Abstract
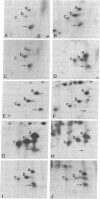
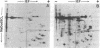
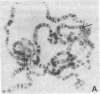
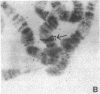
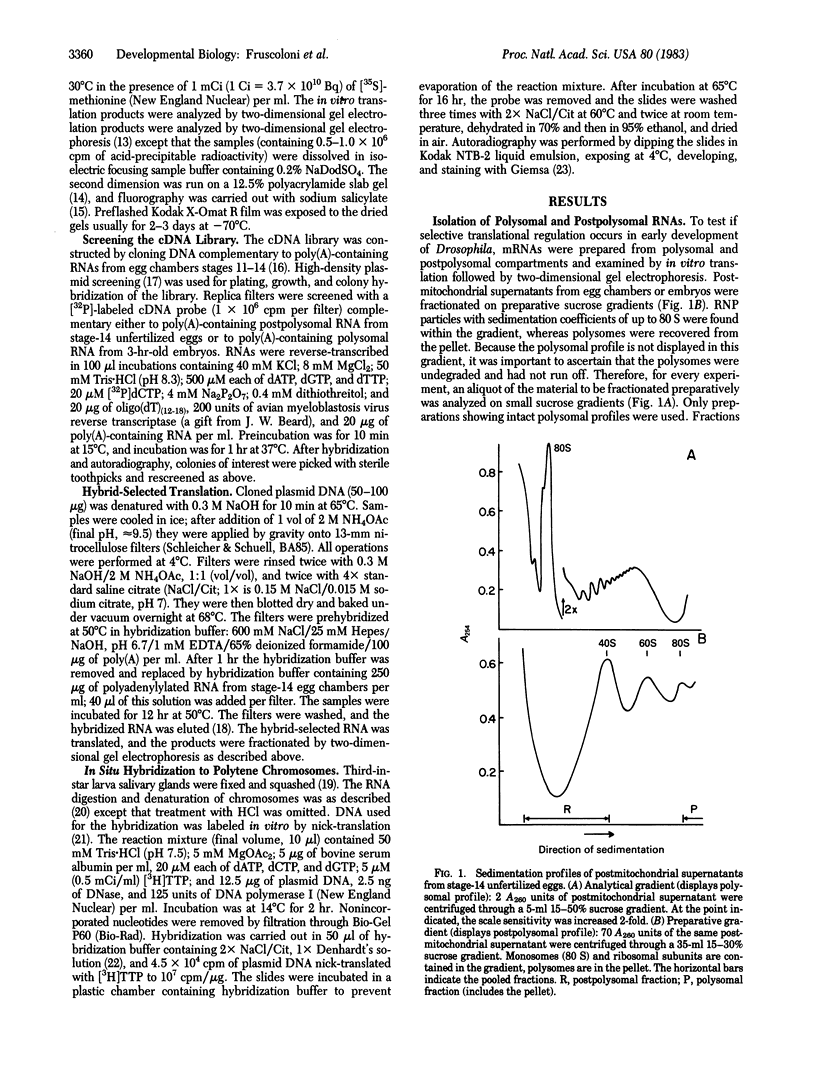
Polysomal and postpolysomal mRNAs were prepared from Drosophila egg chambers or embryos of different developmental stages. Cell-free translation of these mRNAs followed by two-dimensional gel electrophoresis of the products indicated the presence of a specific mRNA that appears to be translated (polysome-associated) during oogenesis. This mRNA, designated T1 mRNA, is selectively excluded from polysomes in 3-hr- and 5-hr-old embryos and is again translated in 18-hr-old embryos. A clone containing DNA complementary to T1 mRNA was selected from a library of recombinant DNA prepared from polyadenylylated ovary RNA. This clone was positively identified by hybrid-selected translation followed by two-dimensional gel electrophoresis and autoradiography. T1 mRNA is polyadenylylated and codes for a small, acidic protein. The cloned probe hybridizes to a unique site (2L-39CD) of the polytene chromosomes, very close to the histone genes. The results suggest that this mRNA is under specific translational regulation in contrast to a background of a large number of other abundant mRNAs that are translated at all developmental stages examined.
Keywords: protein synthesis, development
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. V., Lengyel J. A. Rates of synthesis of major classes of RNA in Drosophila embryos. Dev Biol. 1979 May;70(1):217–231. doi: 10.1016/0012-1606(79)90018-6. [DOI] [PubMed] [Google Scholar]
- Bownes M. A photographic study of development in the living embryo of Drosophila melanogaster. J Embryol Exp Morphol. 1975 Jun;33(3):789–801. [PubMed] [Google Scholar]
- Cascio S. M., Wassarman P. M. Program of early development in the mammal: post-transcriptional control of a class of proteins synthesized by mouse oocytes and early embryos. Dev Biol. 1982 Feb;89(2):397–408. doi: 10.1016/0012-1606(82)90328-1. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Faust C. H., Jr, Diggelmann H., Mach B. Isolation of poly(adenylic acid)-rich ribonucleic acid from mouse myeloma and synthesis of complementary deoxyribonucleic acid. Biochemistry. 1973 Feb 27;12(5):925–931. doi: 10.1021/bi00729a021. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Herlands L., Allfrey V. G., Poccia D. Translational regulation of histone synthesis in the sea urchin strongylocentrotus purpuratus. J Cell Biol. 1982 Jul;94(1):219–223. doi: 10.1083/jcb.94.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough-Evans B. R., Jacobs-Lorena M., Cummings M. R., Britten R. J., Davidson E. H. Complexity of RNA in eggs of Drosophila melanogaster and Musca domestica. Genetics. 1980 May;95(1):81–94. doi: 10.1093/genetics/95.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illmensee K., Mahowald A. P. Transplantation of posterior polar plasm in Drosophila. Induction of germ cells at the anterior pole of the egg. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1016–1020. doi: 10.1073/pnas.71.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Crippa M. Mass fractionation of Drosophila egg chambers. Dev Biol. 1977 Jun;57(2):385–392. doi: 10.1016/0012-1606(77)90223-8. [DOI] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Hough-Evans B. R., Britten R. J., Davidson E. H. Complexity of RNA in developing oocytes of Drosophila melanogaster. Dev Biol. 1980 May;76(2):509–513. doi: 10.1016/0012-1606(80)90399-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Mermod J. J., Crippa M. Variations in the amount of polysomes in mature oocytes of Drosophila melanogaster. Dev Biol. 1978 Oct;66(2):586–592. doi: 10.1016/0012-1606(78)90264-6. [DOI] [PubMed] [Google Scholar]
- Mermod J. J., Schatz G., Crippa M. Specific control of messenger translation in Drosophila oocytes and embryos. Dev Biol. 1980 Mar;75(1):177–186. doi: 10.1016/0012-1606(80)90153-0. [DOI] [PubMed] [Google Scholar]
- Mohler J. D. Developmental genetics of the Drosophila egg. I. Identification of 59 sex-linked cistrons with maternal effects on embryonic development. Genetics. 1977 Feb;85(2):259–272. doi: 10.1093/genetics/85.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Okada M., Kleinman I. A., Schneiderman H. A. Restoration of fertility in sterilized Drosophila eggs by transplantation of polar cytoplasm. Dev Biol. 1974 Mar;37(1):43–54. doi: 10.1016/0012-1606(74)90168-7. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Kedes L. H., Weinberg E. S., Birnstiel M. L. Localization of sequences coding for histone messenger RNA in the chromosomes of Drosophila melanogaster. Chromosoma. 1977 Aug 25;63(2):135–151. doi: 10.1007/BF00292726. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. Isolation of galactose-inducible DNA sequences from Saccharomyces cerevisiae by differential plaque filter hybridization. Cell. 1979 Feb;16(2):443–452. doi: 10.1016/0092-8674(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Thireos G., Griffin-Shea R., Kafatos F. C. Untranslated mRNA for a chorion protein of Drosophila melanogaster accumulates transiently at the onset of specific gene amplification. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5789–5793. doi: 10.1073/pnas.77.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Showman R. M., Klein W. H., Raff R. A. Delayed recruitment of maternal histone H3 mRNA in sea urchin embryos. Nature. 1981 Jul 30;292(5822):477–478. doi: 10.1038/292477a0. [DOI] [PubMed] [Google Scholar]
- Zalokar M., Audit C., Erk I. Developmental defects of female-sterile mutants of Drosophila melanogaster. Dev Biol. 1975 Dec;47(2):419–432. doi: 10.1016/0012-1606(75)90295-x. [DOI] [PubMed] [Google Scholar]
- Zalokar M. Autoradiographic study of protein and RNA formation during early development of Drosophila eggs. Dev Biol. 1976 Apr;49(2):425–437. doi: 10.1016/0012-1606(76)90185-8. [DOI] [PubMed] [Google Scholar]