMed] [Google Scholar]
- 25.Park SK, O’Neill MS, Wright RO, et al. HFE genotype, particulate air pollution, and heart rate variability—A gene-environment interaction. Circulation. 2006;114:2798–2805. doi: 10.1161/CIRCULATIONAHA.106.643197. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz J, Park SK, O’Neill MS, et al. Glutathione-s-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chahine T, Baccarelli A, Litonjua A, et al. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeka A, Sullivan JR, Vokonas PS, et al. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int J Epidemiol. 2006;35:1347–1354. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
- 29.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 30.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferring receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci USA. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergamaschi E, De Palma G, Mozzoni P, et al. Polymorphism of quinone-metabolizing enzymes and susceptibility to ozone-induced acute effects. Am J Respir Crit Care Med. 2001;163:1426–1431. doi: 10.1164/ajrccm.163.6.2006056. [DOI] [PubMed] [Google Scholar]
- 32.Góth L, Vitai M. The effects of hydrogen peroxide promoted by homocysteine and inherited catalase deficiency on human hypocatalasemic patients. Free Radic Biol Med. 2003;35:882–888. doi: 10.1016/s0891-5849(03)00435-0. [DOI] [PubMed] [Google Scholar]
- 33.Hayes JD, McLellan LI. Glutathione and glutathione dependent enzymes respresent a co-ordinately regulated defense against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 34.Bell B, Rose C, Damon A. The veterans Administration longitudinal study of healthly aging. Gerontologist. 1966;6:179–184. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- 35.Tucker KL, Qiao N, Scott T, et al. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 36.Kalkstein L, Valamont K. An evaluation of summer discomfort in the United States using a relative climatologic index. Bull Am Meteorol Soc. 1986;67:842–848. [Google Scholar]
- 37.Gilliland FD, LIYF, Saxon A, et al. Effect of glutathione-S-transferase M1 and P1 genetypes on xenobiotic enhancement of allergic responses: randomized, placebo-controlled crossover study. Lancet. 2004;363:119–125. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 38.Forsberg L, Lyrenäs L, de Faire U, et al. A common functional C-T substitution polymorphisms in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic Biol Med. 2001;30:500–505. doi: 10.1016/s0891-5849(00)00487-1. [DOI] [PubMed] [Google Scholar]
- 39.Rothman N, Smith MT, Hayes RB, et al. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1609C→T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997;57:2839–2842. [PubMed] [Google Scholar]
- 40.Chen YH, Lin SJ, Lin MW, et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetes patients. Hum Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 41.Kaneda H, Ohno M, Taguchi J, et al. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol. 2002;22:1680–1685. doi: 10.1161/01.atv.0000033515.96747.6f. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Ding H, Hung K, et al. A new MALDI-TOF based mini-sequencing assay for genotyping of SNPS. Nucleic Acids Res. 2000;28:e68. doi: 10.1093/nar/28.12.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee LG, Connell CR, Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Res. 1993;21:3761–3766. doi: 10.1093/nar/21.16.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bree A, Verschuren WM, Blom HJ, et al. Lifestyle factors and plasma homocysteine concentrations in a general population sample. Am J Epidemiol. 2001;154:150–154. doi: 10.1093/aje/154.2.150. [DOI] [PubMed] [Google Scholar]
- 45.Ganji V, Kafai MR. Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2003;77:826–833. doi: 10.1093/ajcn/77.4.826. [DOI] [PubMed] [Google Scholar]
- 46.Wood SN. Thin plate regression splines. JR Statist Soc B. 2003;65:95–114. [Google Scholar]
- 47.Daniels MJ, Dominici F, Samet JM, Zeger S. Estimating particulate matter-mortality dose-response curves and threshold levels: an analysis of daily time-series for the 20 largest US cities. Am J Epidemiol. 2000;152:397–406. doi: 10.1093/aje/152.5.397. [DOI] [PubMed] [Google Scholar]
- 48.Ren C, Williams GM, Tong S. Does particulate matter modify the association between temperature and cardiorespiratory diseases? Environ Health Perspect. 2006;114:1690–1696. doi: 10.1289/ehp.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madrigano J, Baccarelli A, Wright RO, Suh H, Sparrow D, Vokonas PS, Schwart J. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med. 2009 doi: 10.1136/oem.2009.046193. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coughlin SS, Hall IJ. Glutathione S-transferase polymorphisms and risk of ovarian cancer: a HuGE review. Genet Med. 2002;4:250–257. doi: 10.1097/00125817-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 52.Hanson EH, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Am J Epidemiol. 2001;154:193–206. doi: 10.1093/aje/154.3.193. [DOI] [PubMed] [Google Scholar]
- 53.Feder JN, Tsuchihashi Z, Irrinki A, et al. The hemochromatosis founder mutation in HLA-H disrupts β2-microglobulin interaction and cell surfaces expression. J Biol Chem. 1997;272:14025–14028. doi: 10.1074/jbc.272.22.14025. [DOI] [PubMed] [Google Scholar]
- 54.Quan F, Korneluk RG, Tropak MB, et al. Isolation and characterization of the human catalase gene. Nucleic Acids Res. 1986;14:5321–5335. doi: 10.1093/nar/14.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mueller S, Riedel HD, Stremmel W. Direct evidence for catalase as the predominat H2O2 removing enzyme in erythrocytes. Blood. 1997;90:4973–4978. [PubMed] [Google Scholar]
- 56.Ahn J, Nowell S, McCann SE, et al. Associations between catalase phenotype and genetype: modification by epidemiologic factors. Cancer Epidemiol Biomarkers Prev. 2006;15:1217–1222. doi: 10.1158/1055-9965.EPI-06-0104. [DOI] [PubMed] [Google Scholar]
- 57.Góth L, Rass P, Páy A. Catalase enzyme mutations and their association with diseases. Mol Diagn. 2004;8:141–149. doi: 10.1007/BF03260057. [DOI] [PubMed] [Google Scholar]
- 58.Capurso C, Solfrizzi V, D’Introno A, et al. Short arm of chromosome 11 and sporadic Alzheimer’s disease: catalase and cathepsin D gene polymorphisms. Neurosci Lett. 2008;432:237–242. doi: 10.1016/j.neulet.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 59.Islam T, McConnell, Gauderman WJ, et al. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 2008;177:388–395. doi: 10.1164/rccm.200706-863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mak JCW, Leung HCM, Ho SP, et al. Polymorphisms in mangases superoxide dismutase and catalase genes: functional study in Hong Kong Chinese asthma patients. Clin Exp Allergy. 2006;36:440–447. doi: 10.1111/j.1365-2222.2006.02458.x. [DOI] [PubMed] [Google Scholar]
- 61.Nadif R, Mintz M, Jedlicka A, et al. Association of CAT polymorphisms with catalase activity and exposure to environmental oxidative stimuli. Free Radic Res. 2005;39:1345–1350. doi: 10.1080/10715760500306711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghio AJ, Piantadosi CA, Wang X, et al. Divalent metal transporter-1 decreases metal-related injury in the lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:460–467. doi: 10.1152/ajplung.00154.2005. [DOI] [PubMed] [Google Scholar]
- 63.Tamagawa E, Bai N, Morimoto K, et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Cell Mol Physiol. 2008;295:79–85. doi: 10.1152/ajplung.00048.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarnat JA, Brown KW, Schwartz J, et al. Ambient Gas Concentrations and Personal Particulate Matter Exposures: Implications for Studying the Health Effects of Particles. Epidemiology. 2005;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]