Abstract
Background
Displaced femoral neck fractures usually are treated with hemiarthroplasty. However, the degree to which the design of the implant used (cemented or uncemented) affects the outcome is not known and may be therapeutically important.
Questions/purposes
In this randomized controlled trial, we sought to compare cemented with cementless fixation in bipolar hemiarthroplasties at 5 years in terms of (1) Harris hip scores; (2) femoral fractures; (3) overall health outcomes using the Barthel Index and EQ-5D scores; and (4) complications, reoperations, and mortality since our earlier report on this cohort at 1-year followup.
Methods
We present followup at a median of 5 years after surgery (range, 56–65 months) from a randomized trial comparing a cemented hemiarthroplasty (112 hips) with an uncemented, hydroxyapatite-coated hemiarthroplasty (108 hips), both with a bipolar head. Results were previously reported at 1-year followup. Harris hip scores, Barthel Index, and EQ-5D scores were assessed by one research nurse and one orthopaedic surgeon. Complications and reoperations were determined by chart review and radiographs examined by three orthopaedic surgeons. Sixty patients (56%) had died in the cemented group and 63 (60%) in the uncemented group. Respectively, three and two patients (2.7% and 1.9%) were completely lost to followup.
Results
Harris hip scores at 5 years were higher in the uncemented group than in the cemented group (86.2 versus 76.3; mean difference 9.9; 95% confidence interval [CI], 1.9–17.9). The prevalence of postoperative periprosthetic femoral fractures was 7.4% in the uncemented group and 0.9% in the cemented group (hazard ratio [HR], 9.3; 95% CI, 1.16–74.5). Barthel Index and EQ-5D scores were not different between the groups. Between 1 and 5 years, we found no additional infections or dislocations. The mortality rate was not different between the groups (HR, 1.2; 95% CI, 0.82–1.7).
Conclusions
Both arthroplasties may be used with good medium-term results after displaced femoral neck fractures. The uncemented hemiarthroplasty may result in higher hip scores but appears to carry an unacceptably high risk of later femoral fractures.
Level of Evidence
Level I, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Many types of arthroplasties are used to treat displaced fractures of the femoral neck in the elderly [1]. There is some evidence of inferior short-term results with decreased mobility and pain when using an uncemented implant, but the diversity of implants used in clinical trials represents a problem [19].
The purpose of this two-center randomized equivalence trial was to compare a hemiarthroplasty using a well-documented cemented femoral stem [7, 12] with a hemiarthroplasty using a well-documented hydroxyapatite-coated proximal press-fit uncemented implant [9, 23, 26]. When we evaluated results of this trial at 1 year, we found comparable outcomes between the two groups for all outcome measures studied [5], and, specifically, the mean Harris hip score was equivalent between the groups at 3 months and 1 year; the duration of surgery and intraoperative blood loss were less in the uncemented group (Table 1). Because few studies have followed randomized hip fracture cohorts up to 5 years, there are limited data on late complications and implant longevity.
Table 1.
Characteristics during and after surgery for patients according to treatment*
| Variable | Cemented | Uncemented | Mean difference or relative risk (95% CI) |
p value |
|---|---|---|---|---|
| Perioperative details | ||||
| Mean (SD) time from admission to surgery (hours) | 21.9 (18.3) (n = 112) | 19.1 (14.4) (n = 108) | 2.8† (−1.6 to 7.1) | 0.22 |
| Mean (SD) duration of surgery (minutes) | 82.6 (19.8) (n = 112) | 70.2 (19.3) (n = 108) | 12.4† (7.2–17.6) | < 0.001 |
| Mean (SD) length of surgical incision (cm) | 14.1 (2.72) (n = 100) | 13.6 (2.60) (n = 97) | 0.47† (−0.28 to 1.22) | 0.22 |
| Main surgeons > 3 years’ experience with procedure | 72 (64) (n = 112) | 60 (56) (n = 108) | 0.86 (0.70–1.08) | 0.22 |
| Mean (SD) intraoperative blood loss (mL) | 390 (183.7) (n = 111) | 300 (171.9) (n = 108) | 89† (42–137) | < 0.001 |
| Mean (SD) postoperative drainage blood loss (mL) | 220 (147.3) (n = 105) | 233 (157.3) (n = 101) | 12.5† (−54.4 to 29.3) | 0.56 |
| Mean (SD) total blood loss (mL) | 598 (278) (n = 111) | 521 (265) (n = 107) | 77† (4.6–149.6) | 0.037 |
| Hospital stay | ||||
| Received blood transfusion while admitted | 47 (42) (n = 111) | 36 (34) (n = 106) | 0.80 (0.57–1.13) | 0.21 |
| Mean (SD) hospital stay (days) | 7.8 (4.11) (n = 109) | 8.4 (9.02) (n = 106) | 0.62† (−2.49 to 1.26) | 0.52 |
Figures are numbers (percentages) of patients unless stated otherwise; * number (n) varies because some information was missing for some patients; †mean difference; CI = confidence interval.
We therefore called in patients from our earlier randomized trial [5] at a median followup of 5 years to compare cemented with cementless fixation in bipolar hemiarthroplasties in terms of (1) Harris hip scores; (2) femoral fractures; (3) overall health outcomes using the Barthel Index and EQ-5D score; and (4) complications, reoperations, and mortality since our earlier report on this cohort at 1-year followup [5].
Patients and Methods
This prospective, randomized equivalence trial was performed at two hospitals; results previously were reported at 1 year [5]. One hundred fifty fractures were included at a district hospital and 80 fractures were included at a university hospital from September 2004 to August 2006. Two hundred twenty-three patients (75% women, mean age at fracture 83 years) were randomized. They were followed at 3 and 12 months and now after 5 years. As previously described [5], patients aged 70 years or older who were admitted to one of the two participating hospitals with a displaced intracapsular femoral neck fracture were eligible for inclusion. Exclusion criteria were being unfit for arthroplasty according to the anesthesiologist on call, previous symptomatic hip pathology such as osteoarthritis, fracture caused by malignant disease, patients with ongoing infectious disease, and patients who were unable to walk before the fracture. Randomization was performed separately for the two hospitals using a computer random number generator with permuted blocks of five. Allocation was undertaken by the surgeon on call using sealed, numbered, opaque envelopes. All patients who were able to provide informed consent did so. Patients who were not able to give their informed consent because of cognitive impairment were included if it was considered to be in their best interest after consultation with their family. The protocol was approved by the regional ethics committee.
Sample size calculation was conducted using the equivalence criterion [22]. Sixty hips in each group were required to show that the mean Harris hip score was the same in both groups with a power of 95% assuming a common SD of 15 and that a difference in means of less than 10 points was unimportant using a two-sided alpha set to 0.05. Equivalence would be demonstrated if the entire two-sided 95% confidence interval for the mean difference in Harris hip score between the groups lied within the equivalence margin of −10 to 10 points. To allow for mortality and loss to followup, 230 hips were included.
Of the 402 intracapsular femoral neck fractures admitted to the two hospitals, 247 were eligible for inclusion and 230 fractures in 223 patients were recruited (Fig. 1). Seven patients were included with both hips; five were included with one hip in each group, one with both hips in the cemented group, and one with both hips in the uncemented group. There were three protocol violations in the cemented group and seven in the uncemented group (Fig. 1), leaving 112 and 108 hips in the respective groups for the per-protocol analyses.
Fig. 1.
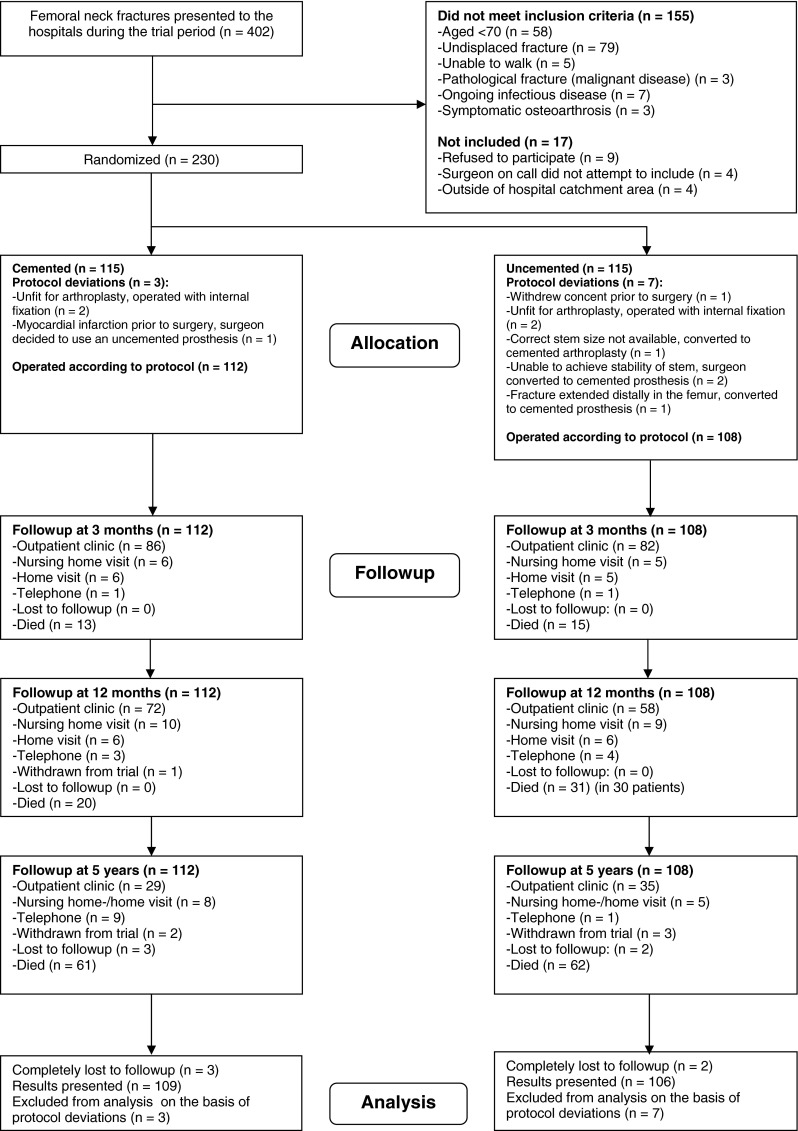
The diagram shows the recruitment and flow of femoral neck fractures during the study.
At 5 years, 61 patients (54%) had died in the cemented group, two (1.9%) withdrew from the trial, and three (2.7%) were completely lost to followup. In the uncemented group, 62 patients (57%) had died, three (2.8%) withdrew from the trial, and two (1.9%) were completely lost to followup. All remaining patients were invited to a followup visit (Fig. 1). New radiographs were obtained of the patients visiting the outpatient clinic. Those who were unable or unwilling to come were visited in their home or nursing home or interviewed by telephone. Telephone interviews were supplemented with information from health personnel and family members whenever possible. The EQ-5D interviews were not conducted by telephone or for patients with impaired mental function.
At baseline, the groups were similar (Table 2).
Table 2.
Baseline and demographic characteristics of patients according to treatment
| Variable | Cemented (n = 112) | Uncemented (n = 108) |
|---|---|---|
| Mean (SD) age at fracture (years) | 83.4 (5.68) | 83.0 (6.29) |
| Women | 87 (78) | 80 (74) |
| ASA Group I or II | 47 (42) | 47 (44) |
| Living in own home | 77 (69) | 76 (70) |
| Mean (SD) preoperative Harris hip score | 82.4 (16.29) | 84.6 (15.05) |
| Able to walk without any aid | 56 (50) | 59 (55) |
| Previously recognized cognitive failure | 26 (23) | 28 (26) |
Values are expressed as mean, with SD in parentheses, or as number of hips, with percentage in parentheses; ASA = American Society of Anesthesiologists.
Thirty-six surgeons performed a median of five operations each (range, 1–17). Patients underwent a bipolar hemiarthroplasty with either a cemented femoral stem (Spectron; Smith & Nephew, Memphis, TN, USA) (Fig. 2) or an uncemented femoral stem (Corail; DePuy/Johnson and Johnson, Leeds, UK) (Fig. 3). The cemented stem is a straight, collared stem made of a cobalt-chromium alloy. Its proximal one-third is grit-blasted. The distal part is smoother and a centralizer is attached to its tip. The uncemented stem is a grit-blasted titanium alloy stem with a proximal press-fit design entirely plasma sprayed with hydroxyapatite. All patients received the appropriate 28-mm cobalt-chromium head from the same manufacturer as the stem. The same bipolar head was used in both groups (Mobile Cup; DePuy/Johnson and Johnson). Surgery was performed through a posterior approach in all patients with the patient in a lateral decubitus position using spinal anesthesia. A third-generation cementing technique was used for the cemented stems [16]. All patients were given 2 g preoperative intravenous cephalothin and a further three doses the first 16 hours after the operation. All patients received 5000 IU low-molecular-weight heparin subcutaneously daily for at least 7 days. The surgeons on call carried out all the procedures with no changes in the departmental routines connected to the study. Early mobilization was encouraged in all patients with weightbearing as tolerated in both groups.
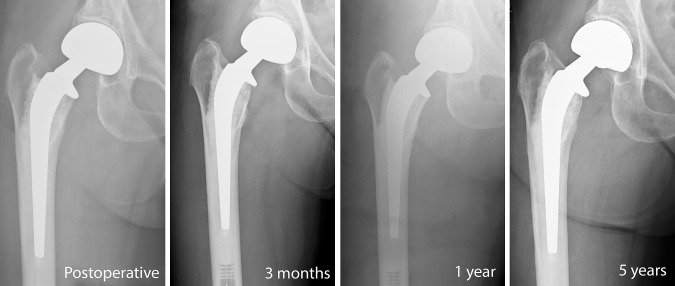
Fig. 2.
A series of radiographs shows the Spectron cemented bipolar hemiarthroplasty at four time intervals.
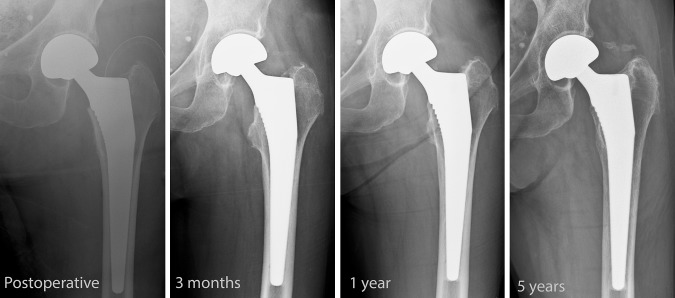
Fig. 3.
A series of radiographs shows the Corail uncemented bipolar hemiarthroplasty at four time intervals.
Hip function was rated with the Harris hip score [6, 10] ranging from 0 to 100 points covering a maximum of 44 points for absence of pain, 47 points for function, and 9 points for ROM and absence of deformity. The primary outcome was the Harris hip score after 5 years. The Barthel Index (BI) was used to rate ability to perform activities of daily living (ADL) [6, 15]. The BI comprises 10 items about basic ADL: feeding, grooming, bathing, dressing, bowel care, bladder care, toilet use, ambulation, transfers, and stairclimbing. The score range of the chosen BI is from 0 to 20. Health-related quality of life was rated by the patient-assessed EQ-5D [4, 6]. We used the EQ-5D index score (range 0 [death] to 1 [best possible health]) as well as the EQ-5D visual analog scale ranging from 0 (worst possible health) to 100 (best possible health) [2].
Data at a median of 5 years after surgery (range, 56–65 months) were collected from Spring 2009 until Summer 2011. All data were collected by one trained research nurse (MF) and one orthopaedic surgeon (EL). The research personnel were not blinded to the intervention.
To minimize the risk of falsely concluding equivalence, all analyses were conducted on a per-protocol basis; that is, participants not operated on according to the allocated treatment were not included in the analysis [22]. The patients representing protocol deviations at the beginning of the trial were not included in the previous publication [5] (Fig. 1), and only one of these patients was alive at 5 years. Therefore, we were not able to include an intention-to-treat analysis. For the equivalence analysis, we used the two-sided confidence interval approach [22]. We used the two-tailed Fisher’s exact test for dichotomous variables and t-tests for numerical variables. Levene’s test was used to assess the equality of variances between the groups. Where there was a significant difference in variance, or if equivalence was not found, the nonparametric Mann-Whitney U test was used. For mortality and postoperative periprosthetic femoral fractures, we used Kaplan-Meier analyses for survival curves and Cox regression analyses for calculation of hazard ratios (HRs). Given the number of patients analyzed at 5 years and the observed SDs, the power of the equivalence test with a 10-point equivalence margin and a two-sided alpha set to 0.05 was 68% for our main end point, Harris hip score (Table 3).
Table 3.
Functional outcomes in patients according to allocated treatment*
| Outcome measure | Cemented | Uncemented | Mean difference or relative risk (95% CI) | p value |
|---|---|---|---|---|
| Mean (SD) Harris hip score | ||||
| Baseline | 82.4 (16.3) (n = 112) | 84.6 (15.1) (n = 108) | 2.3† (−1.9 to 6.4) | 0.29 |
| At 3 months | 70.9 (18.5) (n = 99) | 72.1 (19.7) (n = 90) | 1.2† (−4.3 to 6.7) | 0.67 |
| At 1 year | 78.9 (15.7) (n = 90) | 79.8 (17.6) (n = 77) | 0.9† (−4.2 to 6.0) | 0.73 |
| At 5 years | 76.3 (20.9) (n = 41) | 86.2 (14.1) (n = 37) | 9.9† (1.9–17.9) | 0.027§ |
| Number (%) with Barthel Index of 19 or 20 | ||||
| Baseline | 59 (53) (n = 112) | 58 (54) (n = 108) | 1.02 (0.77–1.36) | 0.89 |
| At discharge (7 days) | 8 (7) (n = 109) | 14 (14) (n = 104) | 1.07 (0.98–1.17) | 0.18 |
| At 3 months | 44 (44) (n = 100) | 45 (50) (n = 90) | 1.12 (0.86–1.47) | 0.47 |
| At 1 year | 45 (50) (n = 91) | 48 (62) (n = 77) | 1.34 (0.94–1.91) | 0.12 |
| At 5 years | 25 (60) (n = 42) | 26 (68) (n = 38) | 1.28 (0.71–2.32) | 0.49 |
| Mean (SD) EQ-5D index score | ||||
| At 3 months | 0.64 (0.26) (n = 73) | 0.58 (0.30) (n = 70) | −0.06† (−0.15 to 0.34) | 0.21 |
| At 1 year | 0.68 (0.23) (n = 56) | 0.61 (0.32) (n = 57) | −0.08† (−0.18 to 0.03) | 0.15 |
| At 5 years | 0.64 (0.35) (n = 35) | 0.73 (0.25) (n = 36) | −0.08† (−0.06 to 0.23) | 0.26 |
| Mean (SD) EQ-5D visual analogue scale | ||||
| At 3 months | 60 (17.7) (n = 78) | 62 (22.4) (n = 68) | 2.3† (−4.2 to 8.9) | 0.49 |
| At 1 year | 61 (17.7) (n = 61) | 65 (20.1) (n = 60) | 3.6† (−3.2 to 10.4) | 0.30 |
| At 5 year | 65 (19.7) (n = 35) | 65 (16.9) (n = 36) | 0.19† (−8.5 to 8.9) | 0.97 |
| Number (%) living in own home | ||||
| Baseline | 77 (69) (n = 112) | 76 (70) (n = 108) | 1.02 (0.86–1.22) | 0.89 |
| At discharge‡ | 4 (4) (n = 109) | 5 (5) (n = 106) | 1.29 (0.36–4.66) | 0.75 |
| At 3 months | 66 (66) (n = 100) | 61 (68) (n = 90) | 1.03 (0.84–1.26) | 0.88 |
| At 1 year | 59 (65) (n = 91) | 59 (77) (n = 77) | 1.18 (0.97–1.44) | 0.13 |
| At 5 years | 26 (63) (n = 41) | 30 (79) (n = 38) | 1.25 (0.94–1.66) | 0.15 |
| Number (%) not in need of any pain medication | ||||
| Baseline | 90 (80) (n = 112) | 90 (83) (n = 108) | 1.04 (0.92–1.18) | 0.60 |
| At discharge‡ | 6 (6) (n = 109) | 5 (5) (n = 106) | 0.86 (0.27–2.72) | 1.00 |
| At 3 months | 60 (60) (n = 100) | 54 (60) (n = 90) | 1.00 (0.79–1.26) | 1.00 |
| At 1 year | 68 (75) (n = 91) | 63 (82) (n = 77) | 1.10 (0.93–1.28) | 0.35 |
| At 5 years | 31 (74) (n = 42) | 28 (74) (n = 38) | 1.00 (0.77–1.30) | 1.00 |
| Number (%) able to walk independently using any aids | ||||
| Baseline | 112 (100) (n = 112) | 108 (100) (n = 108) | – | – |
| At discharge‡ | 88 (81) (n = 109) | 80 (76) (n = 106) | 0.94 (0.81–1.08) | 0.41 |
| At 3 months | 94 (94) (n = 100) | 82 (91) (n = 90) | 0.97 (0.89–1.05) | 0.58 |
| At 1 year | 87 (96) (n = 91) | 71 (92) (n = 77) | 0.96 (0.89–1.04) | 0.52 |
| At 5 years | 34 (83) (n = 41) | 36 (95) (n = 38) | 1.14 (0.98–1.34) | 0.16 |
* Number (n) varies because some information was missing for some patients. Values are expressed as mean with SD in parentheses, or as number of hips, with percentage in parentheses; †mean difference; ‡data collected at discharge from hospital or as close to 7 days as possible; §p value of Mann-Whitney U test; CI = confidence interval.
Results
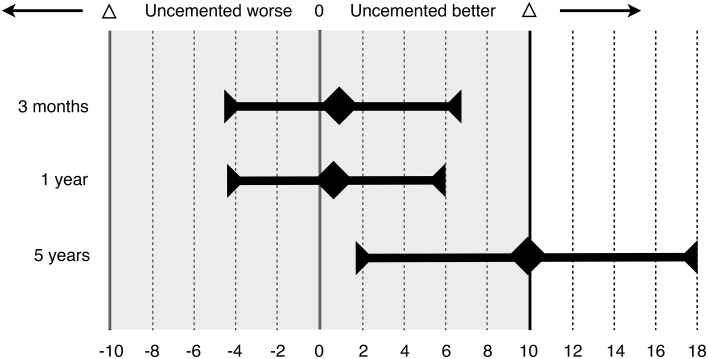
At 5 years, the Harris hip score was higher in the uncemented group than in the cementless group (86.2 versus 76.3; mean difference 9.9; 95% confidence interval [CI], 1.9–17.9) (Fig. 4). Levene’s test showed unequal variance between the groups at 5 years (p = 0.040). However, the Mann-Whitney U test still showed a significant difference for Harris hip score in favor of the uncemented group (p = 0.027). This was in contrast to our results at 3 months and 1 year, when they were equivalent between the groups.
Fig. 4.
The graph shows the mean difference of Harris hip score between the two groups at 3 and 12 months. Error bars indicate 95% CIs. Tinted area indicates zone of equivalence, defined as ± 10 points (Δ). At 3 months and at 1 year, both CIs lie wholly inside of the zone of equivalence and include zero. This shows that the results in the uncemented group were equivalent but not superior to the cemented group. At 5 years, the CI lies wholly above zero, showing that the results in the uncemented group were superior to the cemented group.
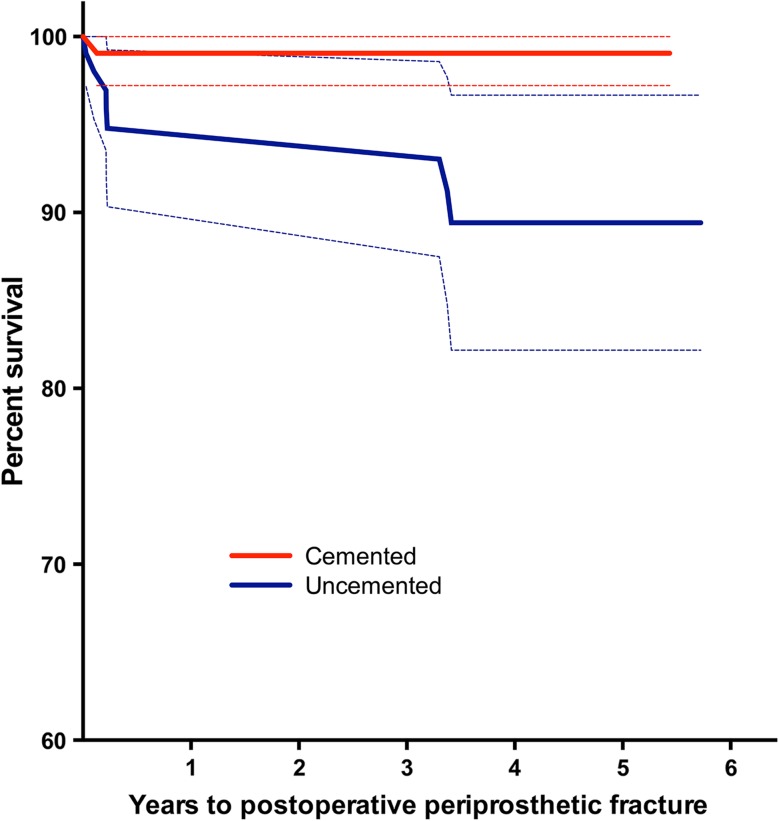
There were more postoperative femoral fractures in the uncemented group than in the cemented group. The total number of postoperative femoral fractures was one (0.9%) in the cemented group and eight (7.4%) in the uncemented group (HR, 9.3; 95% CI, 1.16–74.5; p = 0.035) (Fig. 5). Three of these fractures, all in the uncemented group, occurred after our previous study at 1 year [5]. A total of four patients, all in the uncemented group, had experienced a traumatic periprosthetic fracture that needed surgery.
Fig. 5.
The survival curve with 95% CIs shows the cemented and uncemented hemiarthroplasties with postoperative periprosthetic femoral fracture as the endpoint, censored for death.
There were no differences between the groups on the functional outcomes tools at 5 years (Barthel Index and EQ-5D; Table 3). There were no differences in ability to walk, use of analgesics, or place of living.
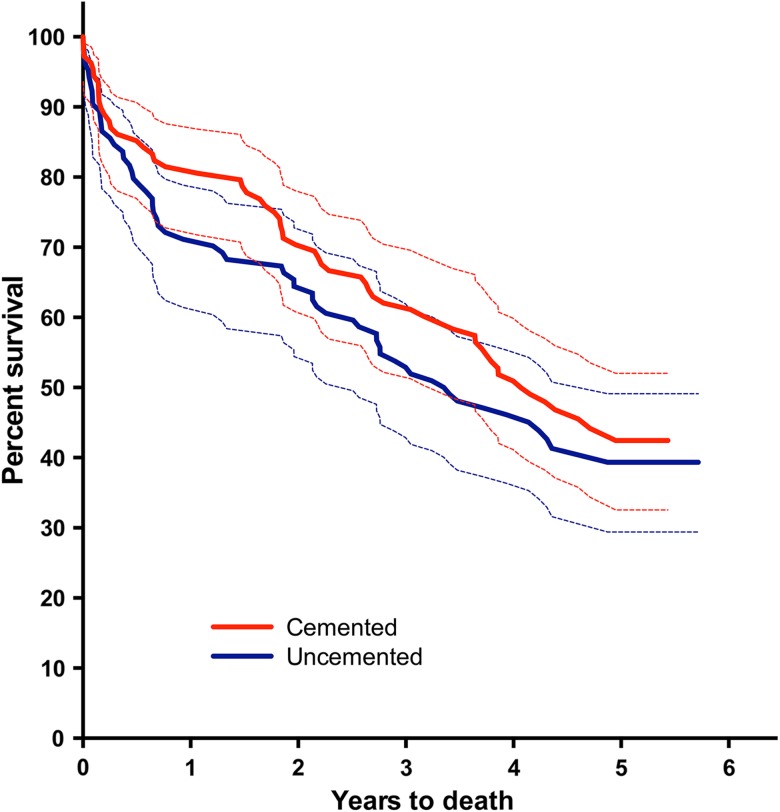
Between 1 and 5 years, we found no additional infections or dislocations, and the 5-year mortality rate was not different between the groups (HR, 1.2; 95% CI, 0.82–1.7; p = 0.38) (Fig. 6). Mortality at 5 years was 56% in the cemented group and 60% in the uncemented group. Also, at 5 years, we found one patient in the cemented group with severe acetabular erosion. The total number of reoperations for any reason was seven (6.3%) in the cemented group and 11 (10.2%) in the uncemented group (relative risk, 1.6; 95% CI, 0.66–4.05; p = 0.33).
Fig. 6.
The survival curve with 95% CIs shows the patients with cemented and uncemented hemiarthroplasties with death as the end point. Seven patients were included with both hips and are only included with their first hip in the mortality analysis.
Discussion
Hemiarthroplasty is the most commonly used treatment for displaced femoral neck fractures in the elderly. Although the number of randomized trials is increasing, there are still problems with diversity of implants that are studied, short followup time, and interpretation of functional results versus reoperation and, maybe most important, rates of subsequent fractures [18, 19, 24]. The aim of this study was to examine whether the uncemented femoral stem used in this trial would perform similarly to the cemented hemiarthroplasty after 5 years. We specifically studied differences in Harris hip scores, femoral fractures, overall health outcomes, complications, reoperations, and mortality.
The difference in Harris hip score should be interpreted with caution, because the sample size was low at 5 years. It is conceivable that had even a few patients with lower scores been available for followup, our findings may have been different; we believe, therefore, that our finding that the Harris hip score favored the uncemented group should be interpreted cautiously. Although our primary functional outcome measure, Harris hip score after 5 years, was higher in the uncemented group, we performed a per-protocol analysis in this study; hip scores may not have been higher in an intention-to-treat analysis, particularly if more patients representing crossover protocol deviations were alive and available for followup. Because the original trial was designed to show equivalence and not superiority, the 10 patients representing protocol deviations (Fig. 1) were not followed and not reported at 1 year [5] and therefore were not included in our followup at 5 years. There were three protocol deviations in the cemented group and seven in the uncemented group (Fig. 1). If we had planned an intention-to-treat trial from the beginning, one patient in the cemented group would have had an uncemented implant and two would have had an internal fixation. In the uncemented group, four would have had a cemented implant and two would have had an internal fixation. For an intention-to-treat versus per-protocol comparison, we would have to compare an analysis with these patients in their original group for the intention-to-treat analysis with the presented per-protocol analysis. As a result of the high mortality in this small group of nine patients, we do not know if there would be a difference in Harris hip score or not in an intention-to-treat analysis. Because the result of the per-protocol analysis is slightly below 10 points and the CI is wide with a lower margin of only 1.9, we believe that it is likely that an intention-to-treat analysis would show that this difference is not statistically significant.
In the most recent Cochrane report on arthroplasties for proximal femoral fractures, the authors conclude that there is reasonable evidence to indicate that cemented prostheses will reduce pain and result in improved mobility. However, they also state that it is possible that these differences may not exist for a hydroxyapatite-coated uncemented prosthesis. They also conclude that trials of older hemiarthroplasties like Thompson and Austin Moore are of less relevance today [19]. In addition, there were methodology issues with several trials regarding concealment of allocation, short followup, and small numbers of patients. Another reported problem was that the level of comparison is questionable, because different types of prostheses were used in the cemented and uncemented groups and may differ in other aspects than the use of cement. The long-term results of the cemented stem used in this trial have been reported to be lower than other cemented stems [3].
In the previously published report of this trial, we found no difference in complications between the two groups [5]. However, at 5 years, the number of subsequent femoral fractures was higher in the uncemented group. This is consistent with findings from a registry study of over 11,000 patients, which found that uncemented hemiarthroplasties had a 2.1 times increased risk of a reoperation compared with cemented prostheses [8]. This increased risk was mainly caused by revisions for periprosthetic fracture and aseptic loosening. A total of 75.5% of the uncemented femoral stems in this register trial was the same implant as in our trial. The same study reported a higher perioperative mortality rate and perioperative complications in the cemented group, but this reversed with longer observation time [8]. A recent randomized controlled trial comparing an Exeter cemented (Stryker Orthopaedics, Mahwah, NJ, USA) and a Zweymüller uncemented (Centerpulse, Zurich, Switzerland) hemiarthroplasty showed a higher rate in the uncemented group of both intraoperative fractures (7.5% versus 0%) and postoperative fractures (15% versus 1.3%) within 2 years [24].
We found no differences in health-related quality of life (BI and EQ-5D), and there were no differences in ability to walk, use of analgesics, or place of living. This does not support the difference found in Harris hip score and indicates that the most important finding in this trial is the increased risk of femoral fractures in the uncemented group. Also, we do not know of any other trial showing superior clinical results using uncemented hemiarthroplasties for femoral neck fractures.
Few national healthcare systems have issued guidelines for the treatment of hip fractures. The England and Wales National Institute for Health and Clinical Excellence (NICE) published its first guidance on hip fracture management in June 2011, specifically stating that a well-proven femoral stem should be used and that it should be cemented [17, 25].
The reported risk of cement-related death is low but not negligible [8, 11, 13, 20, 21]. Whereas some uncemented hemiarthroplasties have shown equivalent clinical results as cemented counterparts, an increased risk of subsequent fractures is evident. The risk of periprosthetic fractures may also differ between cemented femoral stems with different designs [14]. Further studies of specific stem designs of both cemented and uncemented hemiarthroplasties are needed.
The uncemented hemiarthroplasty used in this trial may result in higher hip scores but not in higher overall health outcomes using the BI and EQ-5D scores. However, the uncemented hemiarthroplasty appears to carry an unacceptably high risk of later femoral fractures that was not apparent at 1 year. We still recommend performing hemiarthroplasties using femoral stems that have performed well in THAs, but as the evidence favoring the use of cemented stems is increasing as a result of increased fracture risk throughout the lifetime of uncemented prostheses, we recommend using a cemented stem for treatment of displaced femoral neck fractures in patients aged 70 years or older.
Acknowledgments
We thank research nurse Merete Finjarn for impeccable data collection and patient logistics; and Asbjørn Hjall and Wenche Sørensen for initiation support and making this trial feasible in a busy orthopaedic department. We also thank Are Hugo Pripp PhD, Senior scientist, Department of Biostatistics, Epidemiology and Health Economics, Oslo University Hospital, for expert statistical advice.
Footnotes
The institution of the authors has received funding from Eastern Norway Regional Health Authority (nonprofit, governmental). Four of the authors certify that they (FF, JEM, LN, WF) have received payments or benefits, during the study period, an amount of less than USD 10,000, from Smith & Nephew, Nesbru, Norway, and less than USD 10,000 from Ortomedic, Lysaker, Norway (Ortomedic market orthopaedic implants manufactured by DePuy).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bhandari M, Devereaux PJ, Tornetta P, 3rd, Swiontkowski MF, Berry DJ, Haidukewych G, Schemitsch EH, Hanson BP, Koval K, Dirschl D, Leece P, Keel M, Petrisor B, Heetveld M, Guyatt GH. Operative management of displaced femoral neck fractures in elderly patients. An international survey. J Bone Joint Surg Am. 2005;87:2122–2130. doi: 10.2106/JBJS.E.00535. [DOI] [PubMed] [Google Scholar]
- 2.Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ. 1996;5:141–154. doi: 10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Espehaug B, Furnes O, Engesaeter LB, Havelin LI. 18 years of results with cemented primary hip prostheses in the Norwegian Arthroplasty Register: concerns about some newer implants. Acta Orthop. 2009;80:402–412. doi: 10.3109/17453670903161124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EuroQol_Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed]
- 5.Figved W, Opland V, Frihagen F, Jervidalo T, Madsen JE, Nordsletten L. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures. Clin Orthop Relat Res. 2009;467:2426–2435. doi: 10.1007/s11999-008-0672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frihagen F, Grotle M, Madsen JE, Wyller TB, Mowinckel P, Nordsletten L. Outcome after femoral neck fractures: a comparison of Harris Hip Score, Eq-5d and Barthel Index. Injury. 2008;39:1147–1156. doi: 10.1016/j.injury.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Garellick G, Malchau H, Herberts P. The Charnley versus the Spectron hip prosthesis: clinical evaluation of a randomized, prospective study of 2 different hip implants. J Arthroplasty. 1999;14:407–413. doi: 10.1016/S0883-5403(99)90095-5. [DOI] [PubMed] [Google Scholar]
- 8.Gjertsen JE, Lie SA, Vinje T, Engesaeter LB, Hallan G, Matre K, Furnes O. More re-operations after uncemented than cemented hemiarthroplasty used in the treatment of displaced fractures of the femoral neck: an observational study of 11,116 hemiarthroplasties from a national register. J Bone Joint Surg Br. 2012;94:1113–1119. doi: 10.1302/0301-620X.94B8.29155. [DOI] [PubMed] [Google Scholar]
- 9.Hallan G, Lie SA, Furnes O, Engesaeter LB, Vollset SE, Havelin LI. Medium- and long-term performance of 11,516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg Br. 2007;89:1574–1580. doi: 10.1302/0301-620X.89B12.18969. [DOI] [PubMed] [Google Scholar]
- 10.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 11.Hossain M, Andrew JG. Is there a difference in perioperative mortality between cemented and uncemented implants in hip fracture surgery? Injury. 2012;43:2161–2164. doi: 10.1016/j.injury.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Issack PS, Botero HG, Hiebert RN, Bong MR, Stuchin SA, Zuckerman JD, Di Cesare PE. Sixteen-year follow-up of the cemented spectron femoral stem for hip arthroplasty. J Arthroplasty. 2003;18:925–930. doi: 10.1016/S0883-5403(03)00336-X. [DOI] [PubMed] [Google Scholar]
- 13.Lennox IA, McLauchlan J. Comparing the mortality and morbidity of cemented and uncemented hemiarthroplasties. Injury. 1993;24:185–186. doi: 10.1016/0020-1383(93)90290-M. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl H, Malchau H, Herberts P, Garellick G. Periprosthetic femoral fractures classification and demographics of 1049 periprosthetic femoral fractures from the Swedish National Hip Arthroplasty Register. J Arthroplasty. 2005;20:857–865. doi: 10.1016/j.arth.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 16.Oishi CS, Walker RH, Colwell CW., Jr The femoral component in total hip arthroplasty. Six to eight-year follow-up of one hundred consecutive patients after use of a third-generation cementing technique. J Bone Joint Surg Am. 1994;76:1130–1136. doi: 10.2106/00004623-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Palmer JS, Huber CP. Operative management of hip fractures: a review of the NICE guidelines. Br J Hosp Med. 2012;73:C141–C144. doi: 10.12968/hmed.2012.73.sup9.c141. [DOI] [PubMed] [Google Scholar]
- 18.Parker MI, Pryor G, Gurusamy K. Cemented versus uncemented hemiarthroplasty for intracapsular hip fractures: A randomised controlled trial in 400 patients. J Bone Joint Surg Br. 2010;92:116–122. doi: 10.1302/0301-620X.92B1.22753. [DOI] [PubMed] [Google Scholar]
- 19.Parker MJ, Gurusamy KS, Azegami S. Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database Syst Rev. 2010;6:CD001706. doi: 10.1002/14651858.CD001706.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvizi J, Ereth MH, Lewallen DG. Thirty-day mortality following hip arthroplasty for acute fracture. J Bone Joint Surg Am. 2004;86:1983–1988. doi: 10.2106/00004623-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;369:39–48. doi: 10.1097/00003086-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 23.Reikeras O, Gunderson RB. Excellent results of HA coating on a grit-blasted stem: 245 patients followed for 8–12 years. Acta Orthop Scand. 2003;74:140–145. doi: 10.1080/00016470310013851. [DOI] [PubMed] [Google Scholar]
- 24.Taylor F, Wright M, Zhu M. Hemiarthroplasty of the hip with and without cement: a randomized clinical trial. J Bone Joint Surg Am. 2012;94:577–583. doi: 10.2106/JBJS.K.00006. [DOI] [PubMed] [Google Scholar]
- 25.The management of hip fracture in adults. Clinical guidelines, CG124. Issued: June 2011. Available at: http://guidance.nice.org.Uk/CG124. Accessed February 12, 2013.
- 26.Vidalain JP. Twenty-year results of the cementless Corail stem. Int Orthop. 2011;35:189–194. doi: 10.1007/s00264-010-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]