Abstract
Exercise has been shown to be beneficial for Parkinson’s disease (PD). A major interest in our lab has been to investigate how exercise modulates basal ganglia function and modifies disease progression. Dopamine (DA) depletion leads to loss of dendritic spines within the caudate nucleus and putamen (striatum) in PD and its animal models and contributes to motor impairments. Striatal medium spiny neurons (MSNs) can be delineated into two populations, the dopamine D1 receptor (DA-D1R)-containing MSNs of the direct pathway and dopamine D2 receptor (DA-D2R)-containing MSNs of the indirect pathway. There is evidence to suggest that the DA-D2R-indirect pathway MSNs may be preferentially affected after DA-depletion with a predominate loss of dendritic spine density when compared to MSNs of the DA-D1R-direct pathway in rodents; however, others have reported that both pathways may be affected in primates. The purpose of this study was to investigate the effects of intensive exercise on dendritic spine density and arborization in MSNs of these two pathways in the MPTP mouse model of PD. We found that MPTP led to a decrease in dendritic spine density in both DA-D1R-and DA-D2R-containing MSNs and 30 days of intensive treadmill exercise led to increased dendritic spine density and arborization in MSNs of both pathways. In addition, exercise increased the expression of synaptic proteins PSD-95 and synaptophysin. Taken together these findings support the potential effect of exercise in modifying synaptic connectivity within the DA-depleted striatum and in modifying disease progression in individuals with PD.
Keywords: neuroplasticity, dopamine, synaptogenesis, striatum, basal ganglia, Parkinson’s, GFP
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the loss of midbrain dopaminergic neurons leading to the depletion of striatal dopamine (DA). Individuals with PD manifest both cognitive and motor deficits including balance instability, gait dysfunction, slowness, tremor, and rigidity. While DA replacement therapy remains a cornerstone of treatment, its efficacy diminishes over time. Currently, there is no known cure for PD. A major interest of research in PD is to find treatments that have the capacity to modify disease progression or better prevent disease onset. For example, epidemiological studies have suggested that intensive exercise, especially in males, over a lifetime can influence the occurrence of PD (Chen et al., 2005). Over the last decade a number of investigators have demonstrated exercise to the beneficial for the treatment of PD especially for the treatment of gait and balance (Petzinger et al., 2010; Speelman et al., 2011; Petzinger et al., 2013). In animal models of PD, exercise has been shown to have the capacity to be both neuroprotective, by preventing the loss of DA in lesion models (Gerecke et al., 2010), and neurorestorative, providing enhancement in DA neurotransmission leading to reversal of motor deficits (Fisher et al., 2004; Petzinger et al., 2007). These observations, along with ongoing basic and clinical research, are beginning to investigate the potential role of exercise in modifying disease progression in individuals with PD as well as in animal models of DA-depletion. Towards this goal, a major focus of our lab has been to elucidate the underlying molecular mechanisms by which exercise affects neuroplasticity including synaptic structure and function within the injured basal ganglia in neurotoxin animal models of PD.
In PD and in neurotoxin animal models, the depletion of striatal DA leads to alterations in basal ganglia neurotransmission that manifest in a number of different ways. For example, the striatopallidal (indirect) projection medium spiny neurons (MSNs) become hyperactive and are thought to underlie the onset of akinesia (slowness of movement) (Calabresi et al., 1997). In addition, corticostriatal projections targeting striatal MSNs also display aberrant glutamatergic neurotransmission as demonstrated through electrophysiological techniques (Cepeda et al., 1989; Pisani et al., 2005). Therefore, it is not surprising that striatal MSNs manifest these changes in neurotransmission by alterations in the dendritic spine number and dendritic arborization, the primary morphological correlates of synaptic neurotransmission. For example, studies using Golgi staining have shown a reduction in dendritic arborization and spine density in the caudate nucleus and putamen in tissues from patients with PD (McNeill et al., 1988; Stephens et al., 2005). Similar findings have been made in both the 6-hydroxydopamine (6-OHDA) rat and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) nonhuman primate models of DA-depletion where spine density changes have been reported (Ingham et al., 1989; Ingham et al., 1993; Villalba and Smith, 2010).
The purpose of this study was to determine if intensive exercise in the form of treadmill running leads to a reversal of both dendritic spine loss and the reduction in dendritic arborization in a mouse model of DA-depletion. This speculation is largely based on the fact that we observe exercise enhanced alterations in both glutamate and DA neurotransmission in the MPTP-lesioned mouse model (Kintz et al., 2013). Since dendritic spine density and branching are influenced by experience including environmental enrichment and exercise in several regions of the mammalian brain we were interested in knowing if exercise leads to a similar outcome (Comery et al., 1995; Eadie et al., 2005; Leggio et al., 2005; Stranahan et al., 2007). We also took advantage of the Drd2-eGFP-BAC transgenic mouse strain (Chan et al., 2012; Nelson et al., 2012) in conjunction with biocytin injection methods that allowed us to delineate dopamine D1 receptor (DA-D1R)-containing MSNs and dopamine D2 receptor (DA-D2R)-containing MSNs since some models of DA-depletion show preferential loss of dendritic spine density selectively on DA-D2R-containing MSNs (Day et al., 2006). Utilizing the MPTP mouse model of DA-depletion with either Golgi staining or biocytin labeling we examined the effects of 6 weeks of intensive treadmill running on dendritic spine density and arborization of MSNs within the dorsolateral striatum, a region responsible for motor control and a site where we have documented neuroplastic changes in response to exercise (Petzinger et al., 2007).
Material and Methods
Animals
Twelve C57BL/6J young adult (8 to 10 weeks old) male mice from Jackson Labs (Bar Harbor, Maine) and 52 young adult (8 to 10 weeks old) male Drd2-eGFP-BAC mice (Tg(Drd2-EGFP)118Gsat/Mmnc) supplied from the Mutant Mouse Regional Resource Center of NIH (MMRRC) program at the University of California, Davis (Gong et al., 2003) were used for this study. We have established a colony of Drd2-eGFP-BAC mice that have been backcrossed into C57BL/6J mice in our lab at least 10 times to enhance genomic stability and validate comparison of outcomes with those derived from C57BL/6J mice. Male hemizygous mice Drd2-eGFP-BAC mice continually backcrossed onto C57BL/6J mice were used for this study. Both groups of C57BL/6J and Drd2-eGFP-BAC mice were randomly assigned to one of four treatment groups including: (i) saline, (ii) saline+exercise, (iii) MPTP, and (iv) MPTP+exercise. Mice were group housed with a reverse light cycle (lights off 7 a.m. to 7 p.m.) with ad libitum access to food and water. Experimental procedures were approved by the University of Southern California’s Institutional Animal Care and Use Committee and conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (DHEW Publication 80-23, 2011, Office of Laboratory Animal Welfare, DRR/NIH, Bethesda, MD). All efforts were made to minimize animal suffering and the number of animals used to achieve statistical significance.
Some concern has been raised about the Drd2-eGFP-BAC mouse line and the possibility that this BAC line does not express the physiology and associated behavior seen in C57BL/6J mice (Kramer et al., 2011). However, several reports indicate that Drd2-eGFP-BAC mice backcrossed to C57BL/6J mice display both normal behavior and DA neurotransmission (Taverna et al., 2008; Chan et al., 2012; Nelson et al., 2012). In our hands no differences were detectable between Drd2-eGFP-BAC mice and C57BL/6J mice in maximum running velocity on the treadmill, normal striatal DA-levels, amount of DA-depletion, or degree of substantia nigra pars compacta dopaminergic cell death resulting from systemic injections of MPTP in our striatal lesioning protocol (Jackson-Lewis et al., 1995; Kintz et al., 2013).
MPTP-lesioning
Half of the C57BL/6J and half of the Drd2-eGFP-BAC mice were administered 4 intraperitoneal injections of MPTP at 20 mg/kg (free-base, Sigma, St. Louis, MO) at 2-hour intervals. Remaining mice received intraperitoneal injections of saline. This lesioning regimen results in 90–95% loss of striatal dopamine and 65 to 70% loss of nigrostriatal dopaminergic neurons (Jackson-Lewis et al., 1995; Jakowec et al., 2004). Drd2-eGFP-BAC mice on the C57BL/6J background had DA-depletion and nigrostriatal cell death indistinguishable from wild-type C57BL/6J mice (Kintz et al., 2013). In this study, striatal DA levels of Drd2-eGFP-BAC mice were assessed by high-performance liquid chromatography (HPLC) analysis. HPLC analysis was conducted 5 days after lesioning, corresponding to the start of the treadmill exercise regimen, and at 42 days after lesioning, corresponding to the completion of the treadmill exercise regimen.
Exercise Regimen
1 week before the start of the treadmill exercise regimen (2 days before MPTP lesioning), 8 to 10 week old C57BL/6J and Drd2-eGFP-BAC mice that could maintain a forward position on the 45 cm treadmill belt for 5 minutes at 5.0 m/min were randomly assigned to the 4 groups to ensure that all animals performed similarly on the treadmill task prior to MPTP-lesioning. The treadmill used in these studies was a Model EXER-6M Treadmill manufactured by Columbus Instruments (Columbus, Ohio). A non-noxious stimulus (metal beaded curtain) was used as a tactile incentive to prevent animals from drifting back on the treadmill. The treadmill exercise regimen was conducted as previously described (Fisher et al., 2004). Briefly, exercise was initiated 5 days following MPTP or saline administration, a time point after cell death is complete, and continued 5 days/week for a total of 6 weeks of exercise (corresponding to a final of 42 days after MPTP-lesioning). Exercised mice started at a velocity of 10.0 m/min, which increased to 24.0 m/min by the final week. These velocities were adjusted (either increased or decreased) such that all mice within a group could maintain a forward position on the treadmill for 75% of the running period. At the end of each week, the average achieved velocities were calculated to produce a time course of improvement in running velocity. All mice not exercised were given access to an immobile treadmill for an equivalent amount of time as the running mice.
Golgi Staining
A total of 12 C57BL/6J mouse brains were processed using the Golgi stain. After the final session of running, the C57BL/6 mice were euthanized by cervical dislocation and decapitated to allow extraction of the brain. Golgi-Cox staining (PK401, FD NeuroTechnologies, Ellicott City, MD) was used to visualize neurons. Tissue was impregnated for 2 weeks, sectioned in the coronal orientation in 120 µm thick sections, and processed according to the manufacturer’s specifications. A representative Golgi stained MSN and high magnification images of dendrites from our groups can be found in Figure 3b and 3c.
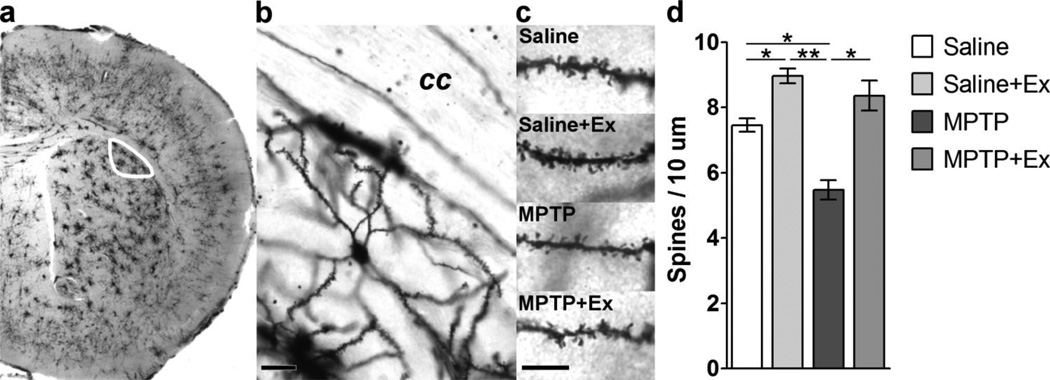
Figure 3. Analysis of Dendritic Spine Density with Golgi Staining.
Panel a shows a representative coronal section of the mid-striatum processed using the Golgi stain. The outline (white) delineates the dorsolateral striatum where MSN dendrites were sampled. Panel b shows a representative high magnification image of a single Golgi stained MSN indicating dendritic arbor and dendritic spine density. The scale bar represents 25 microns. “cc” – corpus callosum. Panel c shows representative dendrites from MSNs of all four groups. Note the reduction in dendritic spine density with MPTP and an increase in spine density with exercise. The scale bar represents 10 microns. Panel d graphically depicts the quantitative data from all four groups. Note the significant increase in dendritic spine density with exercise in both the saline and MPTP mice. The asterisks represent statistical significance at either p < 0.05 “*” or p < 0.01 “**”.
Biocytin Labeling
Drd2-eGFP-BAC mice allowed for the differentiation of the two types of MSNs located in the dorsolateral striatum; DA-D2R-containing MSNs express eGFP and fluoresce green while DA-D1R-containing MSNs do not. 400 µm thick (spanning 0.20 mm to 0.60 mm anterior to bregma), coronal sections were obtained from a recently sacrificed mouse. MSNs were filled with a solution containing 0.5% biocytin (B4261, Sigma-Aldrich, St. Louis, Missouri) using a sharp micropipette. Diffusion of biocytin was assisted with hyperpolarizing pulses for 20 minutes. Tissue sections were fixed in Tris-buffered saline (TBS) containing 4% paraformaldehyde, pH 7.2, cryoprotected in TBS with 30% sucrose, and then sectioned in a cryostat at 60 µm thickness. MSNs were visualized using a protocol based on Wilson and Sachdev (2004) which utilizes the DAB/HRP reaction (PK-4000, Vector Laboratories, Burlingame, CA). A representative DA-D1R MSN and high magnification images of dendrites from our groups can be found in Figure 4a and 4b. A representative DA-D2R MSN and high magnification images of dendrites from our groups can be found in Figure 4c and 4d.
Figure 4. Analysis of Dendritic Spine Density with Biocytin filled DA-D1R or DA-D2R MSNs.
Panel a shows a representative image of a DA-D1R-containing MSN filled with biocytin and processed for visualization. The scale bar represents 25 microns. Panel b shows representative images of dendrites from biocytin-filled DA-D1R-containing MSNs in each of the four groups. Note the reduction of dendritic spines with MPTP and an increase in dendritic spine density with exercise. The scale bar represents 10 microns. Panel e graphically depicts the quantitation of these data showing a significant reduction in dendritic spine density with MPTP and its increase with exercise in DA-D1R-containing MSNs. Panel c shows a representative image of a DA-D2R-containing MSN filled with biocytin and processed for visualization. The scale bar represents 25 microns. Panel d shows representative images of dendrites from biocytin-filled DA-D2R-containing MSNs in each of the four groups. Note the reduction of dendritic spines with MPTP and an increase in dendritic spine density with exercise. The scale bar represents 10 microns. Panel f graphically depicts the quantitation of these data showing a significant reduction in dendritic spine density with MPTP and its increase with exercise in DA-D2R-containing MSNs. The asterisks represent statistical significance at either p < 0.05 “*” or p < 0.01 “**”.
Morphological Analyses
For Golgi stained tissue, MSNs located in the dorsolateral striatum between 0.14 mm to 0.62 mm anterior to bregma (Figure 3a), with the following properties were analyzed based on (i) soma that was round or ovoid with a diameter of 11 to 20 µm, (ii) three to eight visible primary dendrites, (iii) and sparse dendritic spines on primary branches with an increase in dendritic spines from second or third order branches onward (Rafols et al., 1989). High magnification image of representative dendrites are in Figure 1c. Dendritic spine density was calculated by dividing a MSN’s traced dendritic length by its dendritic spine count total along those traced dendrites. Dendritic length was obtained by tracing dendrites of a MSN starting about 30 µm away from the soma, a distance where spines are abundant, until the dendrite was cut off or at 15 µm from the terminus, an area were spine density tapers off. Spine counts were obtained by manually counting dendritic spines along the traced dendrites. This analysis was performed on Golgi stained and biocytin filled MSNs. Complete tracings of biocytin filled MSNs allowed for utilization of the Sholl analysis to determine branching characteristics of a neuron at distances away from the soma (Sholl, 1953). Sholl analysis was only performed on biocytin filled cells. All morphological data was collected at 80× or 120× magnification with an Olympus Cooperation BX50 microscope (Shinjuku, Tokyo, Japan) using a QImaging QIClick camera (Surrey, British Columbia, Canada) to acquire images that were analyzed using BIOQUANT Life Science 2011 V 11.2.60 (BIOQUANT Image Analysis Corporation, Nashville, TN) or Fiji (Schindelin et al., 2012). Data were gathered by an individual blinded to the experimental conditions.
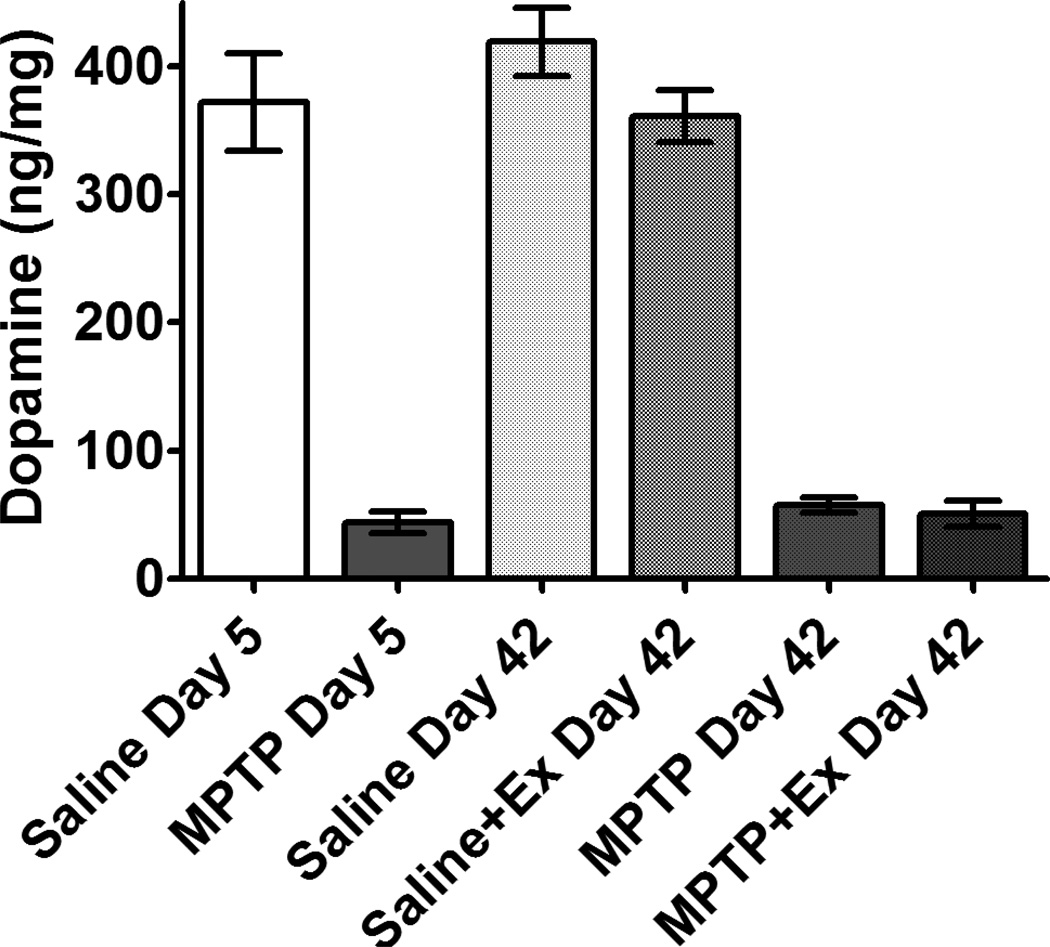
Figure 1. MPTP-lesioning leads to early DA depletion which is sustained out to 42 days post-lesion.
Striatal dopamine levels of Drd2-eGFP-BAC mice are reduced at 5 days after MPTP-lesioning. This loss is sustained in these mice even after 30 days of treadmill exercise (42 days post-lesion). DA levels of control mice were unchanged after 30 days of treadmill exercise.
Immunohistochemistry
12 Drd2-eGFP-BAC mice (3 per experimental group) were used for immunohistochemical analysis. Immunohistochemistry was performed on 20 µm-thick slices containing the striatum between 0.14 mm to 0.62 mm anterior to bregma. 8 tissue sections per animal were washed 3 times for 15 minutes in TBS pH 7.2 and blocked for 1 hour at room temperature in 6% normal goat serum (NGS) and in TBS with 0.05% Triton X-100 (TX). The following antibodies were used to detect PSD-95 and synaptophysin: mouse anti-PSD-95 IgG (1:2500, EMD Millipore, Billerica, MA), rabbit anti-synaptophysin IgG (1:10000, Epitomics, Burlingame, CA), and biotinylated goat anti-mouse IgG (1:5000, Vector Laboratories, Inc., Burlingame, CA). Antibody specificity was validated by subjecting sections to the same IHC protocol but without the addition of primary and/or secondary antibody. Primary antibodies were diluted in TBS with 2% NGS and 0.05% TX. Following incubation in the primary antibody solution overnight at 4°C, tissue was washed three times in TBS. The tissue was then incubated in biotinylated antibody solution consisting of TBS with 2% NGS and 0.05% TX overnight at 4°C and subsequently rinsed 3 times in TBS. From this point on the tissue was protected from light. Sections were then incubated with Cy5-conjugated streptavidin (1:5000, Rockland Immunochemicals, Inc., Gilbertsville, PA) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:10000, Invitrogen, Grand Island, NY) diluted in TBS with 3% NGS and 0.05% TX for 1 hour at room temperature. Sections were mounted on gelatin-subbed slides, dried, and then coverslipped using Vectashield Mounting Medium with DAPI (Vector Laboratories, Inc., Burlingame, CA). Representative high magnification images of PSD-95 and synaptophysin staining can be found in Figure 5a and 5b.
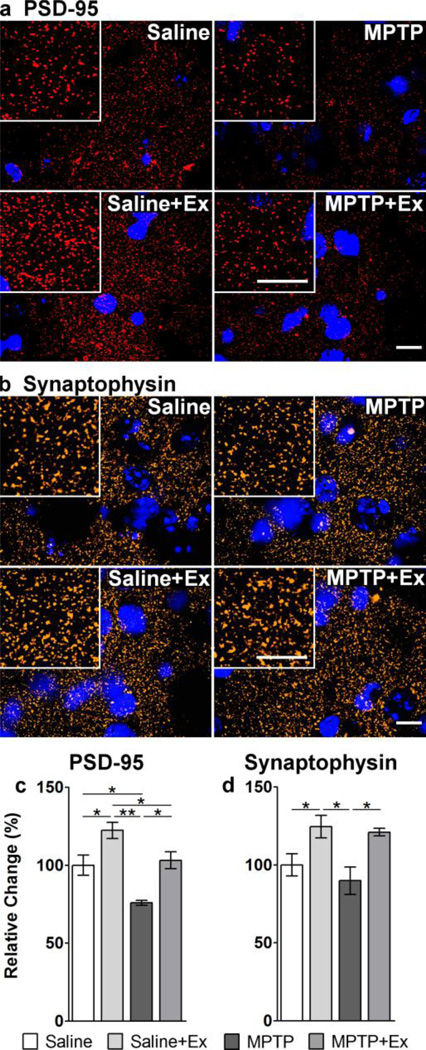
Figure 5. Immunohistochemical staining of synaptic proteins PSD-95 and synaptophysin.
Panel a shows representative immunohistochemical staining of dorsolateral striatal tissues from all four groups with an antibody against PSD-95 protein (red) and the nuclear stain DAPI (blue). The insert boxes in each panel are images at a higher magnification showing distinct puncta (synaptic staining). The scales bars in each set of panels represent 10 microns. Quantitation of immunofluorescence for PSD-95 is shown in Panel c indicating a decrease in the number of synapses with MPTP. With exercise there is a significant increase in the number of synapses in both saline and MPTP mice. Panel b shows representative immunohistochemical staining of dorsolateral striatal tissues from all four groups with an antibody against synaptophysin protein (red) and the nuclear stain DAPI (blue). Quantitation of immunofluorescence for synaptophysin is shown in Panel d indicating a significant increase in the number of synapses in exercised mice compared to their non-exercised counterparts. The asterisks represent statistical significance at either p < 0.05 “*” or p < 0.01“**”.
Immunofluorescence intensity was captured at 100× magnification with an Olympus BX61 microscope (Shinjuku, Tokyo, Japan) equipped with a Disk Scanning Unit (spinning disk confocal) and 100 W mercury light source (U-LH100HG) using a Hamamatsu Photonics ORCA-R2 camera (Hamamatsu, Japan) and analyzed using Metamorph Advanced 7.7.2.0 (Molecular Devices, LLC, Sunnyvale, CA). One z-stack was taken from the dorsolateral striatum of each brain slice and deconvolved using Metamorph Autoquant Deconvolution software to remove background noise. The frame of best focus, defined as the frame in which the largest portion of the fluorescing areas of the image is in focus, was then selected from the stack. In order to account for striatal bundles, cell bodies, or unfocused areas, data was collected from three regions of interest placed randomly on areas of fluorescing tissue. A threshold was automatically performed using Metamorph’s internal algorithm, which thresholds for the brightest pixels in the right tail of the pixel brightness histogram, leaving out the majority of pixels in the image. Regional statistics were then collected via the "Integrated Morphometry" function of Metamorph.
Statistical analyses
SPSS Statistics 21 (IBM, Armonk, NY) was used to compare means of data acquired. For HPLC analysis, a Mann-Whitney t-test was performed for comparisons between MPTP versus saline treated mice: (1) day 5 post-lesion MPTP non-exercised, (2) day 42 post-lesion MPTP non-exercised, (3) day 42 post-lesion MPTP exercise. A one-way ANOVA was performed to compare differences in striatal DA across the MPTP groups: (1) day 5 post-lesion MPTP, (2) day 42 post-lesion MPTP non-exercised, (3) day 42 post-lesion MPTP+exercise. Tukey’s honest significance test was used to perform post hoc analyses of the dendritic spine density data. Fisher’s least significant difference was used to perform post hoc analyses for data from the Sholl Analysis and immunohistochemistry. Figures were made with GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA) and GIMP (GNU Image Manipulation Program, www.gimp.org)
Results
MPTP administration leads to loss of striatal DA
In the non-exercised Drd2-eGFP-BAC mice, striatal DA was significantly reduced (p = 0.002) at 5 days post-lesioning by 88.2% in MPTP (43.8 ± 8.5 ng/mg protein ± SEM) compared to saline mice (372 ± 38) and still significantly reduced (p = 0.001) at 42 days post-lesioning by 86.3% in MPTP (57.4 ± 5.7) compared to saline mice (419 ± 27). In the exercised Drd2-eGFP-BAC mice, striatal DA was significantly reduced at 42 days post-lesioning by 86.0% in MPTP+exercise (50.7 ± 10.2) compared to saline+exercise mice (361 ± 20). No significant difference in DA levels was observed between day 5 post-lesion MPTP non-exercised, day 42 post-lesion MPTP non-exercised, and day 42 post-lesion MPTP+exercise mice (F(2,19) = 0.594, p = 0.562). No significant difference in DA levels was observed between day 5 post-lesion saline non-exercised, day 42 post-lesion saline non-exercised, and Day 42 post-lesion saline+exercise mice (F(2,20) = 0.846, p = 0.444). Importantly, Drd2-eGFP-BAC mice and C57BL/6 mice were similarly sensitive to MPTP. HPLC analysis of C57BL/6 mice lesioned with MPTP showed a decline in striatal DA compared to saline mice at similar levels (VanLeeuwen et al., 2010; Kintz et al., 2013). Striatal dopamine levels remained unchanged in both the saline and MPTP-lesioned groups during the 6-week period of these studies.
Intensive treadmill exercise attenuates initial running speed deficit of MPTP-lesioned mice
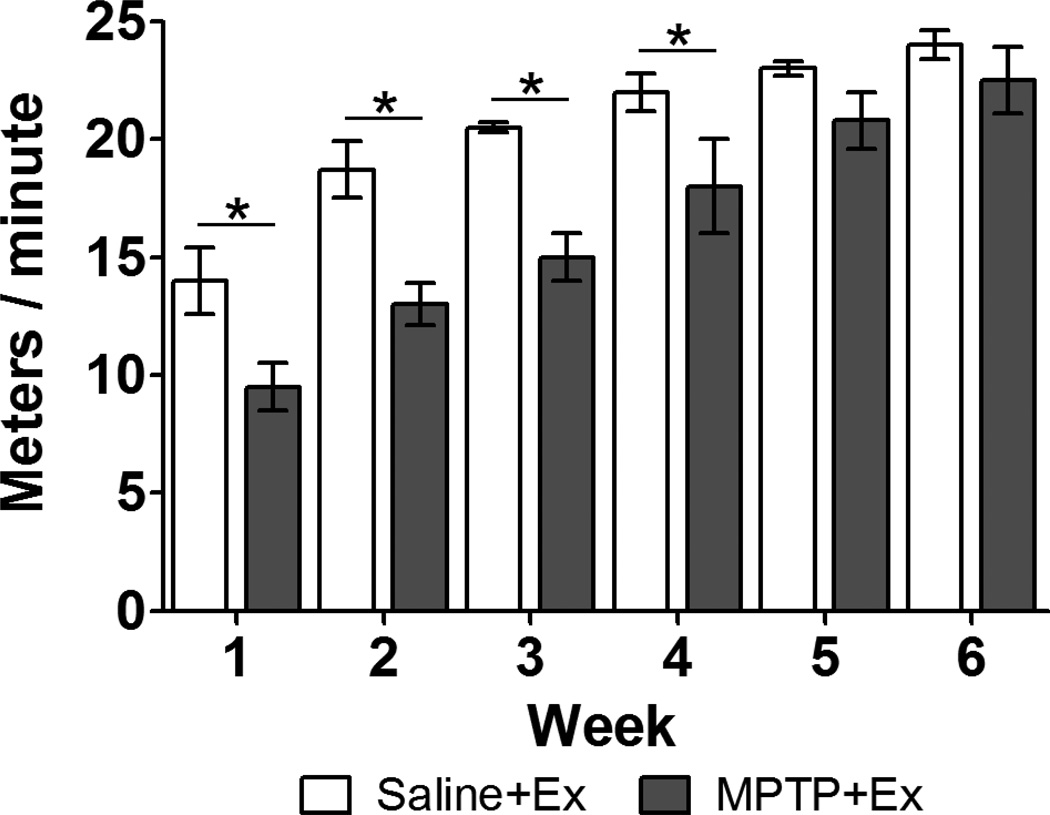
Similar to our previous findings (Fisher et al., 2004; Petzinger et al., 2007), our recent cohort of MPTP+exercise mice needed 5 weeks of intensive treadmill running to no longer be significantly different than saline+exercise mice in their achievable treadmill velocity. The time course of improvement in running velocity is shown in Figure 2. The week 1 assessment showed that MPTP+exercise mice (9.5 ± 1.0, meters / minute ± SEM) were significantly slower (p < 0.05) than saline+exercise mice (14 ± 1.4). However, the week 5 assessment showed that MPTP+exercise mice (21 ± 1.2) were no longer significantly different (p > 0.05) than saline+exercise mice (23 ± 0.6) and this restoration of motor ability persisted through week 6.
Figure 2. MPTP mice are able to achieve running velocities no different than control mice after 5 weeks of treadmill exercise.
MPTP-lesioning initially leads to the failure of mice to achieve running velocities similar to control mice. After 5 weeks of treadmill running there was no longer a significant difference between the groups showing that MPTP mice are no longer impaired. The asterisks represent statistical significance at p < 0.05 “*”.
Exercise increases MSN spine densities in MPTP-lesioned mice
A two-way ANOVA of Golgi stained MSNs within the dorsolateral striatum revealed a statistically significant difference between the groups (F(3,49) = 23.3, p < 0.001) as well as significant effects of exercise (F(3,49) = 45.6, p < 0.001) and lesion (F(3,49) = 15.78, p < 0.001), with an interaction between the variables (F(1,49) = 4.56, p = 0.038).
Post hoc analyses (Figure 3d) revealed the following differences between specific treatment groups. MPTP-lesioning led to a significant decrease (p = 0.001) of total dendritic spine densities of MSNs compared to saline controls (MPTP 5.47 ± 0.30, n = 9; Saline 7.45 ± 0.21, n = 10; mean spines / 10 µm ± SEM). Exercise led to a significant increase (p < 0.001) in spine densities observed between the MPTP groups (MPTP+Ex 8.36 ± 0.46, n = 10). Exercise also led to a significant increase (p = 0.004) in spine densities between the saline groups (Saline+Ex 8.96 ± 0.23, n = 24). There were no significant differences in total spine density between MPTP+exercise and either of the saline groups (Saline p = 0.264; Saline+Ex p = 0.475).
Exercise increases dendritic spine density in DA-D1R- and DA-D2R-containing MSNs in MPTP Mice
Analysis of DA-D1R-containing MSNs
In order to further delineate exercise effects on dendritic spine densities in MSNs of both the DA-D1R-direct and the DA-D2R-indirect pathways, individual MSNs were electrophoretically filled with biocytin. A two-way ANOVA of DA-D1R-containing MSNs revealed a statistically significant difference between the groups (F(3,10) = 8.41, p = 0.004) with an exercise effect (F(3,10) = 5.65, p = 0.039), a trend towards a lesion effect (F(3,10) = 3.93, p = 0.076), and an interaction between the variables (F(3,10) = 14.8, p = 0.003).
Post hoc analyses revealed (Figure 4e) the following differences between specific treatment groups. MPTP mice (5.08 ± 0.61, n = 3; mean spines / 10 µm ± SEM) had significant decreases (p = 0.021) in dendritic spine densities compared to saline-injected controls (8.39 ± 0.35, n = 2). MPTP+exercise mice (8.62 ± 0.58; n = 5) had significantly greater (p = 0.003) spine densities than MPTP mice. There was not a statistically significant difference in spine densities between MPTP+exercise mice and either of the saline groups (Saline p = 0.993; Saline+Ex 7.56 ± 0.29, n = 4, p = 0.434). Also, there was no significant difference (p = 0.774) between the saline groups.
Analysis of DA-D2R-containing MSNs
A two-way ANOVA of DA-D2R-containing MSNs revealed a statistically significant difference between the groups (F(3,16) = 4.49, p = 0.018) with a lesion effect (F(3,16) = 4.81, p = 0.043), and an interaction between the variables (F(3,16) = 5.78, p = 0.029), but not an exercise effect (F(3,16) = 2.85, p = 0.111).
Post hoc analyses (Figure 4f) revealed the following differences between specific treatment groups. MPTP mice (6.42 ± 0.86, n = 5; mean spines / 10 µm ± SEM) had significant decreases (p = 0.032) in dendritic spine densities compared to saline mice (9.25 ± 0.72, n = 4). MPTP+exercise mice (8.94 ± 0.35, n = 6) had significantly more spines than MPTP mice (p = 0.034). There was no significant difference in spine densities between MPTP+exercise mice and either of the saline groups (Saline p = 0.984; Saline+Ex 8.81 ± 0.50, n = 6, p = 0.999). There was no significant difference (p = 0.962) between saline mice groups.
Exercise increases PSD-95 expression in MPTP-lesioned mice
A two-way ANOVA of PSD-95 expression in the dorsolateral striatum revealed a statistically significant difference between the groups (F(3,8) = 14.2, p = 0.001) with an exercise effect (F(3,8) = 24.0, p = 0.001), a lesion effect (F(3,8) = 18.2, p = 0.003), but no interaction between the variables (F(3,8) = 0.246, p = 0.633).
Post hoc analyses (Figure 5c) revealed the following differences between specific treatment groups. PSD-95 expression in MPTP mice (75.9 ± 1.63, n = 3; relative % of saline ± SEM) was significantly lower (p = 0.010) than expression in saline controls (100 ± 6.53, n = 3). MPTP+exercise mice (103 ± 5.42, n = 3) had significantly greater (p = 0.005) expression than MPTP mice. Saline+exercise mice (122 ± 5.26, n = 3) had significantly greater expression than all other groups (Saline p = 0.014; MPTP p < 0.001; MPTP+Ex p = 0.028).
Exercise increases synaptophysin expression in MPTP-lesioned mice
A two-way ANOVA of synaptophysin expression in the dorsolateral striatum revealed a statistically significant difference between the groups (F(3,8) = 5.97, p = 0.019) with an exercise effect (F(3,8) = 16.7, p = 0.004), but no lesion effect (F(3,8) = 1.00, p = 0.346), nor interaction of the variables (F(3,8) = 0.235, p = 0.641).
Post hoc analyses (Figure 5d) of synaptophysin expression in MPTP mice (89.8 ± 8.88, n = 3; relative % of saline ± SEM) was not significantly different (p = 0.324) than saline-injected controls (100 ± 7.05, n = 3). However, exercise led to a significantly greater (p = 0.012) expression of synaptophysin in MPTP+exercise (121 ± 2.47, n = 3) versus MPTP mice. MPTP+exercise mice had greater expression than saline-injected controls that was trending towards significance (p = 0.061). Exercise also led to a significantly greater expression (p = 0.035) in the saline-injected controls (Saline+Ex 125 ± 7.14, n = 3).
Exercise increases dendritic arborization in MPTP-lesioned mice
Analysis of DA-D1R-containing MSNs
A two-way ANOVA of the distal sections (defined as 80 to 140 µm from the soma) of the areas under the Sholl curves yielded a statistically significant difference among the DA-D1R-containing MSN groups (F(3,19) = 4.68, p = 0.013). A statistically significant effect of exercise was observed (F(3,19) = 8.67, p = 0.008). The effect of lesion was trending towards significance (F(3,19), p = 0.093) and an interaction between the variables was trending towards significance as well (F(3,19), p = 0.087). The Sholl curve can be found in Figure 6a.
Figure 6. Sholl analysis of Dendritic Arborization.
Panel a shows quantitation of the mean number of intersections from the soma in biocytin filled DA-D1R-containing MSNs from all four groups. Panel b shows area under the curve quantitation of DA-D1R-containing MSNs for proximal (10 to 70 µm) and distal (80 to 140 µm) sections of the Sholl analysis. Note significant differences of the distal section between MPTP+exercise mice and all other groups. Panel d shows quantitation of the mean number of intersections from the soma in biocytin filled DA-D2R-containing MSNs from all four groups. Panel e shows area under the curve quantitation of DA-D2R-containing MSNs for proximal (10 to 70 µm) and distal (80 to 140 µm) sections of the Sholl analysis. Note a significant increase in the proximal section of MPTP+exercise mice compared to MPTP mice. Panel c shows the quantitative data for the longest dendrite of DA-D1R-containing MSNs as an indicator of dendritic pruning. There is no difference in longest dendrite length in DA-D1R-containing MSNs. Panel f shows the quantitative data for the longest dendrite in DA-D2R-containing MSNs. Note MPTP leads to pruning of the dendritic arbor in DA-D2R-containing MSNs. The asterisks represent statistical significance at either p < 0.05 “*” or p < 0.01“**”.
Post hoc analyses (Figure 6b) revealed that distal sections that MPTP+exercise mice (151 ± 11.8, n = 5; arbitrary units ± SEM) had significantly greater arborization than all other groups (Saline 100 ± 6.90, n = 4, p = 0.007; Saline+Ex 112 ± 6.46, n = 8, p = 0.014; MPTP 99.6 ± 8.82, n = 6, p = 0.003).
A two-way ANOVA of the proximal sections (defined as 10 to 70 µm from the soma) did not reveal a significant difference among the groups (F(3,19) = 0.162, p = 0.921).
Analysis of DA-D2R-containing MSNs
A two-way ANOVA of proximal sections (defined as 10 to 70 µm from the soma) of the area under the Sholl curves of DA-D2R-containing MSNs revealed statistically significant differences between the groups (F(3,17) = 3.10, p = 0.05). There was a statistically significant interaction between the variables (F(3,17) = 9.14, p = 0.008). We observed no significant effects of exercise and MPTP alone (Exercise F(3,17) = 0.21, p = 0.652; Lesion F(3,17) = 0.001, p = 0.979). The Sholl curve can be found in Figure 6d.
Post hoc analyses (Figure 6e) of proximal sections revealed that saline mice (100 ± 9.91, n = 5; arbitrary units ± SEM) had greater arborization than MPTP mice (75.0 ± 13.3, n = 4) that was trending towards significance (p = 0.059). MPTP+exercise mice (104 ± 8.09, n = 5) had significantly greater (p = 0.034) arborization that MPTP mice. Saline mice also had greater arborization than exercised controls (Saline+Ex 73.7 ± 4.94, n = 7) that was trending towards significance (p = 0.068).
A two-way ANOVA of the distal sections (defined as 80 to 140 µm from the soma) did not yield a significant result (F(3,17) = 0.702, p = 0.564).
Exercise does not offset distal pruning of DA-D2R MSN dendrites in MPTP-lesioned mice
A two-way ANOVA of DA-D2R-containing MSNs of MPTP mice did not show a significant difference between the groups (F(3,18) = 2.06, p = 0.141). However, the ANOVA revealed a statistically significant effect of lesion (F(3,18) = 5.97, p = 0.025).
Post hoc analyses (Figure 6f) showed that MPTP mice (136 ± 6.0, n = 7; µm ± SEM) had significantly shorter (p = 0.044) dendrites than saline-injected controls (Saline 157 ± 5.4, n = 4). There were no other significant differences between the groups. Also, there were no significant differences in longest dendrite lengths among the DA-D1R-containing MSN groups (Figure 6c).
Discussion
Intensive treadmill exercise results in an increase in overall dendritic spine density within the striatum of the MPTP mouse model. DA-depletion led to a decrease in spine density of both DA-D1R- and DA-D2R-containing MSNs of the direct and indirect pathways, respectively, while exercised mice did not show this loss in either pathway. Treadmill running has been shown to increase dendritic spine density within the striatum in a hemorrhagic stroke model in the rodent (Takamatsu et al., 2010); however, to the best of our knowledge this is the first report of an exercise induced increase in spine density in an animal model of PD. Increased striatal dendritic spine density has also been reported in the context of environmental enrichment in non-injured, intact animals. Specifically, Comery et al. (1995) reported that rodents exposed to 30 days of a complex environment demonstrated a 30% increase in spine density of MSNs. In this report we also observed an increase in dendritic spine density in striatal MSNs in saline+exercise mice supporting the fact that exercise has a positive effect on basal ganglia connectivity in the healthy and intact brain. In addition, exercise and/or environmental enrichment have been shown in healthy animals to increase spine density in extra-striatal regions including the CA3 hippocampal neurons, layer III pyramidal of the cortex, and cerebellar Purkinje cells (Comery et al., 1995; Eadie et al., 2005; Leggio et al., 2005; Stranahan et al., 2007).
We found that the effects of exercise on spine density were associated with increased expression of two synaptic proteins, PSD-95 and synaptophysin, in MPTP mice. PSD-95, a structural anchoring protein, is localized to the post-synaptic terminal, while synaptophysin, a vesicle associated protein, is localized to the pre-synaptic terminal. Several studies have reported that synaptogenesis occurs in the context of exercise and motor skill learning (Black et al., 1990; Takamatsu et al., 2010; Pagnussat et al., 2012). In our study the exercise induced increase in PSD-95 and synaptophysin expression along with the increase in dendritic spine density is consistent with increased synaptogenesis in the dorsolateral striatum. This may then underlie improved motor performance with exercise that we have previously reported in the MPTP-lesioned mouse model (Fisher et al., 2004; Petzinger et al., 2007; VanLeeuwen et al., 2010; Kintz et al., 2013).
Sholl analysis, a measure of neuronal dendritic arborization, revealed that exercise led to an increase in arborization of both pathways in MPTP-lesioned mice. These effects were specific to either proximal (DA-D2R) or distal (DA-D1R) segments of the dendritic field. In addition, there was no effect of exercise on arborization in saline animals. No effect of exercise was observed on the longest dendritic length in any group. Consistent with our findings, exercise increased arborization of striatal neurons in a model of intracerebral hemorrhagic stroke with no effects on arborization in sham animals (Takamatsu et al., 2010). Interestingly, exercise effects on spine density and arborization within the hippocampus are similar to those observed within the striatum. Specifically, while exercise led to a general and widespread increase in spine density within many areas of the hippocampus, the exercise effects on gross morphological changes to dendritic arborization were limited to a small number of regions (Stranahan et al., 2007).
Dendritic spine loss after DA-depletion has been reported to occur either exclusively in DA-D2R-containing MSNs in rodents or in both DA-D1R- and DA-D2R-containing MSNs in monkeys. For example, Day et al. (2006) reported a selective loss of spine density in DA-D2R-containing MSNs of the indirect pathway in both reserpine and 6-hydroxydopamine (6-OHDA) rodent models of DA-depletion. Our findings differ from Day et al. (2006) in that we observed spine density changes in both DA-D1R and DA-D2R-containing MSNs utilizing the MPTP-lesioned mouse. However, our findings are in agreement with Villalba et al. (2010) where loss of spine density in both DA-D1R- and DA-D2R-containing MSNs were observed several months after MPTP-lesioning in non-human primates. Differences that may account for variability between studies could be due to a number of parameters including the neurotoxin lesioning paradigm (type of toxin, dosage, and route of administration), the time between lesion and analysis of tissue, and the animal model with respect to age, sex, or species.
In this study, we observed that DA-depletion led to a decrease in dendritic spine density 42 days after MPTP administration. Published studies using a number of DA-depleting agents suggest that spine loss may take several days to weeks to occur after lesioning: 6-OHDA (12 days) (Ingham et al., 1989), reserpine (5 days) (Day et al., 2006), MPP+ (2 weeks) (Neely et al., 2007). In light of these reports and our current findings we hypothesize that the most parsimonious mechanism by which exercise may alter dendritic spine density is by reversing spine loss. Since DA-depletion leads to the loss of dendritic spines and our exercise protocol was started after MPTP-induced cell death and spine loss had occurred, exercise may then reverse this effect by promoting new spine formation. This interpretation is based on our observation that exercise promotes dendritic spine formation in both saline+exercise and MPTP+exercise mice. In future studies we propose to investigate mechanisms involved in dendritic spine formation including a precise analysis of spine density in a time course fashion as well as investigate underlying dynamic changes (nascent versus mature) that may occur in models of DA-depletion and exercise.
There are a number of potential mechanisms by which exercise may modulate an increase in spine density within the striatum. One mechanism could be through neurotrophic factors (Cotman and Berchtold, 2002). Exercise has been shown to elevate the expression of a number of neurotrophic factors, including brain-derived neurotrophic factor, and these factors are known to regulate spine formation. Alternatively, exercise effects on spine density may be through glutamate modulation (Calabresi et al., 1997). Exercise has been reported to alter glutamate neurotransmission, including AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor subtypes in DA-depleted rodent models. Exercise induced alterations in glutamatergic neurotransmission may serve to mitigate excitotoxicity due to DA-depletion. Other receptors that are known to be modulate spine loss in the context of DA-depletion, such as the L-type calcium channel may play a role in exercise mediated effects on spine density (Day et al., 2006). Finally, exercise may lead to changes in a number of factors affecting general brain health including the activation of astrocytes or microglia, alterations in the permeability of the blood brain barrier, increased vasculature (angiogenesis), and blood flow, all of which can influence neurotrophic factor expression or delivery and provide an environment promoting spine growth and spine maintenance (Li et al., 2005; Yang et al., 2007; Al-Jarrah et al., 2010; Kawanishi et al., 2010; Bernardi et al., 2013).
A question not examined in this study, though highly relevant, is how does exercise modulate MSN dendritic spine morphology with dopamine-depletion? To our knowledge, no studies have investigated the dynamics of spine morphology on striatal MSNs in the context of exercise. However, other groups have reported the effects of exercise on spine morphology in other regions of the brain. Gonzalez-Burgos et al. (2011) reported that in non-injured adult rat cerebellar Purkinje cells, 4 weeks of treadmill running led to increased quantities of stubby, mushroom, and wide dendritic spine subtypes compared to non-exercised controls. In the hippocampus, Stranahan et al. (2007) reported that average dendritic spine surface area of CA1 pyramidal neurons decreased in rats that had access to a running wheel compared to sedentary controls, suggesting that exercise led to an increase in filopodia-like spines. As a future study we plan to determine more precisely potential morphological changes that may take place on dendritic MSNs and to determine if any changes underlie the behavioral benefits seen with intensive exercise in the dopamine-depleted striatum.
A limitation of our study was that we did not observe an increase in the dendritic spine density in saline+exercise compared to saline sedentary groups using biocytin labeling. This is in contrast to analysis of Golgi stained MSNs where we observed an exercise induced increase in spine density in intact saline animals. While the discrepancy between these two labeling methods is not clear one potential reason may be due to differences in the mechanisms by which Golgi impregnation and biocytin labeling differ. For example, Golgi impregnation requires tissues to be prefixed in formaldehyde (or a related fixative) prior to deposition of the silver chromate precipitate. This is in contrast to biocytin filling of striatal MSNs after electrophysiological recording or patch clamping where biocytin (a 372 molecular weight amide of biotin conjugated to L-lysine) diffuses into a live cell from the recording pipette. A number of studies have reported differences in neuron morphology when comparing biocytin labeling and Golgi staining. For example, Coleman et al. (1992) using biocytin infusion showed extensive dendritic morphology of the immature cat lateral geniculate nucleus which was in contrast to reports by Mason (1983) who indicated a lack of dendrites using Golgi impregnation in this same region. Similar discrepancies have been reported in a number of brain regions using these two stains to reveal dendritic morphology (Conley and Wilson, 1992; Bannister and Larkman, 1995; Li et al., 2012).
Conclusion
In conclusion, this study demonstrates an exercise induced increase on dendritic spine density and arborization in an animal model of PD. Increased expression of proteins involved in synaptic connectivity observed in the MPTP mouse model may contribute to exercise related benefits in motor skill learning and performance in the DA-depleted state. Future studies will examine if changes in spine morphology are associated with alterations in the electrophysiological properties of striatal MSNs and the exercise-dependent mechanisms involved in spine maintenance and/or formation. In addition, findings from these studies may be translated to clinical exercise studies in individuals with PD that investigate exercise related alterations in motor circuit connectivity within the dorsal basal ganglia using fMRI. Finally, exercise induced alterations in spine density help support the potential role of exercise in modifying disease progression in individuals with PD through neuroplastic changes including synaptogenesis and motor circuitry.
Highlights.
MPTP leads to reduced spine density in MSNs in both pathways of the basal ganglia
Treadmill exercise increases spine density in MSNs of both pathways in MPTP mice
Exercise increases dendritic arborization of striatal MSNs in MPTP mice
MPTP induced DA depletion leads to selective pruning of DA-D2R-containing MSNs
Exercise increases expression of proteins PSD-95 and synaptophysin in MPTP mice
Acknowledgments
Grant Information: The authors would like to acknowledge the support of the NINDS RO1 NS44327, U.S. Army NETRP (Grant # W81XWH-04-1-0444), Zumberge Foundation of USC, and Southern California Clinical and Translational Science Institute and University of Southern California, Keck School of Medicine RR031986. A special thanks to Friends of the USC Parkinson’s Disease Research Group including George and Mary Lou Boone, Walter and Susan Doniger, Edna and John Ball, Team Parkinson, Team 4BA, and the family of Don Gonzalez Barrera.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interest.
References
- Al-Jarrah M, Jamous M, Al Zailaey K, Bweir SO. Endurance exercise training promotes angiogenesis in the brain of chronic/progressive mouse model of Parkinson's Disease. NeuroRehabilitation. 2010;26:369–373. doi: 10.3233/NRE-2010-0574. [DOI] [PubMed] [Google Scholar]
- Bannister NJ, Larkman AU. Dendritic morphology of CA1 pyramidal neurones from the rat hippocampus: I. Branching patterns. The Journal of Comparative Neurology. 1995;360:150–160. doi: 10.1002/cne.903600111. [DOI] [PubMed] [Google Scholar]
- Bernardi C, Tramontina AC, Nardin P, Biasibetti R, Costa AP, Vizueti AF, Batassini C, Tortorelli LS, Wartchow KM, Dutra MF, Bobermin L, Sesterheim P, Quincozes-Santos A, de Souza J, Goncalves CA. Treadmill exercise induces hippocampal astroglial alterations in rats. Neural Plasticity. 2013;2013:709–732. doi: 10.1155/2013/709732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Sciences. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neuroscience & Biobehavioral Reviews. 1997;21:519–523. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Walsh JP, Hull CD, Howard SG, Buchwald NA, Levine MS. Dye-Coupling in the neostriatum of the rat: I. Modulation by dopamine-depleting lesions. Synapse. 1989;4:229–237. doi: 10.1002/syn.890040308. [DOI] [PubMed] [Google Scholar]
- Chan CS, Peterson JD, Gertler TS, Glajch KE, Quintana RE, Cui Q, Sebel LE, Plotkin JL, Shen W, Heiman M, Heintz N, Greengard P, Surmeier DJ. Strain-Specific Regulation of Striatal Phenotype in Drd2-eGFP BAC Transgenic Mice. The Journal of Neuroscience. 2012;32:9124–9132. doi: 10.1523/JNEUROSCI.0229-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Coleman L-A, Friedlander MJ. Intracellular injections of permanent tracers in the fixed slice: a comparison of HRP and biocytin. Journal of Neuroscience Methods. 1992;44:167–177. doi: 10.1016/0165-0270(92)90009-3. [DOI] [PubMed] [Google Scholar]
- Comery TA, Shah R, Greenough WT. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiology of Learning and Memory. 1995;63:217–219. doi: 10.1006/nlme.1995.1025. [DOI] [PubMed] [Google Scholar]
- Conley M, Wilson KF. Dendritic organization of class II (inter)neurons in the dorsal lateral geniculate nucleus of the tree shrew: Observations based on Golgi, immunocytochemical, and biocytin methods. The Journal of Comparative Neurology. 1992;319:51–65. doi: 10.1002/cne.903190107. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature Neuroscience. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of Comparative Neurology. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. Journal of Neuroscience Research. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Research. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Gonzalez-Tapia D, Zamora DA, Feria-Velasco A, Beas-Zarate C. Guided motor training induces dendritic spine plastic changes in adult rat cerebellar purkinje cells. Neuroscience Letters. 2011;491:216–220. doi: 10.1016/j.neulet.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Research. 1989;503:334–338. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Maldegem B, Weenink A, Arbuthnott GW. Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Experimental Brain Research. 1993;93:17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. Journal of Neuroscience Research. 2004;76:539–550. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exercise Immunology Review. 2010;16:105–118. [PubMed] [Google Scholar]
- Kintz N, Petzinger GM, Akopian G, Ptasnik S, Williams C, Jakowec MW, Walsh JP. Exercise modulates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic-acid receptor (AMPAR) expression selectively on striatopallidal indirect projection pathway neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-(MPTP)-lesioned mouse model of basal ganglia Injury. Journal of Neuroscience Research. 2013 doi: 10.1002/jnr.23260. In Press. [DOI] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. The Journal of Neuroscience. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behavioural Brain Research. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Li J, Ding YH, Rafols JA, Lai Q, McAllister JP, 2nd, Ding Y. Increased astrocyte proliferation in rats after running exercise. Neuroscience Letters. 2005;386:160–164. doi: 10.1016/j.neulet.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Li J, Liu N, Lu K, Zhang L, Gu J, Guo F, An S, Zhang L, Zhang L. Cocaine-induced dendritic remodeling occurs in both D1 and D2 dopamine receptor-expressing neurons in the nucleus accumbens. Neuroscience Letters. 2012;517:118–122. doi: 10.1016/j.neulet.2012.04.040. [DOI] [PubMed] [Google Scholar]
- Mason CA. Postnatal maturation of neurons in the cat's lateral geniculate nucleus. The Journal of Comparative Neurology. 1983;217:458–469. doi: 10.1002/cne.902170410. [DOI] [PubMed] [Google Scholar]
- McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson's disease. Brain Research. 1988;455:148–152. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience. 2007;149:457–464. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hang GB, Grueter BA, Pascoli V, Luscher C, Malenka RC, Kreitzer AC. A Comparison of Striatal-Dependent Behaviors in Wild-Type and Hemizygous Drd1a and Drd2 BAC Transgenic Mice. The Journal of Neuroscience. 2012;32:9119–9123. doi: 10.1523/JNEUROSCI.0224-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat AS, Simao F, Anastacio JR, Mestriner RG, Michaelsen SM, Castro CC, Salbego C, Netto CA. Effects of skilled and unskilled training on functional recovery and brain plasticity after focal ischemia in adult rats. Brain Research. 2012;1486:53–61. doi: 10.1016/j.brainres.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. The Lancet Neurology. 2013;12:716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, Holschneider DP, Nacca A, Walsh JP, Jakowec MW. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Movement Disorders. 2010;25(Suppl 1):S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. The Journal of Neuroscience. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Centonze D, Bernardi G, Calabresi P. Striatal synaptic plasticity: implications for motor learning and Parkinson's disease. Movement Disorders. 2005;20:395–402. doi: 10.1002/mds.20394. [DOI] [PubMed] [Google Scholar]
- Rafols JA, Cheng HW, McNeill TH. Golgi study of the mouse striatum: Age-related dendritic changes in different neuronal populations. The Journal of Comparative Neurology. 1989;279:212–227. doi: 10.1002/cne.902790205. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Erwin Frise VK, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfel S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. Journal of Anatomy. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nature Reviews Neurology. 2011;7:528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, Bell JE, Kilford L, Kingsbury AE, Daniel SE, Ingham CA. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience. 2005;132:741–754. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu Y, Ishida A, Hamakawa M, Tamakoshi K, Jung CG, Ishida K. Treadmill running improves motor function and alters dendritic morphology in the striatum after collagenase-induced intracerebral hemorrhage in rats. Brain Research. 2010;1355:165–173. doi: 10.1016/j.brainres.2010.07.070. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. The Journal of Neuroscience. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLeeuwen J-E, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Journal of Neuroscience Research. 2010;88:650–668. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Striatal spine plasticity in Parkinson's Disease. Frontiers in Neuroanatomy. 2010;4 doi: 10.3389/fnana.2010.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Sachdev RN. Intracellular and juxtacellular staining with biocytin. Current Protocols in Neuroscience. 2004;Chapter 1(Unit 1):12. doi: 10.1002/0471142301.ns0112s26. [DOI] [PubMed] [Google Scholar]
- Yang J, Sadler TR, Givrad TK, Maarek JMI, Holschneider DP. Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats. Neuroimage. 2007;36:755–773. doi: 10.1016/j.neuroimage.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]