Abstract
Purpose
Epigenetic silencing of several Wnt pathway related genes has been reported in renal cancer. Except for the TCF4 gene, there are no reports regarding Wnt pathway gene polymorphisms in renal cancer. Therefore, we hypothesized that the polymorphisms in Wnt signaling genes may be risk factors for renal cancer.
Experimental Design
A total of 210 patients (145 male and 65 female) with pathologically confirmed renal cell carcinoma (RCC), and 200 age- and sex-matched control individuals were enrolled in this study. We genotyped 14 SNPs in six genes including DKK2 (rs17037102, rs419558, rs447372), DKK3 (rs3206824, rs11022095, rs1472189, rs7396187, rs2291599), DKK4 (rs2073664), sFRP4 (rs1802073, rs1802074), SMAD7 (rs12953717), DAAM2 (rs6937133, rs2504106) using PCR-RFLP and direct sequencing in RCC and age-matched healthy subjects. We also tested the relationship between these polymorphisms and clinicopathologic data including gender, grade, tumor stage, lymph-node involvement, distant metastasis, and overall survival.
Results
A significant decrease in the frequency of the G/A+A/A genotypes in the DKK3 codon335 rs3206824 was observed in RCC patients compared with controls. The frequency of the rs3206824 (G/A) A- rs7396187 (G/C) C haplotype was significantly lower in RCC compared with other haplotypes. We also found that DKK3 rs1472189 C/T is associated with distant metastasis and furthermore, DKK2 rs17037102 G homozygous patients had a decreased risk for death by multivariate Cox regression analysis.
Conclusions
This is the first report documenting that DKK3 polymorphisms are associated with RCC and that the DKK2 rs17037102 polymorphism may be a predictor for survival in RCC patients after radical nephrectomy.
Keywords: Polymorphism, Wnt, DKK, sFRP, RCC
INTRODUCTION
Renal cell carcinoma (RCC) is the third leading cause of death among urological tumors, accounting for about 2% of adult malignancies.[1] Although, the rate of detection of RCC has increased with improved diagnostic techniques, metastatic lesions are still found at diagnosis in about 25% of RCC patients. Moreover, in RCC patients, distant metastases are sometimes found long after surgical removal of the primary tumor. After detecting these metastases, the 5 years survival rate is generally less than 10%.[2] The standard treatment for localized renal cancer is surgical removal,[2] while immunotherapy is used for metastatic disease because of its multi-drug resistance. Interleukin (IL)-2 is the most common immunotherapy for RCC but is effective in only 10 to 15% of patients.[3]
Wnt/β-catenin signaling is involved in numerous processes in development, is strongly implicated in tumorigenesis and highly related to tumor invasion and metastasis.[4]
Wnt signals transduced by the canonical pathway play a role in determining cell fate and those by the noncanonical pathway are important for control of cell movement and tissue polarity.[5]
Canonical Wnt ligands bind to frizzled (FZD) family receptors and the LRP5/LRP6 co-receptor, which stabilize β-catenin. Subsequently β-catenin interacts with members of the lymphoid enhancer factor 1/T-cell factor (LEF1/TCF) family, resulting in generation of a functional transcription factor complex and the expression of downstream target genes. [5] Non-canonical Wnt ligands bind to FZD family receptors, and ROR2 and RYK co-receptors.[5–7] This signaling is mainly involved in cytoskeletal reorganization during cancer cell invasion and metastasis.[6][7] At present, five Wnt antagonist families have been described, namely, secreted frizzled-related protein (sFRP), Wnt inhibitory factor 1 (Wif1), Xenopus Cerberus, Wise and Dickkopf (DKK) families.[8]
The sFRP family (sFRP1-sFRP5) and Wnt inhibitory factor-1 (Wif-1) are involved in inhibiting Wnt signaling by directly binding to Wnt molecules.[8] The DKK family (DKK1-DKK4) inhibits Wnt signaling by binding to the LRP5/LRP6 component of the Wnt receptor complex.[8]
Transforming growth factor-β (TGF-β) also regulates cell fate and proliferation co-operatively with Wnt proteins.[9][10] The Smad proteins transduce signaling from the activated TGF-β –TGF receptor complex.[11] Smad proteins, together with β–catenin, act as a transcriptional regulator in the TGF-β and Wnt signaling pathways.[11]
Epigenetic silencing of several Wnt pathway genes has been reported in renal cancer. [12–15] However, there have been no reports regarding Wnt pathway related gene polymorphisms in renal cancer except for the TCF4 gene.[16]
Therefore, with this evidence, we hypothesized that gene polymorphisms associated with Wnt (canonical and noncanonical) and TGF-β cascades may be associated with renal cancer risk. To test this hypothesis, we conducted a case-control study with respect to 14 single nucleotide polymorphisms (SNPs) including DKK2 (rs17037102, rs419558, rs447372), DKK3 (rs3206824, rs11022095, rs1472189, rs7396187, rs2291599), DKK4 (rs2073664), sFRP4 (rs1802073, rs1802074), DAAM2 (rs6937133, rs2504106), and SMAD7 (rs12953717). We selected these polymorphic sites based on previous reports and HapMap data (http://www.hapmap.org/),[17][18] which are composed of possibly functional (non-synonymous and 5’- or 3’-untranslated region SNPs) or disease-associated SNPs. We then tested the relationship between these polymorphisms and clinicopathologic data including gender, grade, tumor stage, lymph-node involvement, distant metastasis, and overall survival. We also investigated the relationship between Wnt antagonist genes polymorphisms and the expression of the beta-catenin, which is one of the downstream targets of Wnt signaling.
Materials and Methods
Samples
A total of 210 patients (145 male and 65 female) with pathologically confirmed conventional RCC, and 200 age- and sex-matched control individuals were enrolled in this study. Genomic DNA was extracted from the peripheral blood of 154 patients and 200 healthy individuals (Shimane University Hospital, Izumo, Japan), and from the paraffin-embedded non-cancerous kidney tissue of 56 patients (Toho University Hospital, Tokyo, Japan). A DNA mini kit (Qiagen, Valencia, CA) was used to extract DNA from normal tissue and peripheral blood according to the manufacturers' protocols. The mean ages of the patient and control groups were 62.0 and 61.0 years, respectively (Table 1, p = 0.35). All of the patients (n = 210) tested were diagnosed with RCC on the basis of histopathological findings. They were classified according to the WHO criteria and staged according to the tumor-node-metastasis (TNM) classification. Healthy controls consisted of volunteers with no apparent abnormal findings upon medical examination at Shimane University Hospital. These samples are same as previously reported ones. [19]
Table 1.
Characteristics of RCC patients and controls
| Cases (n=210) |
Controls (n=200) |
P-value | ||
|---|---|---|---|---|
| Age(y)(mean ± SD) | 62.1 ± 12 | 60.1 ± 16 | 0.35 | |
| Gender | Males | 145(69 %) | 151 (75%) | 0.23 |
| Females | 65 (31 %) | 49 (25 %) | ||
| Grade | 1 | 55 (26 %) | ||
| 2 | 130 (62 %) | |||
| 3+4 | 25(12 %) | |||
| pT | 1 | 114 (54 %) | ||
| 2 | 45(21 %) | |||
| 3 | 48 (23%) | |||
| 4 | 3(2%) | |||
| pN | (−) | 200 (95 %) | ||
| (+) | 10 (5%) | |||
| pM | (−) | 191 (91 %) | ||
| (+) | 19(9 %) | |||
| Pathology | ||||
| Clear cell carcinoma | 198 (94 %) | |||
| Granular cell carcinoma | 10 (5 %) | |||
| Chromophobe cell carcinoma | 2(1 %) |
Abbreviations: pT, pathological tumor classification; pN, lymph node invasion; pM, distant metastasis
To ascertain that volunteers were healthy and free of cancer, they all underwent various tests that included physical exams, questionnaires about their health and history, chest X-rays, blood and urine tests for various tumor markers, and abdominal ultrasound, gastric endoscopy, and colon enema. Peripheral blood samples were obtained from the patients and controls after written informed consent was obtained in Shimane University Hospital and Toho University hospital.
Genotyping
Polymorphic sites with minor allele frequencies (MAF) of more than 0.1 in Japanese populations were selected from HapMap data. Information regarding functional polymorphisms (non-synonymous, synonymous, 5’- and 3’- untranslated region,) is added to Table 2 and diagrams of the DKK2, DKK3, and sFRP4 genes showing their functional domains and polymorphic sites are in Figure 1. Polymorphisms were analyzed by PCR-RFLP. Genotyping methods, primer sets, and annealing temperatures used for RFLP are shown in Table 2. Each PCR reaction was carried out in a total volume of 20 µL consisting of 0.3 µL of a 10 µmol/L solution of each primer, 1.5 mmol/L MgCl2, 0.8 mmol/L deoxynucleotide triphosphate, 0.5 unit RedTaq DNA polymerase (Sigma), 1 µL of genomic DNA (30 ng/µL), and 15.6 µL H2O using a PTC 200 Thermal Cycler (MJ Research). All reactions were subjected to two rounds of amplification using a nested primer approach. The first and second PCR annealing temperatures and PCR cycles were 52 °C, 48 cycles, and 58 °C, 45 cycles, respectively. The second PCR products were digested with each restriction enzyme and temperature (New England BioLabs, Waltham, MA) for 3 hours, separated on a 2.0 % agarose gel and subsequently stained with ethidium bromide.
Table 2.
Information about SNP ID, function variation, primers sequence, product size, PCR conditions, and restriction enzymes for the target genes
| gene | SNP ID | function variation | primer sequence | product size | annealing Tem (cycle number) | restriction enzyme | ||
|---|---|---|---|---|---|---|---|---|
| DKK2 | rs17037102 | non synonymous | 1 st | F; | 5'-TGGCTTCATATTTCACATCAAGA-3' | 52 (48) | ||
| exon3 Arg146Glu | R; | 5'-TGTGTGGTCTTCCTAGATTCTGC-3' | ||||||
| 2 nd | F; | 5'-TGATCATCTCCAGGCATCTG-3' | 132bp | 58 (40) | DdeI | |||
| R; | 5'-ATTCTGCCATCCCAAGTCAT-3' | |||||||
| DKK2 | rs419558 | 3'UTR | 1 st | F; | 5'-TACGGACACAGGACCTCACA-3' | 52 (48) | ||
| R; | 5'-TCCAATATATGTGGGAAAAGAGC-3' | |||||||
| 2 nd | F; | 5'-TCCCCTGGTTTCAAAGATGA-3' | 251bp | 58 (40) | AflII | |||
| R; | 5'-TGGGAAAAGAGCTAACAGAGAGA-3' | |||||||
| DKK2 | rs447372 | intron1 | 1 st | F; | 5'-TGCTCAAAAAGCAGCATCTC-3' | 52 (48) | ||
| R; | 5'-AGCTAACGGTCACTCAACCAA-3' | |||||||
| 2 nd | F; | 5'-TCAAAAAGCAGCATCTCAGG-3' | 249bp | 58 (40) | EcoRV | |||
| R; | 5'-CTCCAAATTTTGCCCTACCA-3' | |||||||
| DKK3 | rs3206824 | non synonymous | 1 st | F; | 5'-GAGGTCCCCGATGAGTATGA-3' | 52 (48) | ||
| exon7 Arg335Gly | R; | 5'-TAGGAAGAAGCCTGGTCAGC-3' | ||||||
| 2 nd | F; | 5'-GGTCCCCGATGAGTATGAAG-3' | 209bp | 58 (40) | DdeI | |||
| R; | 5'-AGCACACACCTGGGGAAATA-3' | |||||||
| DKK3 | rs11022095 | intron 6 A/G | 1 st | F; | 5'-TCAAGCAATCCTCCTGCATT-3' | 52 (48) | ||
| R; | 5'-AGTGGATGGTCCATGGAGAG-3' | |||||||
| 2 nd | F; | 5'-TTACAGGTGTGAGCCACTGC-3' | 288bp | 58 (40) | BssSI | |||
| R; | 5'-CTCCCCAGACCTGTCACAAT-3' | |||||||
| DKK3 | rs1472189 | 3' near gene C/T | 1 st | F; | 5'-TGGGGGACCTAGTTTTATCTCA-3' | 52 (48) | ||
| R; | 5'-ACCATCAGGACCACCCTACA-3' | |||||||
| 2 nd | F; | 5'-CTTCCTCAAGCTTTCCTTGC-3' | 58 (40) | BslI | ||||
| R; | 5'-TACCTTGAAGGCATCCCAGT-3' | |||||||
| DKK3 | rs7396187 | intron4 G/C | 1 st | F; | 5'-TTCCTTAGGTCCCTAGGTCCA-3' | 52 (48) | ||
| R; | 5'-AGGGCAAAGGAGACTCTTCA-3' | |||||||
| 2 nd | F; | 5'-ACAGGGCATGGCAGTTAGAG-3' | 245bp | 58 (40) | Fnu4HI | |||
| R; | 5'-CTCTTCACCCAACAGGCATT-3' | |||||||
| DKK3 | rs2291599 | intron4 C/T | 1 st | F; | 5'-CAGAGGACATGGGGTGGAT-3' | 52 (48) | ||
| R; | 5'-CTGCTGCTCTGCTCTCCATT-3' | |||||||
| 2 nd | F; | 5'-CTTGTCCTCCAGGAGTCAGC-3' | 250bp | 58 (40) | AvaI | |||
| R; | 5'-CATCATCGACGAGGACTGTG-3' | |||||||
| DKK4 | rs2073664 | synonymous | 1 st | F; | 5'-GCCATGGCATTACTGCTTTT-3' | 52 (48) | ||
| exon 4 V169V | R; | 5'-ATTGCTGGTCAATTGGCTTC-3' | ||||||
| 2 nd | F; | 5'-CTGCGTGCTGTGTCTGTTTT-3' | 292bp | 58 (40) | EcoNI | |||
| R; | 5'-AACGCTGGAAGATTTCTGGA-3' | |||||||
| SFRP4 | rs1802073 | non synonymous | 1 st | F; | 5'-GAGCACCATAAAGGGGTGAG-3' | 52 (48) | ||
| exon6 P320T | R; | 5'-GGGCACATGGCCTTACATAG-3' | ||||||
| 2 nd | F; | 5'-ACAGCGGAGAACAGTTCAGG-3' | 246bp | 58 (40) | HinfI | |||
| R; | 5'-TGGCCTTACATAGGCTGTCC-3' | |||||||
| SFRP4 | rs1802074 | non synonymous | 1 st | F; | 5'-AAGAGAGGCTGCAGGAACAG-3' | 52 (48) | ||
| exon6 R340K | R; | 5'-TCTGTACCAAAGGGCAAACC-3' | ||||||
| 2 nd | F; | 5'-AGAGCGGAGAACAGTTCAGG-3' | 246bp | 58 (40) | EarI | |||
| R; | 5'-TGGCCTTACATAGGCTGTCC-3' | |||||||
| SMAD7 | rs12953717 | intron3 C/T | 1 st | F; | 5'-GTGCCACAGGGTCTCCTTC-3' | 52 (48) | ||
| R; | 5'-GGATGTGGAGACAATCAGGAA-3' | |||||||
| 2 nd | F; | 5'-GCTTCGTTTCCACCCCTTAG-3' | 272bp | 58 (40) | Fnu4HI | |||
| R; | 5'-AACCCAGGAGCCTCAGAGAT-3' | |||||||
| DAAM2 | rs6937133 | intron1 A/G | 1 st | F; | 5'-GCACTGGTGCTCACTCCTCT-3' | 52 (48) | ||
| R; | 5'-TTCCTGCACAGCTGAGTGTC-3' | |||||||
| 2 nd | F; | 5'-TTGCAGTTAAACCTGGGTGA-3' | 175bp | 58 (40) | TaqI | |||
| R; | 5'-TGTTCGCTGCCCAGTTTAGT-3' | |||||||
| DAAM2 | rs2504106 | intron1 C/T | 1 st | F; | 5'-TGGCCTCTCATACATCATGC-3' | 52 (48) | ||
| R; | 5'-CCCTCTTTCCTCCTTTTCCA-3' | |||||||
| 2 nd | F; | 5'-GCCCAGAAAAGCCTCAAATA-3' | 215bp | 58 (40) | SfcI | |||
| R; | 5'-GGTCATTTCCCAAATGGTCA-3' |
Figure 1. Diagrams of DKK2, DKK3, and sFRP4 genes and polymorphic sites.
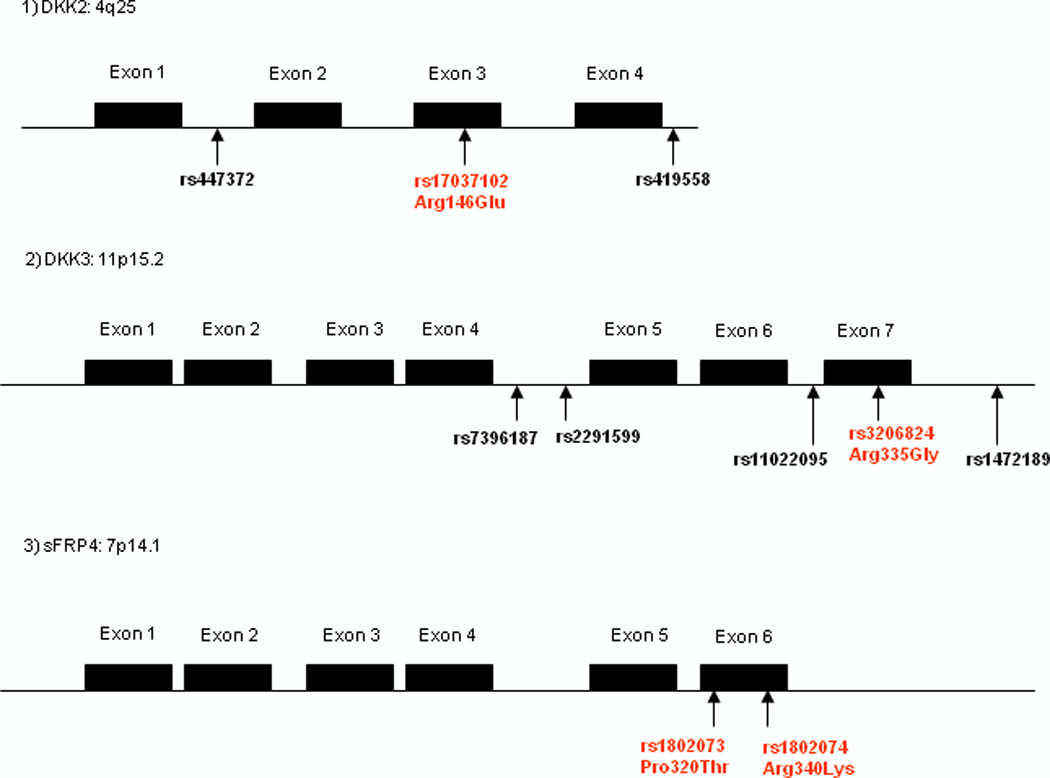
1) DKK2 2) DKK3 3) sFRP4
All assays were conducted blindly without the knowledge of case or control status. Two researchers carried out RFLP and reading of the gels. All samples were retested and the results were 100% concordant. To confirm the genotype ascribed by PCR–RFLP, approximately 50% of the PCR sample products were randomly selected and subjected to direct sequencing using an ABI PRISM 377 DNA sequencer (Applied Biosystems, Inc., Foster City, CA). There were no discrepancies in the results.
Immunohistochemical study
We performed immunohistochemistry of the Wnt downstream target beta-catenin in formalin-fixed, paraffin-embedded (FFPE) specimens using rabbit polyclonal antibody against human beta-catenin (#9562, Cell signaling Technology, Beverly, MA). The staining procedure was according to a commercial kit (Lab vision, Fremont, CA). We investigated the relationship between Wnt antagonist gene genotypes and beta-catenin expression. The sections were counterstained with Harris' hematoxylin. A typical representative staining is shown in Figure 2.
Figure 2. Representative immunohistochemical stains of beta-catenin in RCC tissues.
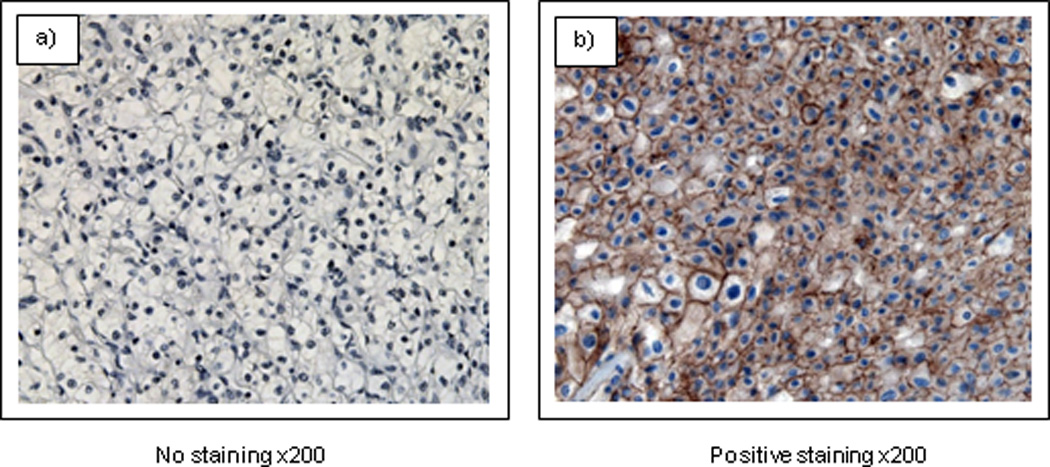
RCC cells showed mainly cytoplasmic and membranous expression.
Magnification is ×200
Statistical analysis
The common homozygote was used as the reference for calculation genotype specific odds ratio. Hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated from the proportional hazard assumption of the Cox regression model including multivariate analysis. The probability of overall survival time was estimated using Kaplan-Meier plots and the log-rank test. Hardy–Weinberg equilibrium and haplotype analysis were evaluated by SNPAlyze version 2.2 (DYNACOM, Tokyo, Japan), utilizing an EM method. The chi-square test was used to compare the genotype frequency between patients and controls. All statistical analyses were performed using StatView (version 5; SAS Institute Inc., NC). We adopted false discovery rate (FDR) according to Benjamini and Hochberg [20][21] and set the statistically significant level as P < 0.05. An FDR of 0.05 was used as a critical value for assessment whether the obtained P value was significant. The genotype frequencies of the polymorphisms in control samples (n = 200) and case samples (n = 210) did not deviate from Hardy-Weinberg equilibrium (P > 0.05).
RESULTS
Comparison of genotype distribution between RCC cases and controls
The genotype distributions of the DKK2 (rs17037102, rs419558, rs447372), DKK3 (rs3206824, rs11022095, rs1472189, rs7396187, rs2291599), DKK4 (rs2073664), sFRP4 (rs1802073, rs1802074), SMAD7 (rs12953717), DAAM2 (rs6937133, rs2504106) polymorphisms between renal cancer cases and healthy controls are shown in Table 3. A significant decrease in the frequency of the G/A + A/A genotypes of DKK3 rs3206824 (non synonymous Arg335Gly) was observed in renal cancer patients compared with controls (OR = 0.43; 95% CI, 0.29–0.65) (Table 3).
Table 3.
Association between polymorphisms in Wnt signaling pathway genes and renal cancer
| Gene polymorphism | Genotype | renal cancer (n=210) | control (n=200) | OR (95% CI) | p-value | FDR adjusted p-value* |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| DKK2 (rs17037102) | GG | 105 (50) | 106 (53) | 1 (reference) | ||
| GA | 92 (44) | 82 (41) | 1.13 (0.76–1.69) | 0.54 | ||
| AA | 12 (6) | 12 (06) | 1.01 (0.43–2.34) | 0.98 | ||
| GA+AA | 104 (50) | 94 (47) | 1.11 (0.76–1.65) | 0.57 | 0.61 | |
| DKK2 (rs419558) | CC | 119 (57) | 88 (44) | 1 (reference) | ||
| CT | 63 (30) | 88 (44) | 0.53 (0.34–0.81) | 0.003 | ||
| TT | 28 (13) | 24 (12) | 0.86 (0.45–1.58) | 0.64 | ||
| CT+TT | 91 (43) | 112 (56) | 0.60 (0.41–0.88) | 0.01 | 0.028a | |
| DKK2 (rs447372) | GG | 117 (56) | 88 (44) | 1 (reference) | ||
| GA | 65 (31) | 88 (44) | 0.56 (0.36–0.84) | 0.0063 | ||
| AA | 28 (13) | 24 (12) | 0.88 (0.47–1.62) | 0.67 | ||
| GA+AA | 93 (44) | 112 (56) | 0.62 (0.42–0.92) | 0.01 | 0.0238a | |
| DKK3 (rs3206824) | GG | 150 (71) | 104 (52) | 1 (reference) | ||
| GA | 53 (25) | 82 (41) | 0.45 (0.29–0.69) | 0.0001 | ||
| AA | 7 (4) | 14 (7) | 0.35 (0.14–0.89) | 0.02 | ||
| GA+AA | 60 (29) | 96 (48) | 0.43 (0.29–0.65) | 0.0001 | 0.0014a | |
| DKK3 (rs11022095) | GG | 28 (13) | 23 (11) | 1 (reference) | ||
| GA | 88 (42) | 87 (44) | 0.83 (0.44–1.55) | 0.56 | ||
| AA | 94 (45) | 90 (45) | 0.85 (0.46–1.59) | 0.63 | ||
| GA+AA | 182 (87) | 177 (89) | 0.84 (0.47–1.52) | 0.57 | 0.57 | |
| DKK3 (rs1472189) | CC | 155 (74) | 134 (67) | 1 (reference) | ||
| CT | 51 (24) | 59 (30) | 0.74 (0.48–1.16) | 0.19 | ||
| TT | 4 (2) | 7 (3) | 0.49 (0.14–1.72) | 0.26 | ||
| CT+TT | 55 (26) | 66 (33) | 0.72 (0.47–1.10) | 0.13 | 0.18 | |
| DKK3 (rs7396187) | GG | 138 (66) | 102 (51) | 1 (reference) | ||
| GC | 58 (27) | 75 (38) | 0.57 (0.37–0.88) | 0.01 | ||
| CC | 14 (7) | 23 (11) | 0.45 (0.22–0.92) | 0.02 | ||
| GC+CC | 72 (34) | 98 (49) | 0.54 (0.37–0.81) | 0.002 | 0.01a | |
| DKK3 (rs2291599) | CC | 101 (48) | 113 (57) | 1 (reference) | ||
| CT | 81 (39) | 62 (31) | 1.46 (0.95–2.24) | 0.08 | ||
| TT | 28 (13) | 25 (12) | 1.25 (0.69–2.29) | 0.46 | ||
| CT+TT | 109 (52) | 87 (43) | 1.41 (0.95–2.07) | 0.08 | 0.14 | |
| DKK4 (rs2073664) | CC | 144 (68) | 145 (72) | 1 (reference) | ||
| CT | 40 (19) | 46 (23) | 0.88 (0.54–1.42) | 0.58 | ||
| TT | 26 (13) | 9 (5) | 2.91 (1.32–6.42) | 0.006 | ||
| CT+TT | 66 (32) | 55 (28) | 1.21 (0.79–1.85) | 0.38 | 0.48 | |
| sFRP4 (rs1802073) | CC | 51 (24) | 36 (18) | 1 (reference) | ||
| CA | 82 (39) | 112 (56) | 0.52 (0.31–0.86) | 0.01 | ||
| AA | 77 (37) | 52 (26) | 1.04 (0.61–1.82) | 0.87 | ||
| CA+A | 159 (76) | 164 (82) | 0.68 (0.42–1.11) | 0.12 | 0.19 | |
| sFRP4 (rs1802074) | AA | 36 (17) | 18 (9) | 1 (reference) | ||
| AG | 69 (33) | 69 (34) | 0.51 (0.26–0.96) | 0.04 | ||
| GG | 105 (50) | 113 (56) | 0.46 (0.25–0.87) | 0.01 | ||
| AG+GG | 174 (83) | 182 (91) | 0.48 (0.26–0.87) | 0.01 | 0.04a | |
| SMAD7 (rs12953717) | CC | 125 (59) | 139 (70) | 1 (reference) | ||
| CT | 68 (32) | 48 (24) | 1.57 (1.01–2.45) | 0.04 | ||
| TT | 17 (9) | 13 (6) | 1.45 (0.68–3.11) | 0.33 | ||
| CT+TT | 85 (41) | 61 (30) | 1.55 (1.03–2.33) | 0.03 | 0.06 | |
| DAAM2 (rs6937133) | GG | 30 (14) | 33 (16) | 1 (reference) | ||
| GA | 65 (31) | 65 (33) | 1.1(0.61–2.01) | 0.75 | ||
| AA | 115 (55) | 102 (51) | 1.24 (0.71–2.17) | 0.45 | ||
| GA+AA | 180 (86) | 167 (84) | 1.18 (0.69–2.03) | 0.53 | 0.62 | |
| DAAM2 (rs2504106) | TT | 72 (34) | 37 (17) | 1 (reference) | ||
| TC | 84 (40) | 118 (59) | 0.36 (0.23–0.59) | 0.0001 | ||
| CC | 54 (26) | 45 (23) | 0.62 (0.35–1.08) | 0.08 | ||
| TC+CC | 138 (66) | 163 (82) | 0.44 (0.28–0.69) | 0.0002 | 0.0014a | |
Note; FDR means false discovery rate, FDR adjusted P value using the Benjamini-Hochberg methods.
FDR adjusted p-value of < 0.05 was regarded as statistically significant,
Remain significant after FDR adjustment
We also found a significant decrease in the frequency of G/C+C/C genotypes of the DKK3 rs7396187 in patients (OR = 0.54; 95% CI, 0.37–0.81) (Table 3). There was also a significant increased frequency of the A/A genotype of rs1802074 SNP of sFRP4 gene (OR, 2.15; 95% CI, 1.15–4.02), increased frequency of the T/T genotype of rs2073664 of DKK4 (OR, 2.91; 95% CI, 1.32–6.42), and a significant increase in the T/C + C/C genotypes of DAAM2 rs2504106 (OR, 0.44; 95% CI, 0.28–0.69) in patients (Table 3). However, no significant difference was observed in the genotype distribution of other genotypes between patients and controls (Table 3).
Next, we investigated gene-gene interaction using DKK2 rs419558, DKK2 rs447372, DKK3 rs3206824, DKK3 rs7396187, SMAD7 rs12953717, sFRP4 rs1802074, and DAAM2 rs2504106 because we found a significant difference in the genotype distribution between cases and controls. Among these combinations, when the combined effect of 2 polymorphisms (DKK3 rs3206824 and sFRP4 rs1802074) was evaluated, a decreased renal cancer risk was found for only the DKK3 rs3206824 GA + AA and sFRP4 rs1802074 A/G + G/G genotypes (OR, 0.19; 95% CI, 0.09–0.45; P < 0.0001) (Table 4).
Table 4.
Gene-gene interaction analysis in DKK3 rs3206824-sFRP4 rs1802074
| DKK3 A (GG/GA+AA)-sFRP4 B (AA/AG+GG) | renal cancer (n=210) | control (n=200) | OR (95% CI) | p-value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| GG-AA | 27 (13) | 9 (4.5) | 1 (reference) | |
| GG-AG/GG | 123 (58) | 95 (47.5) | 0.43 (0.19–0.96) | 0.04 |
| GA/AA-AA | 9 (5) | 9 (5) | 0.33 (0.10–0.20) | 0.06 |
| GA/AA-AG/GG | 51 (24) | 87 (43.5) | 0.19 (0.09–0.45) | <0.0001 |
Linkage disequilibrium and haplotype analysis in DKK3 and DKK2 gene polymorphisms
The DKK3 rs3206824 polymorphism was in linkage disequilibrium (LD) with rs7396187 (D = 0.1627). Therefore, the frequency of each haplotype including rs3206824 (G/A) and rs7396187 (G/C) was calculated between RCC patients and controls. The frequency of the A–C haplotype was significantly lower in RCC (P < 0.0001) compared with other haplotypes (Table 5).
Table 5.
Haplotype analysis and renal cancer risk
A) DKK3 rs3206824-rs7396187
B) DKK2 rs419558-rs447372
| A) | |||||
|---|---|---|---|---|---|
| Haplotype | Overall | case | cont | p-value | |
| DKK3 A (G/A)-F (G/C) | (%) | (%) | |||
| G-G | 0.719 | 0.7661 | 0.6701 | <0.001 | |
| A-C | 0.1873 | 0.1321 | 0.2447 | <0.001 | |
| G-C | 0.0651 | 0.0736 | 0.0563 | 0.3155 | |
| A-G | 0.0285 | 0.0281 | 0.0289 | 0.9451 | |
| B) | |||||
|---|---|---|---|---|---|
| Haplotype | Overall | case | control | p-value | |
| DKK2 A (C/T)- B (G/A) | (%) | (%) | |||
| C-G | 0.6866 | 0.7119 | 0.66 | 0.109 | |
| T-A | 0.311 | 0.2833 | 0.34 | 0.0796 | |
| C-A | 2.44E-03 | 4.76E-03 | 5.10E-28 | 0.167 | |
Note; A: rs3206824, F: rs7396187
Note; A: rs419558, B: rs447372
The DKK2 rs419558 polymorphism was in linkage disequilibrium (LD) with rs447372 (D = 0.2244). However we did not find a significant difference between case and control in the haplotype analysis using rs419558 and rs447372 (Table 5).
Relationship of genotype distribution with clinicopathological characteristics
We investigated the effect of the twelve SNPs on clinicopathological factors including sex, grade, pathological stage (pT), pN, and pM. Regarding gender, pT, and pN, there were no significant effects of SNPs (Table 6). We found a higher frequency of C/T + T/T genotypes in DKK3 rs1472189 in patients with distant metastasis (pM (+)).
Table 6.
Comparison between gene genotypes and clinical parameters
| gender |
Grade |
Stage |
pN |
pM |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene (SNP ID) | Genotype | female | male | p value | 1+2 | 3+4 | p value | pT1+pT2 | pT3+pT4 | p value | (−) | (+) | p value | (−) | (+) | p value |
| n=65 | n=145 | n=185 | n=25 | n=159 | n=51 | n=200 | n=10 | n=191 | n=19 | |||||||
| DKK2 (rs17037102) | GG | 35 | 70 | reference | 92 | 13 | reference | 79 | 26 | reference | 99 | 6 | reference | 94 | 11 | reference |
| GA | 24 | 68 | 0.27 | 82 | 10 | 0.74 | 70 | 22 | 0.89 | 89 | 3 | 0.41 | 85 | 7 | 0.49 | |
| AA | 6 | 7 | 0.36 | 11 | 2 | 0.76 | 10 | 3 | 0.89 | 12 | 1 | 0.78 | 12 | 1 | 0.75 | |
| GA+AA | 30 | 75 | 0.45 | 93 | 12 | 0.83 | 80 | 25 | 0.87 | 101 | 4 | 0.52 | 97 | 8 | 0.47 | |
| DKK2 (rs419558) | CC | 39 | 80 | reference | 107 | 12 | reference | 91 | 28 | reference | 118 | 1 | reference | 113 | 6 | reference |
| CT | 17 | 46 | 0.42 | 56 | 7 | 0.83 | 48 | 15 | 0.96 | 57 | 6 | 0.004 | 54 | 9 | 0.03 | |
| TT | 9 | 19 | 0.94 | 22 | 6 | 0.11 | 20 | 8 | 0.58 | 25 | 3 | 0.004 | 24 | 4 | 0.08 | |
| CT+TT | 26 | 65 | 0.51 | 78 | 13 | 0.35 | 68 | 23 | 0.77 | 82 | 9 | 0.002 | 78 | 13 | 0.02 | |
| DKK2 (rs447372) | GG | 39 | 78 | reference | 105 | 12 | reference | 90 | 27 | reference | 116 | 1 | reference | 111 | 6 | reference |
| GA | 17 | 48 | 0.31 | 58 | 7 | 0.91 | 49 | 16 | 0.82 | 59 | 6 | 0.005 | 56 | 9 | 0.04 | |
| AA | 9 | 19 | 0.91 | 22 | 6 | 0.11 | 20 | 8 | 0.54 | 25 | 3 | 0.004 | 24 | 4 | 0.09 | |
| GA+AA | 26 | 67 | 0.41 | 80 | 13 | 0.41 | 69 | 24 | 0.65 | 84 | 9 | 0.003 | 80 | 13 | 0.03 | |
| DKK3 (rs3206824) | GG | 50 | 100 | reference | 136 | 14 | reference | 116 | 34 | reference | 143 | 7 | reference | 138 | 12 | reference |
| GA | 13 | 40 | 0.23 | 43 | 10 | 0.06 | 37 | 16 | 0.28 | 50 | 3 | 0.77 | 46 | 7 | 0.26 | |
| AA | 2 | 5 | 0.79 | 6 | 1 | 0.66 | 6 | 1 | 0.61 | 7 | 0 | 7 | 0 | |||
| GA+AA | 15 | 45 | 0.24 | 49 | 11 | 0.07 | 43 | 17 | 0.39 | 57 | 3 | 0.92 | 53 | 7 | 0.41 | |
| DKK3 (rs11022095) | GG | 11 | 17 | reference | 25 | 3 | reference | 21 | 7 | reference | 27 | 1 | reference | 23 | 5 | reference |
| GA | 28 | 60 | 0.47 | 76 | 12 | 0.68 | 67 | 21 | 0.91 | 83 | 5 | 0.61 | 80 | 8 | 0.21 | |
| AA | 26 | 68 | 0.24 | 84 | 10 | 0.99 | 71 | 23 | 0.95 | 90 | 4 | 0.87 | 88 | 6 | 0.06 | |
| GA+AA | 54 | 128 | 0.31 | 160 | 22 | 0.83 | 138 | 44 | 0.93 | 173 | 9 | 0.75 | 168 | 14 | 0.08 | |
| DKK3 (rs1472189) | CC | 47 | 108 | reference | 138 | 17 | reference | 119 | 36 | reference | 150 | 5 | reference | 146 | 9 | reference |
| CT | 16 | 35 | 0.89 | 46 | 5 | 0.82 | 38 | 13 | 0.74 | 46 | 5 | 0.05 | 41 | 10 | 0.003 | |
| TT | 2 | 2 | 0.41 | 1 | 3 | 0.0001 | 2 | 2 | 0.21 | 4 | 0 | 4 | 0 | |||
| CT+TT | 18 | 37 | 0.74 | 47 | 8 | 0.48 | 40 | 15 | 0.55 | 50 | 5 | 0.07 | 45 | 10 | 0.005 | |
| DKK3 (rs7396187) | GG | 46 | 92 | reference | 127 | 11 | reference | 108 | 30 | reference | 132 | 6 | reference | 127 | 11 | reference |
| GC | 16 | 42 | 0.43 | 46 | 12 | 0.01 | 40 | 18 | 0.11 | 54 | 4 | 0.46 | 51 | 7 | 0.36 | |
| CC | 3 | 11 | 0.36 | 12 | 2 | 0.42 | 11 | 3 | 0.92 | 14 | 0 | 13 | 1 | 0.92 | ||
| GC+CC | 19 | 53 | 0.31 | 58 | 14 | 0.014 | 51 | 21 | 0.14 | 68 | 4 | 0.69 | 64 | 8 | 0.45 | |
| DKK3 (rs2291599) | CC | 34 | 67 | reference | 93 | 8 | reference | 76 | 25 | reference | 95 | 6 | reference | 90 | 11 | reference |
| CT | 26 | 55 | 0.82 | 70 | 11 | 0.21 | 62 | 19 | 0.84 | 77 | 4 | 0.77 | 74 | 7 | 0.61 | |
| TT | 5 | 23 | 0.11 | 22 | 6 | 0.04 | 21 | 7 | 0.98 | 28 | 0 | 27 | 1 | 0.24 | ||
| CT+TT | 31 | 78 | 0.41 | 92 | 17 | 0.08 | 83 | 26 | 0.88 | 105 | 4 | 0.44 | 101 | 8 | 0.37 | |
| DKK4 (rs2073664) | CC | 40 | 104 | reference | 127 | 17 | reference | 107 | 37 | reference | 137 | 7 | reference | 129 | 15 | reference |
| CT | 18 | 22 | 0.03 | 35 | 5 | 0.91 | 30 | 10 | 0.92 | 37 | 3 | 0.52 | 36 | 4 | 0.94 | |
| TT | 7 | 19 | 0.93 | 23 | 3 | 0.96 | 22 | 4 | 0.26 | 26 | 0 | 26 | 0 | |||
| CT+TT | 25 | 41 | 0.14 | 58 | 8 | 0.95 | 52 | 14 | 0.48 | 63 | 3 | 0.92 | 62 | 4 | 0.31 | |
| sFRP4 (rs1802073) | CC | 15 | 36 | reference | 42 | 9 | reference | 37 | 14 | reference | 47 | 4 | reference | 43 | 7 | reference |
| CA | 25 | 57 | 0.89 | 70 | 12 | 0.64 | 65 | 17 | 0.37 | 79 | 3 | 0.29 | 76 | 6 | 0.21 | |
| AA | 25 | 52 | 0.71 | 73 | 4 | 0.02 | 57 | 20 | 0.85 | 74 | 3 | 0.34 | 71 | 6 | 0.26 | |
| CA+AA | 50 | 109 | 0.78 | 143 | 16 | 0.15 | 122 | 37 | 0.54 | 153 | 6 | 0.24 | 147 | 12 | 0.17 | |
| sFRP4 (rs1802074) | AA | 15 | 21 | reference | 32 | 4 | reference | 28 | 8 | reference | 35 | 1 | reference | 35 | 1 | reference |
| AG | 15 | 54 | 0.03 | 65 | 4 | 0.33 | 53 | 16 | 0.92 | 67 | 2 | 0.97 | 64 | 5 | 0.35 | |
| GG | 35 | 70 | 0.36 | 88 | 17 | 0.09 | 78 | 27 | 0.68 | 98 | 7 | 0.38 | 92 | 13 | 0.09 | |
| AG+GG | 50 | 124 | 0.13 | 153 | 21 | 0.87 | 131 | 43 | 0.75 | 165 | 9 | 0.54 | 156 | 18 | 0.15 | |
| SMAD7 (rs12953717) | CC | 46 | 79 | reference | 108 | 17 | reference | 94 | 31 | reference | 119 | 6 | reference | 111 | 14 | reference |
| CT | 13 | 55 | 0.01 | 61 | 7 | 0.51 | 51 | 17 | 0.98 | 65 | 3 | 0.91 | 65 | 3 | 0.11 | |
| TT | 6 | 11 | 0.91 | 16 | 1 | 0.37 | 14 | 3 | 0.52 | 16 | 1 | 0.85 | 15 | 2 | 0.95 | |
| CT+TT | 19 | 66 | 0.03 | 77 | 8 | 0.36 | 65 | 20 | 0.83 | 81 | 4 | 0.97 | 80 | 5 | 0.19 | |
| DAAM2 (rs6937133) | GG | 12 | 18 | reference | 26 | 4 | reference | 22 | 8 | reference | 29 | 1 | reference | 26 | 4 | reference |
| GA | 20 | 45 | 0.37 | 59 | 6 | 0.54 | 48 | 17 | 0.96 | 62 | 3 | 0.77 | 56 | 9 | 0.95 | |
| AA | 33 | 82 | 0.23 | 100 | 15 | 0.97 | 89 | 26 | 0.64 | 109 | 6 | 0.67 | 109 | 6 | 0.12 | |
| GA+AA | 53 | 127 | 0.25 | 159 | 21 | 0.79 | 137 | 43 | 0.74 | 171 | 9 | 0.69 | 165 | 15 | 0.38 | |
| DAAM2 (rs2504106) | TT | 19 | 53 | reference | 64 | 8 | reference | 57 | 15 | reference | 68 | 4 | reference | 66 | 6 | reference |
| TC | 29 | 55 | 0.27 | 73 | 11 | 0.71 | 62 | 22 | 0.43 | 80 | 4 | 0.83 | 73 | 11 | 0.34 | |
| CC | 17 | 37 | 0.53 | 48 | 6 | 0.99 | 40 | 14 | 0.51 | 52 | 2 | 0.63 | 52 | 2 | 0.29 | |
| TC+CC | 46 | 92 | 0.31 | 121 | 17 | 0.79 | 102 | 36 | 0.39 | 132 | 6 | 0.69 | 125 | 13 | 0.79 | |
note; Considering multiple comparison,
p-value of < 0.05 was regarded as statistically significant
Relationship of various Wnt-antagonist genes genotype with the expression of beta-catenin
We investigated the relationship between the Wnt-antagonist genes genotypes and beta-catenin expression. However, we could not find any relationship between these polymorphisms and beta-catenin expression (Table 7).
Table 7.
Relationship of Wnt antagonist genes distribution with the expression of beta-catenin
| beta catenin expression (IHC) |
||||
|---|---|---|---|---|
| NO (n=25) | YES (n=31) | p value | ||
| Gene polymorphism | Genotype | n (%) | n (%) | |
| DKK2 (rs17037102) | GG | 18(72) | 21(68) | reference |
| GA | 5(20) | 9(29) | 0.49 | |
| AA | 2(8) | 1(3) | 0.49 | |
| GA+AA | 7(28) | 10(32) | 0.73 | |
| DKK2 (rs419558) | CC | 16(64) | 22(71) | reference |
| CT | 6(24) | 3(10) | 0.18 | |
| TT | 3(12) | 6(19) | 0.63 | |
| CT+TT | 9(36) | 9(29) | 0.58 | |
| DKK2 (rs447372) | GG | 16(64) | 22(71) | reference |
| GA | 7(28) | 3(10) | 0.12 | |
| AA | 2(8) | 6(19) | 0.37 | |
| GA+AA | 9(36) | 9(29) | 0.58 | |
| DKK3 (rs3206824) | GG | 21(84) | 24(77) | reference |
| GA | 4(16) | 6(19) | 0.72 | |
| AA | 0(0) | 1(4) | ||
| GA+AA | 4(16) | 7(23) | 0.54 | |
| DKK3 (rs11022095) | GG | 5(20) | 4(13) | reference |
| GA | 7(35) | 10(32) | 0.48 | |
| AA | 13(45) | 17(55) | 0.52 | |
| GA+AA | 20(80) | 27(87) | 0.47 | |
| DKK3 (rs1472189) | CC | 23(92) | 23(74) | reference |
| CT | 2(8) | 8(26) | 0.08 | |
| TT | 0(0) | 0 | ||
| CT+TT | 2(8) | 8(26) | 0.08 | |
| DKK3 (rs7396187) | GG | 21(84) | 23(74) | reference |
| GC | 4(16) | 5(16) | 0.85 | |
| CC | 0(0) | 3(10) | ||
| GC+CC | 4(16) | 8(26) | 0.38 | |
| DKK3 (rs2291599) | CC | 11(44) | 15(48) | reference |
| CT | 9(36) | 11(35) | 0.86 | |
| TT | 5(20) | 5(16) | 0.67 | |
| CT+TT | 14(56) | 16(52) | 0.74 | |
| DKK4 (rs2073664) | CC | 16(64) | 21(68) | reference |
| CT | 6(24) | 3(10) | 0.21 | |
| TT | 3(12) | 7(22) | 0.45 | |
| CT+TT | 9(36) | 10(32) | 0.77 | |
| sFRP4 (rs1802073) | CC | 6(24) | 10(32) | reference |
| CA | 8(32) | 11(35) | 0.78 | |
| AA | 11(44) | 10(32) | 0.37 | |
| CA+AA | 19(76) | 21(68) | 0.49 | |
| sFRP4 (rs1802074) | AA | 8(32) | 9(29) | reference |
| AG | 12(48) | 11(35) | 0.75 | |
| GG | 5(20) | 11(35) | 0.35 | |
| AG+GG | 17(68) | 22(71) | 0.82 | |
Multivariate Cox proportional hazard analysis for overall survival in RCC patients
The prognostic values for overall survival using parameters such as gender, age at diagnosis, tumor grade, pTNM, 14 SNPs were analyzed using Kaplan-Meier survival curves, and it was observed that higher grade (grade 3 + 4), pT3 + pT4, pN (+), pM (+), and DKK2 rs17037102 G/A+A/A genotypes were associated with shorter survival (Fig. 3).
Figure 3. Kaplan-Meier survival curve for overall survival for 160 RCC patients.
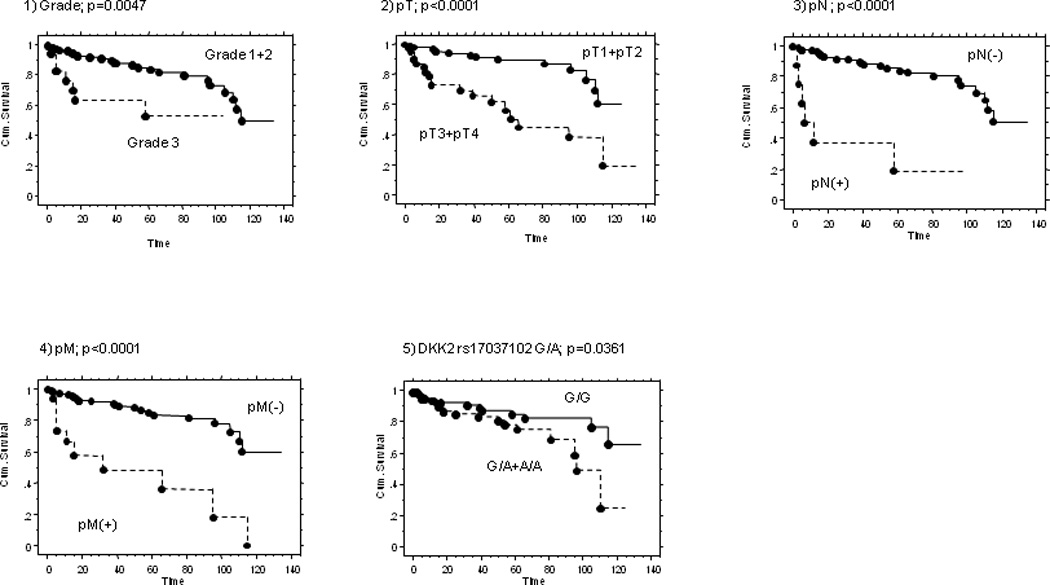
(1) grade (2) pT (3) pN (4) pM (5) DKK2 rs17037102
Higher grade (grade 3 + 4), pT3 + pT4, pN(+), pM(+), and DKK2 rs17037102 G/A+A/A genotypes are independent predictors of overall survival.
In addition to pT3 + pT4, pN (+) and pM (+), the DKK2 rs17037102 G/G genotype was found to be a independent favorable factor for survival after multivariate analysis (OR, 0.407; 95% CI, 0.156–1.062; p P = 0.0489) (Table 8).
Table 8.
Multivariate Cox proportional hazards analysis for overall survival after radical nephrectomy (n=160)
| parameters | Hazard Ratio (95% CI) | p value |
|---|---|---|
| grade; 3 vs 1+2 | 1.515 (0.411–5.584) | 0.5324 |
| pT; pT3+4 vs pT1+pT2 | 4.017 (1.469–10.986) | 0.0067 |
| pN; pN(+) vs pN(−) | 8.885 (1.368–57.713) | 0.0221 |
| pM; pM(+) vs pM(−) | 7.312 (2.003–26.692) | 0.0026 |
| DKK2 rs17037102 G/G vs G/A+A/A | 0.407 (0.156–1.062) | 0.0489 |
Discussion
In this study, we found a significant decrease in the frequency of the G/A+A/A genotypes of the DKK3 rs3206824 (non synonymous Arg335Gly) in RCC patients compared with controls (OR = 0.43; 95% CI, 0.29–0.65). The functions of DKK1 and DKK3 have been investigated and various studies have found them to be tumor suppressor genes. [12][22–32] DKK3 is a member of the Dickkopf family and regulates cell proliferation and apoptosis as a tumor suppressor gene both in vitro and in vivo.[22][23]
DKK3 mRNA and protein levels were significantly decreased in human renal cancer tissues compared to normal kidney tissues.[22] Regarding the mechanism of down-regulation of DKK3, Kobayashi et al. detected hypermethylation in the promoter region in human cancer cell lines in which the expression of DKK3 was decreased.[25]
They also detected codon335 SNP (rs3206824) in DKK3 gene in mutation analysis and compared the distribution of genotypes between 200 healthy controls and 56 lung cancer patients. However there was no significant difference between the two groups.[25] Except for this previous report, there have been no significant findings regarding codon335 in SNP studies.
As a next step, we selected 4 polymorphic sites in the DKK3 gene based on HapMap data, and conducted linkage disequilibrium (LD) and haplotype analysis. The DKK3 rs3206824 (non synonymous Arg335Gly) was in linkage disequilibrium with rs7396187, and the frequency of each haplotype including rs3206824 (G/A) and rs7396187 (G/C) was calculated between RCC patients and controls. The frequency of the A–C haplotype was significantly lower in RCC (P < 0.0001) compared with other haplotypes. An apparent added effect was also observed in the haplotype analysis. As far as we know, this is the first report that has shown the association of the DKK3 gene haplotype with renal cancer.
We also selected 3 polymorphic sites in the DKK2 gene and DKK2 rs419558 was in linkage disequilibrium with rs447372, but there was no significant difference among the frequency of each haplotype including these two polymorphisms between RCC patients and controls. Interestingly, we found that DKK2 rs17037102 (non synonymous Arg146Glu) G/A+A/A genotype carriers were associated with shorter survival in all patients. In addition the DKK2 rs17037102 GG genotype was a favorable factor for survival by multivariate Cox regression analysis, suggesting that the DKK2 rs17037102 G/A+A/A genotypes will be a useful parameter to detect high-risk RCC patients.
However, this polymorphism was not associated with other clinical variables including grade, stage, nodes or metastases. Prognostic factors are usually associated with some clinico-pathological features. However, it has been shown that functions of the Wnt signaling pathway are extremely diverse,[33] suggesting that it is currently not known whether conventional clinicopathological factors reflect all of those functions at present. This will require further study. However there has been a recent report showing that a SNP can be associated with metastasis, but not with prognosis.[34]
There are no reports regarding the DKK2 gene polymorphism in various cancers including renal cancer, therefore we could not compare our results to other studies. Regarding DKK2 function, Kremen proteins, which are Dickkopf receptors, modulate DKK2 activity during Wnt/LRP6 signaling.[35][36] DKK2 can either activate or inhibit the Wnt/β-catenin pathway, depending on cellular context.[4] The role of DKK2 itself on deregulation in cancer is not well understood since there are no other reports regarding DKK2 SNPs and cancer susceptibility.
There was a marginal significance to the increased frequency of the A/A genotype of rs1802074 (sFRP4) in renal cancer patients compared with controls (Table 3).
We tested the gene-gene interactions among gene-gene interaction using DKK2 rs419558, DKK2 rs447372, DKK3 rs3206824, DKK3 rs7396187, DKK4 rs2073664, sFRP4 rs1802074, and DAAM2 rs2504106, in which we found a significant difference in the genotype distribution between cases and controls. There was a strong correlation however between DKK3 rs3206824 GA/AA and sFRP4 rs1802074 AG/GG (Table 4) suggesting that these two polymorphisms are related to RCC susceptibility.
The sFRP family has been commonly reported to be down-regulated by epigenetic inactivation in various cancers.[25][37–39] Urakami et al. also investigated the methylation frequency of the sFRP family (sFRP1-sFRP5) in renal cancer tissue and adjacent normal kidney tissues and found that the methylation level of sFRP1 was significantly higher in renal cancer tissues.[12] In other SNP studies of the sFRP family, Caldwell et al. found one polymorphic site on exon1 in sFRP1, but no significant association with the development of colorectal cancer.[38] The sFRP4 protein directly binds Wnt7a to inhibit activation of β-catenin/canonical signaling,[40][41] and was shown to be down-regulated in renal cancer tissues by epigenetic mechanisms.[12] However at present, there are no reports investigating the potential effect of sFRP4 gene polymorphisms on RCC.
Recently Lee et al. found that DKK3 was a negative regulator of beta-catenin.[42]
In order to investigate the effect of Wnt-antagonist polymorphisms on Wnt-signaling, we did an immunohistochemical analysis of beta-catenin expression and compared the relationship between beta-catenin expression levels and Wnt-antagonist polymorphisms. However, we did not find any relationship between beta-catenin expression and the polymorphisms. The detailed molecular mechanisms involved in how these polymorphisms have an effect on renal cancer is unclear. Nonsynonymous single nucleotide polymorphisms (nsSNPs) introduce amino acid changes and may affect protein function.[43] Therefore, it is generally believed that nsSNPs may be associated with cancer susceptibility.[43] PolyPhen (http://genetics.bwh.harvard.edu/pph/) is a computer program which is used to predict the effect of nonsynonymous coding SNPs on protein structure and function. When we used this program, DKK3 Arg335Gly (rs3206824), DKK2 Arg146Glu (rs17037102) and sFRP4 Arg340Lys (rs1802074) were judged to be benign, while sFRP4 Pro320Thr (rs1802073) showed a high PolyPhen score (1.577) and was judged as “possibly damaging”. Also XRCC1 Arg399Gln has been reported to be associated with the survival and prognosis of various cancer patients yet is judged to be benign by the PolyPhen program.[44][45] In addition the synonymous SNPs can alter mRNA folding and reduce mRNA stability thereby altering translation through structural changes in the RNA.[46] There is accumulating evidence regarding the functional effects of synonymous mutations. [47][48]
The polymorphisms associated with the 'Odds' of RCC are not associated with the clinical and pathological factors or survival. Similarly the polymorphisms associated with clinical and pathological features are not associated with survival. It is reasonable to consider that the functional role of a SNP as a risk factor is not always the same as that of a prognostic factor, because a risk SNP may contribute to the early stage of carcinogenesis of nearly normal cells whereas a prognostic SNP may be involved in the progression of fully transformed cells. Indeed, there have been many examples that a risk SNP is of no significance as a prognostic SNP, and vice versa.[49][50]
In conclusion, this is the first report documenting that the DKK2 rs17037102 polymorphism may be a predictor for survival after radical nephrectomy. Although further studies with a larger sample size are necessary, our present findings contribute to an understanding of individual survival differences after nephrectomy.
Acknowledgements
We thank Dr. Roger Erickson for his support and assistance with the preparation of the manuscript. This study was supported by Grants RO1CA130860, RO1CA111470, RO1CA108612, T32-DK07790 from the NIH, VA Research Enhancement Award Program (REAP), Merit Review grants, and Yamada Science Foundation.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics. 2000, CA. Cancer J Clin. 2004;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101–3106. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 3.Kudo-Saito C, Wansley EK, Gruys ME, Wiltrout R, Schlom J, Hodge JW. Combination therapy of an orthotopic renal cell carcinoma model using intratumoral vector-mediated costimulation and systemic interleukin-2. Clin Cancer Res. 2007;13:1936–1946. doi: 10.1158/1078-0432.CCR-06-2398. [DOI] [PubMed] [Google Scholar]
- 4.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 5.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 10.Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- 11.Edlund S, Lee SY, Grimsby S, et al. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2005;25:1475–1488. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urakami S, Shiina H, Enokida H, et al. Wnt antagonist family genes as biomarkers for diagnosis, staging, and prognosis of renal cell carcinoma using tumor and serum DNA. Clin Cancer Res. 2006;12:6989–6997. doi: 10.1158/1078-0432.CCR-06-1194. [DOI] [PubMed] [Google Scholar]
- 13.Dahl E, Wiesmann F, Woenckhaus M, et al. Frequent loss of SFRP1 expression in multiple human solid tumours: association with aberrant promoter methylation in renal cell carcinoma. Oncogene. 2007;26:5680–5691. doi: 10.1038/sj.onc.1210345. [DOI] [PubMed] [Google Scholar]
- 14.Gumz ML, Zou H, Kreinest PA, et al. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:4740–4749. doi: 10.1158/1078-0432.CCR-07-0143. [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto K, Hirata H, Kikuno N, Tanaka Y, Nakagawa M, Dahiya R. DNA methylation and histone modifications cause silencing of Wnt antagonist gene in human renal cell carcinoma cell lines. Int J Cancer. 2008;123:535–542. doi: 10.1002/ijc.23514. [DOI] [PubMed] [Google Scholar]
- 16.Shiina H, Igawa M, Breault J, et al. The human T-cell factor-4 gene splicing isoforms, Wnt signal pathway, and apoptosis in renal cell carcinoma. Clin Cancer Res. 2003;9:2121–2132. [PubMed] [Google Scholar]
- 17.Frank B, Burwinkel B, Bermejo JL, et al. Ten recently identified associations between nsSNPs and colorectal cancer could not be replicated in German families. Cancer Lett. 2008;271:153–157. doi: 10.1016/j.canlet.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 18.Proitsi P, Li T, Hamilton G, et al. Positional pathway screen of wnt signaling genes in schizophrenia: association with DKK4. Biol Psychiatry. 2008;63:13–16. doi: 10.1016/j.biopsych.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Hiroshi H, Zhong C, et al. Polymorphisms of Catechol-O-methyltransferase in Men with Renal Cell Cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:92–97. doi: 10.1158/1055-9965.EPI-06-0605. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y, Pan W, Khodursky AB. A note on using permutation-based false discovery rate estimates to compare different analysis methods for microarray data. Bioinformatics. 2005;21:4280–4288. doi: 10.1093/bioinformatics/bti685. [DOI] [PubMed] [Google Scholar]
- 22.Kurose K, Sakaguchi M, Nasu Y, et al. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol. 2004;171:1314–1318. doi: 10.1097/01.ju.0000101047.64379.d4. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh SY, Hsieh PS, Chiu CT, Chen WY. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene. 2004;23:9183–9189. doi: 10.1038/sj.onc.1208138. [DOI] [PubMed] [Google Scholar]
- 24.Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25:5027–5036. doi: 10.1038/sj.onc.1209508. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashia K, Ouchida M, Tsuji T, et al. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene. 2002;282:151–158. doi: 10.1016/s0378-1119(01)00838-1. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, Toyota M, Caraway H, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98:1147–1156. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato H, Suzuki H, Toyota1 M, et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007;28:2459–2466. doi: 10.1093/carcin/bgm178. [DOI] [PubMed] [Google Scholar]
- 28.Roman-Gomez J, Jimenez-Velasco A, Catillejo JA, et al. Promoter hypermethylation of cancer-related genes: a strong independent prognostic factor on acute lymphoblastic leukemia. Blood. 2004;104:2492–2498. doi: 10.1182/blood-2004-03-0954. [DOI] [PubMed] [Google Scholar]
- 29.Aguilera O, Fraga MF, Ballestar E, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25:4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 30.Urakami S, Shiina H, Enokida H, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin Cancer Res. 2006;12:2109–2116. doi: 10.1158/1078-0432.CCR-05-2468. [DOI] [PubMed] [Google Scholar]
- 31.Zenzmaier C, Untergasser G, Hermann M, Dirnhofer S, Sampson N, Berger P. Dysregulation of Dkk-3 expression in benign and malignant prostatic tissue. Prostate. 2008;68:540–547. doi: 10.1002/pros.20711. [DOI] [PubMed] [Google Scholar]
- 32.Yue W, Sun Q, Dacic S, et al. Downregulation of Dkk3 activates β-catenin/TCF-4 signaling in lung cancer. Carcinogenesis. 2008;29:84–92. doi: 10.1093/carcin/bgm267. [DOI] [PubMed] [Google Scholar]
- 33.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 34.Tse KP, Tsang NM, Chen KD, Li HP, Liang Y, Hsueh C, Chang KP, Yu JS, Hao SP, Hsieh LL, Chang YS. MCP-1 Promoter Polymorphism at 2518 is associated with metastasis of nasopharyngeal carcinoma after treatment. Clin Cancer Res. 2007;13:6320–6326. doi: 10.1158/1078-0432.CCR-07-1029. [DOI] [PubMed] [Google Scholar]
- 35.Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signaling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 36.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 37.Chim CS, Pang R, Fung TK, Choi CL, Liang R. Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia. 2007;21:2527–2536. doi: 10.1038/sj.leu.2404939. [DOI] [PubMed] [Google Scholar]
- 38.Caldwell GM, Jones C, Gensberg K, et al. The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res. 2004;64:883–888. doi: 10.1158/0008-5472.can-03-1346. [DOI] [PubMed] [Google Scholar]
- 39.Fukui T, Kondo M, Maeda O, et al. Transcriptional silencing of secreted frizzled related protein 1 (SFRP1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24:6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- 40.Horvath LG, Henshall SM, Kench JG, et al. Membranous expression of secreted frizzled-related protein 4 predicts for good prognosis in localized prostate cancer and inhibits PC3 cellular proliferation in vitro. Clin Cancer Res. 2004;10:615–625. doi: 10.1158/1078-0432.ccr-0707-03. [DOI] [PubMed] [Google Scholar]
- 41.Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–1028. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- 42.Lee EJ, Jo M, Rho SB, et al. Dkk3, downregulated in cervical cancer, functions as a negative regulator of beta-catenin. Int J Cancer. 2009;124:287–297. doi: 10.1002/ijc.23913. [DOI] [PubMed] [Google Scholar]
- 43.Ng PC, Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet. 2006;7:61–80. doi: 10.1146/annurev.genom.7.080505.115630. [DOI] [PubMed] [Google Scholar]
- 44.Giachino DF, Ghio P, Regazzoni S, et al. Prospective assessment of XPD Lys751Gln and XRCC1 Arg399Gln single nucleotide polymorphisms in lung cancer. Clin Cancer Res. 2007;13:2876–2881. doi: 10.1158/1078-0432.CCR-06-2543. [DOI] [PubMed] [Google Scholar]
- 45.Berndt SI, Huang WY, Fallin MD, et al. Genetic variation in base excision repair genes and the prevalence of advanced colorectal adenoma. Cancer Res. 2007;67:1395–1404. doi: 10.1158/0008-5472.CAN-06-1390. [DOI] [PubMed] [Google Scholar]
- 46.Shen LX, Basilion JP, Stanton VP Jr. Single-nucleotide polymorphisms can cause different structural folds of mRNA. Proc Natl Acad Sci USA. 1999;96:7871–7876. doi: 10.1073/pnas.96.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duan J, Wainwright MS, Comeron JM, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- 48.Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. Silent polymorphisms speak: how they affect pharmacogenomics and the treatment of cancer. Cancer Res. 2007;67:9609–9612. doi: 10.1158/0008-5472.CAN-07-2377. [DOI] [PubMed] [Google Scholar]
- 49.Holt SK, Karyadi DM, Kwon EM, Stanford JL, Nelson PS, Ostrander EA. Association of megalin genetic polymorphisms with prostate cancer risk and prognosis. Clin Cancer Res. 2008;14:3823–3831. doi: 10.1158/1078-0432.CCR-07-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa BM, Ferreira P, Costa S, et al. Association between functional EGF+61 polymorphism and glioma risk. Clin Cancer Res. 2007;13:2621–2626. doi: 10.1158/1078-0432.CCR-06-2606. [DOI] [PubMed] [Google Scholar]


