Abstract
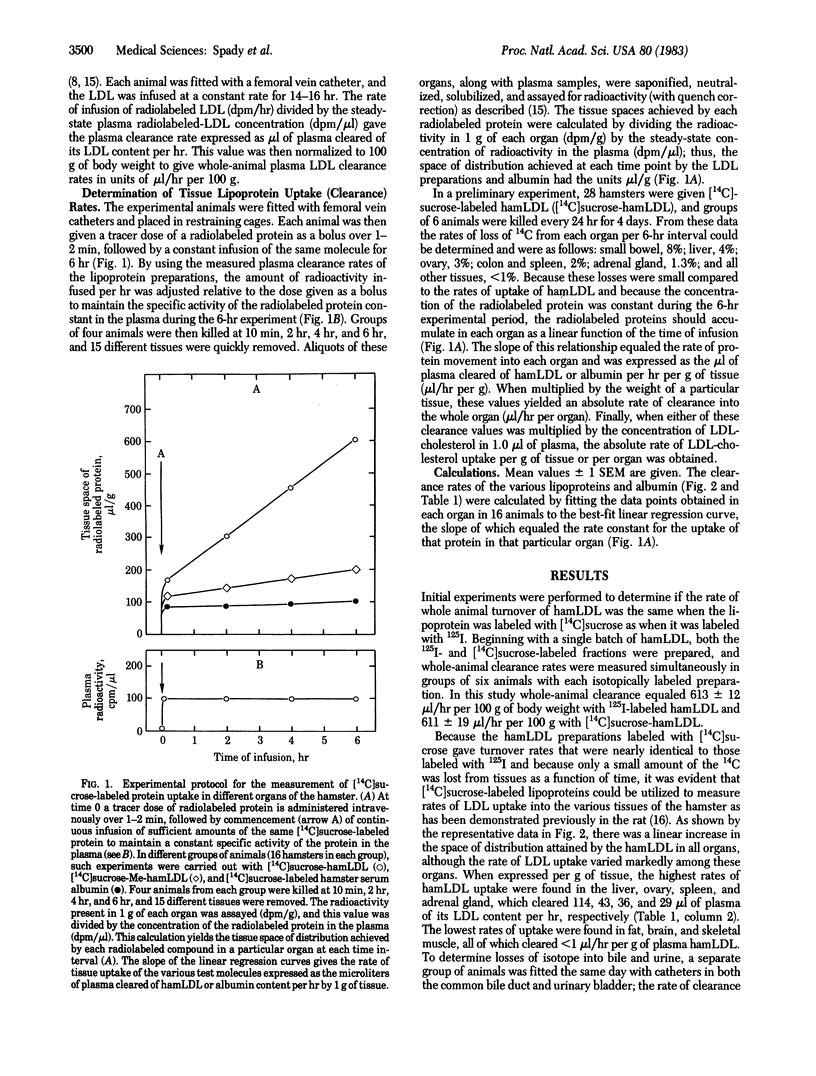
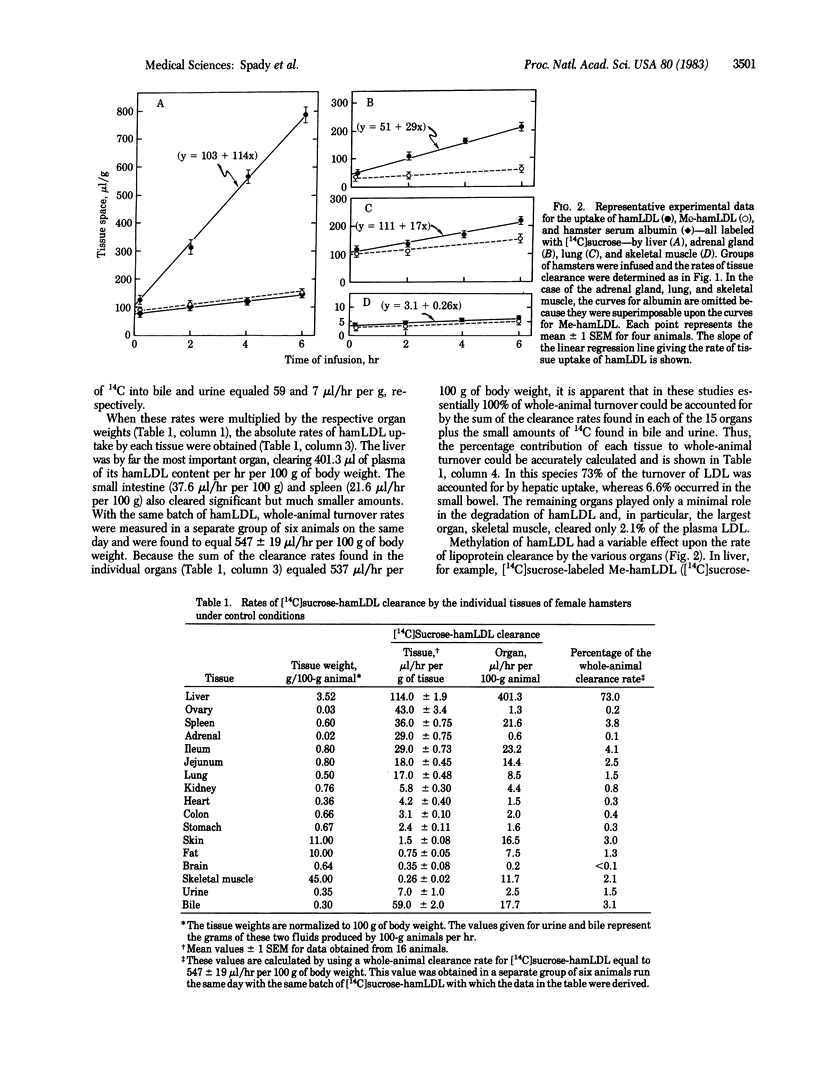
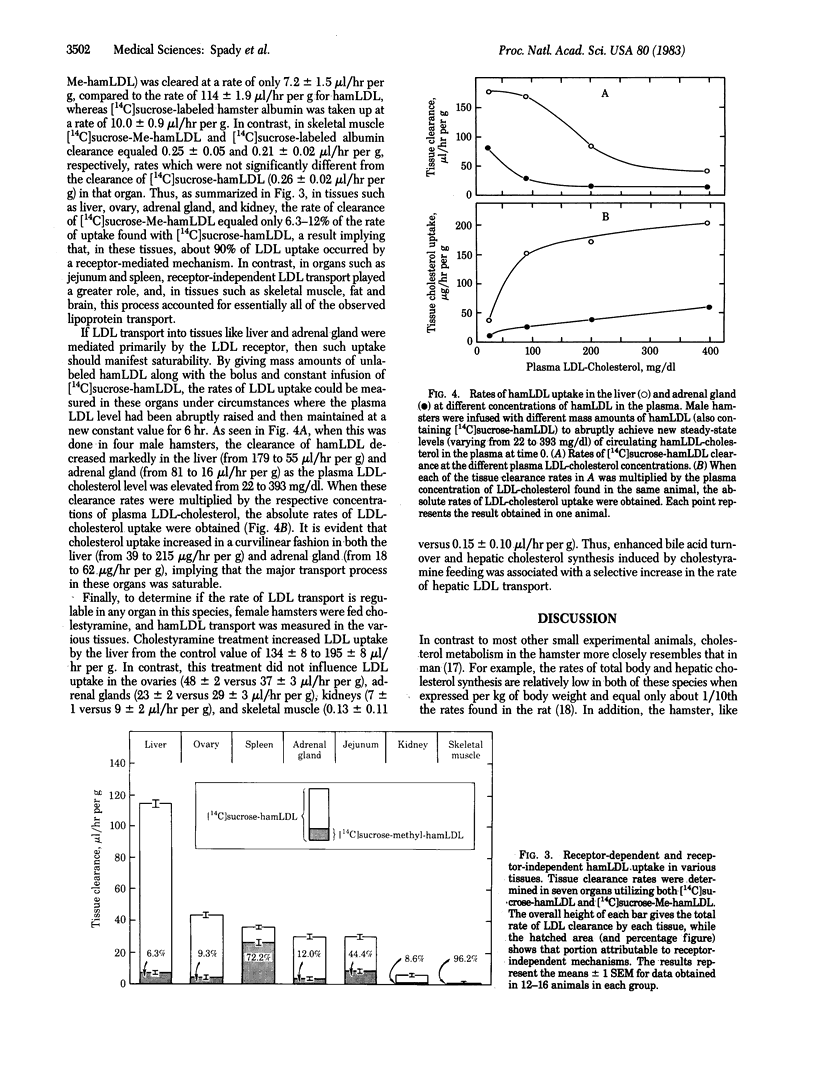
By using a constant infusion technique in the hamster, rates of uptake of [14C]sucrose-labeled hamster low density lipoprotein (hamLDL) and methylated hamster LDL (MehamLDL) were directly measured in 15 tissues. From these measurements the magnitude of LDL receptor-dependent and receptor-independent lipoprotein transport was calculated. The whole-animal clearance of hamLDL equaled 547 microliters/hr per 100 g of body weight. LDL clearance per g of tissue was highest in the liver (114 microliters/hr per g), ovary (43), spleen (36), adrenal gland (29), and intestine (24) and was lowest in fat (0.75), brain (0.35), and muscle (0.26). When adjusted for organ weight, the sum of the absolute clearance rates in all of the tissues examined equaled the rate of whole-animal LDL turnover. Liver accounted for 73%, and the jejunum and ileum combined accounted for 7% of whole-animal clearance. The 12 other tissues each accounted for only a minor portion of LDL clearance. Rates of uptake of Me-hamLDL were much less in many tissues and accounted for only 6-12% of the uptake of LDL in the liver, ovary, adrenal gland, lung, and kidney. However, this receptor-independent uptake was quantitatively more important in the intestine (44%) and spleen (72%) and accounted for essentially all LDL uptake in organs such as muscle, skin, and brain. Thus, in the hamster, most LDL is taken up and degraded by the liver. This uptake process is greater than 90% mediated by the LDL receptor and manifests saturation kinetics. Finally, cholestyramine feeding increases receptor-mediated LDL transport in the liver but in no other tissue studied.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Watanabe Y., Kita T. Impaired receptor-mediated catabolism of low density lipoprotein in the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1982 May;79(10):3305–3309. doi: 10.1073/pnas.79.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The LDL pathway in human fibroblasts: a receptor-mediated mechanism for the regulation of cholesterol metabolism. Curr Top Cell Regul. 1976;11:147–181. doi: 10.1016/b978-0-12-152811-9.50011-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Disparities in the interaction of rat and human lipoproteins with cultured rat fibroblasts and smooth muscle cells. Requirements for homology for receptor binding activity. J Biol Chem. 1980 Dec 10;255(23):11163–11172. [PubMed] [Google Scholar]
- Koelz H. R., Sherrill B. C., Turley S. D., Dietschy J. M. Correlation of low and high density lipoprotein binding in vivo with rates of lipoprotein degradation in the rat. A comparison of lipoproteins of rat and human origin. J Biol Chem. 1982 Jul 25;257(14):8061–8072. [PubMed] [Google Scholar]
- Kovanen P. T., Basu S. K., Goldstein J. L., Brown M. S. Low density lipoprotein receptors in bovine adrenal cortex. II. Low density lipoprotein binding to membranes prepared from fresh tissue. Endocrinology. 1979 Mar;104(3):610–616. doi: 10.1210/endo-104-3-610. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Melchior G. W., Innerarity T. L., Holcombe K. S. Inhibition of receptor-mediated clearance of lysine and arginine-modified lipoproteins from the plasma of rats and monkeys. Proc Natl Acad Sci U S A. 1980 Jan;77(1):225–229. doi: 10.1073/pnas.77.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Andersen J. M., Dietschy J. M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981 Dec;68(6):1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn S. H., Newton R. S., Chang C. M., Weinstein D. B., Steinberg D. Receptor-mediated catabolism of homologous low density lipoproteins in cultured pig hepatocytes. J Biol Chem. 1981 Apr 10;256(7):3340–3347. [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of catabolism of rat and human low density lipoproteins in rats. Biochim Biophys Acta. 1982 Jan 15;710(1):7–14. doi: 10.1016/0005-2760(82)90183-7. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of degradation of low density lipoprotein: application of a method for determining the fate of plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5345–5349. doi: 10.1073/pnas.76.10.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J., Bicker S., Lorimer A. R., Packard C. J. Receptor-mediated low density lipoprotein catabolism in man. J Lipid Res. 1979 Nov;20(8):999–1006. [PubMed] [Google Scholar]
- Sigurdsson G., Noel S. P., Havel R. J. Catabolism of the apoprotein of low density lipoproteins by the isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):628–634. [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983 Mar;24(3):303–315. [PubMed] [Google Scholar]
- Turley S. D., Andersen J. M., Dietschy J. M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981 May;22(4):551–569. [PubMed] [Google Scholar]
- Turley S. D., Spady D. K., Dietschy J. M. Alteration of the degree of biliary cholesterol saturation in the hamster and rat by manipulation of the pools of preformed and newly synthesized cholesterol. Gastroenterology. 1983 Feb;84(2):253–264. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]



