Abstract
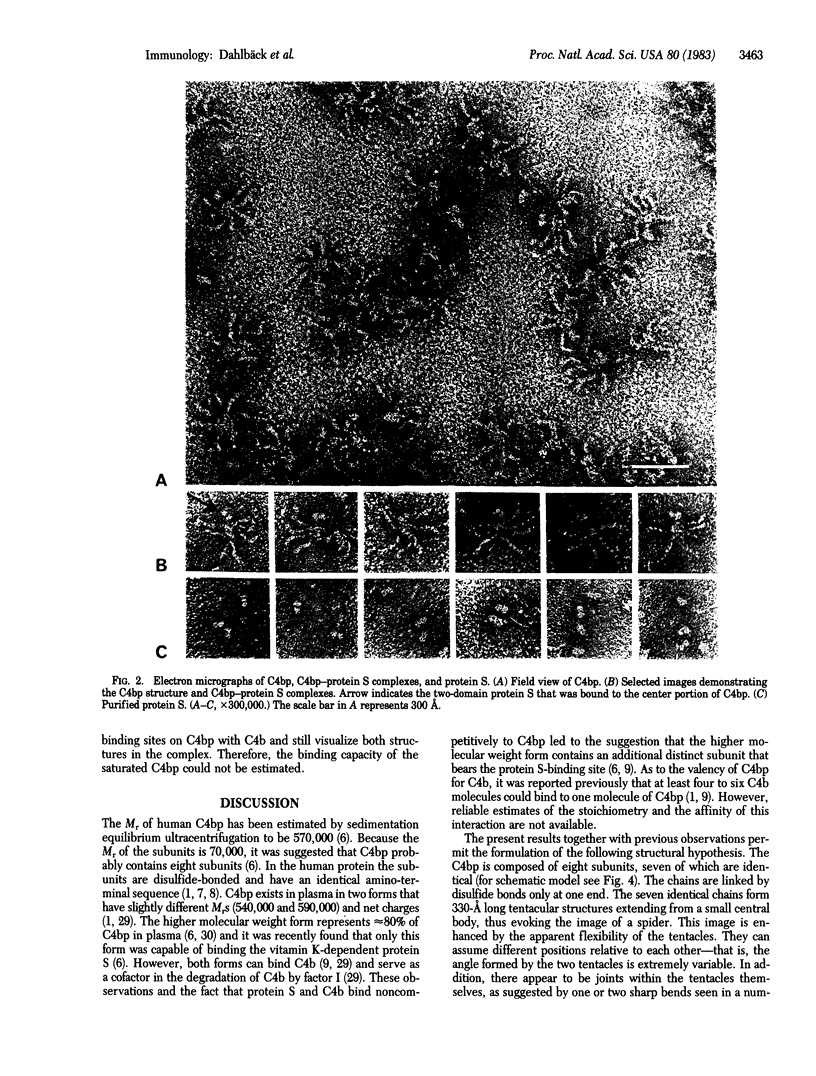
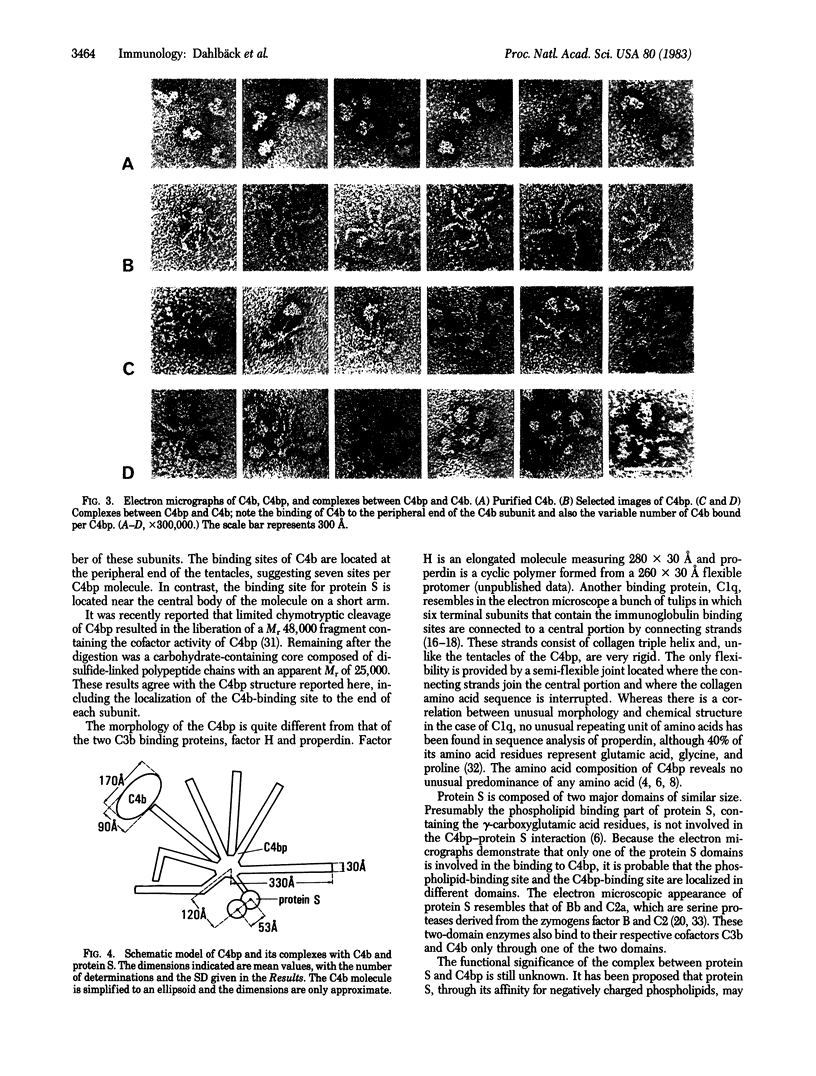
C4b-binding protein (C4bp) participates in the regulation of the C3 convertase of the classical pathway of complement. By binding to C4b, which is one of the structural subunits of this enzyme, C4bp accelerates the decay-dissociation of the enzyme and renders C4b susceptible to degradation by factor I (C3b inactivator). C4bp is a high molecular weight plasma protein (Mr = 570,000) composed of apparently identical subunits (Mr = 70,000) linked by disulfide bonds. In plasma and in purified form C4bp also forms a bimolecular complex (Kd = 0.9 X 10(-7) M) with protein S, a recently identified vitamin K-dependent plasma protein. The binding sites on C4bp for protein S and C4b are distinct and noncompetitive and protein S does not influence the function of C4bp as a regulator of the C3 convertase. C4bp, C4b, and protein S were visualized by electron microscopy by negative staining. C4bp was found to have an unusual spider-like structure. It is composed of seven thin (30 A), elongated (330 A), and flexible subunits that are linked to a small central body. Protein S exhibited two globular domains of equal size with a center-to-center distance of approximately equal to 50 A. Protein S was found to bind to the C4bp through only one of its domains by attaching to a short subunit that is distinct from the other seven subunits. C4b imaged as an irregular, relatively compact molecule. It was found to interact with the peripheral ends of the elongated subunits, suggesting seven C4b-binding sites per molecule of C4bp.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Hildebrand B. Degradation of human complement component C4b in the presence of the C4b-binding protein-protein S complex. Biochem J. 1983 Mar 1;209(3):857–863. doi: 10.1042/bj2090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B. Purification of human C4b-binding protein and formation of its complex with vitamin K-dependent protein S. Biochem J. 1983 Mar 1;209(3):847–856. doi: 10.1042/bj2090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B. Purification of human vitamin K-dependent protein S and its limited proteolysis by thrombin. Biochem J. 1983 Mar 1;209(3):837–846. doi: 10.1042/bj2090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Stenflo J. High molecular weight complex in human plasma between vitamin K-dependent protein S and complement component C4b-binding protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2512–2516. doi: 10.1073/pnas.78.4.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- DiScipio R. G., Davie E. W. Characterization of protein S, a gamma-carboxyglutamic acid containing protein from bovine and human plasma. Biochemistry. 1979 Mar 6;18(5):899–904. doi: 10.1021/bi00572a026. [DOI] [PubMed] [Google Scholar]
- Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978 Oct 1;148(4):1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Nussenzweig V. The role of C4-binding protein and beta 1H in proteolysis of C4b and C3b. J Exp Med. 1979 Aug 1;150(2):267–276. doi: 10.1084/jem.150.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Fujita T., Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6596–6600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidoh T., Natsuume-Sakai S., Takahashi M. Murine binding protein of the fourth component of complement: structural polymorphism and its linkage to the major histocompatibility complex. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3794–3798. doi: 10.1073/pnas.78.6.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundwall A., Malmheden I., Stålenheim G., Sjöquist J. Isolation of component C4 of human complement and its polypeptide chains. Eur J Biochem. 1981 Jun;117(1):141–146. doi: 10.1111/j.1432-1033.1981.tb06312.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nagasawa S., Mizuguchi K., Ichihara C., Koyama J. Limited chymotryptic cleavage of human C4-binding protein: isolation of a carbohydrate-containing core domain and an active fragment. J Biochem. 1982 Oct;92(4):1329–1332. doi: 10.1093/oxfordjournals.jbchem.a134052. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J Biol Chem. 1982 Dec 25;257(24):15204–15212. [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Gagnon J. Human C4-binding protein: N-terminal amino acid sequence analysis and limited proteolysis by trypsin. FEBS Lett. 1982 Jan 11;137(1):75–79. doi: 10.1016/0014-5793(82)80318-9. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Johnson D. M., Gagnon J., Phohaska R. Preparation of human factor D of the alternative pathway of complement. Methods Enzymol. 1981;80(Pt 100):134–143. doi: 10.1016/s0076-6879(81)80013-4. [DOI] [PubMed] [Google Scholar]
- Scharfstein J., Ferreira A., Gigli I., Nussenzweig V. Human C4-binding protein. I. Isolation and characterization. J Exp Med. 1978 Jul 1;148(1):207–222. doi: 10.1084/jem.148.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker V. N., Poon P. H., Seegan G. W., Smith C. A. Semi-flexible joint in the C1q subunit of the first component of human complement. J Mol Biol. 1981 May 15;148(2):191–197. doi: 10.1016/0022-2836(81)90511-8. [DOI] [PubMed] [Google Scholar]
- Seegan G. W., Smith C. A., Schumaker V. N. Changes in quaternary structure of IgG upon reduction of the interheavy-chain disulfide bond. Proc Natl Acad Sci U S A. 1979 Feb;76(2):907–911. doi: 10.1073/pnas.76.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Vogel C. W., Müller-Eberhard H. J. Ultrastructure of cobra venom factor-dependent C3/C5 convertase and its zymogen, factor B of human complement. J Biol Chem. 1982 Sep 10;257(17):9879–9882. [PubMed] [Google Scholar]
- Stenflo J., Jönsson M. Protein S, a new vitamin K-dependent protein from bovine plasma. FEBS Lett. 1979 May 15;101(2):377–381. doi: 10.1016/0014-5793(79)81048-0. [DOI] [PubMed] [Google Scholar]
- Strang C. J., Siegel R. C., Phillips M. L., Poon P. H., Schumaker V. N. Ultrastructure of the first component of human complement: electron microscopy of the crosslinked complex. Proc Natl Acad Sci U S A. 1982 Jan;79(2):586–590. doi: 10.1073/pnas.79.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Podack E. R., Müller-Eberhard H. J. Ultrastructure of the membrane attack complex of complement: detection of the tetramolecular C9-polymerizing complex C5b-8. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7474–7478. doi: 10.1073/pnas.79.23.7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Villiers M. B., Thielens N. M., Reboul A., Colomb M. G. A study of a covalent-like interaction between soluble nascent C4b and C4-binding protein. Biochim Biophys Acta. 1982 Jun 4;704(2):197–203. doi: 10.1016/0167-4838(82)90146-7. [DOI] [PubMed] [Google Scholar]
- Walker F. J. Regulation of activated protein C by a new protein. A possible function for bovine protein S. J Biol Chem. 1980 Jun 25;255(12):5521–5524. [PubMed] [Google Scholar]
- Walker F. J. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J Biol Chem. 1981 Nov 10;256(21):11128–11131. [PubMed] [Google Scholar]