Abstract
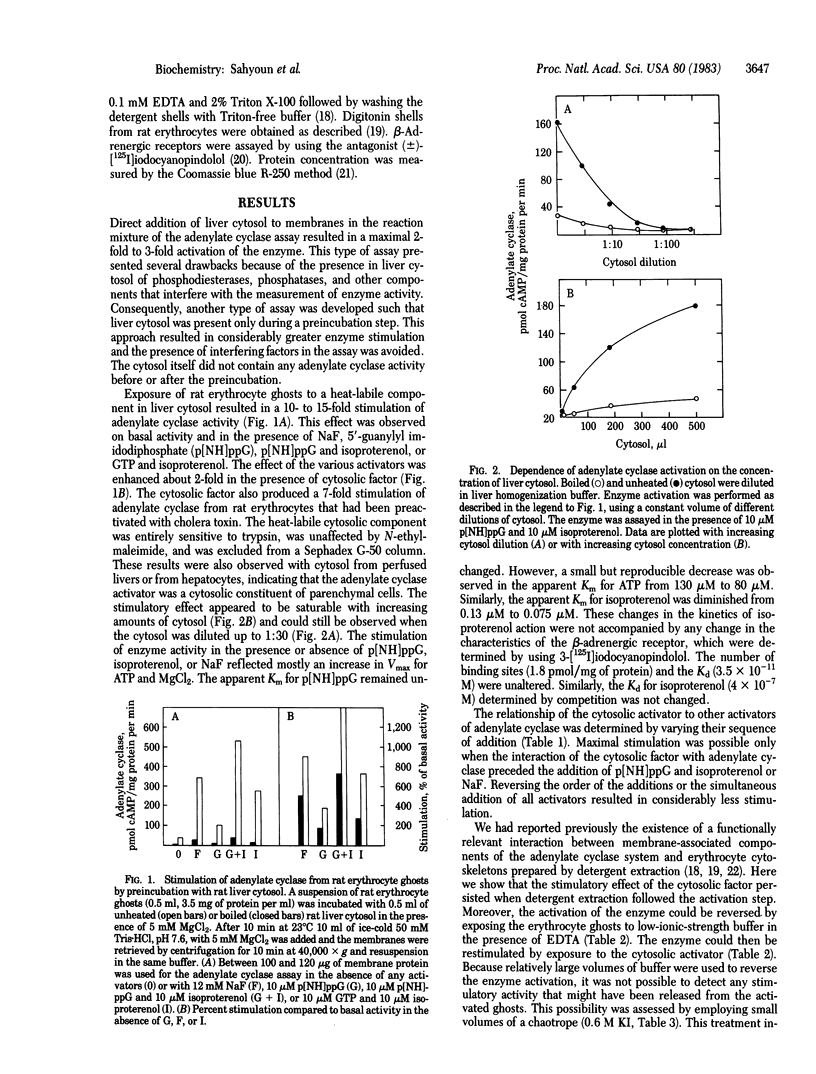
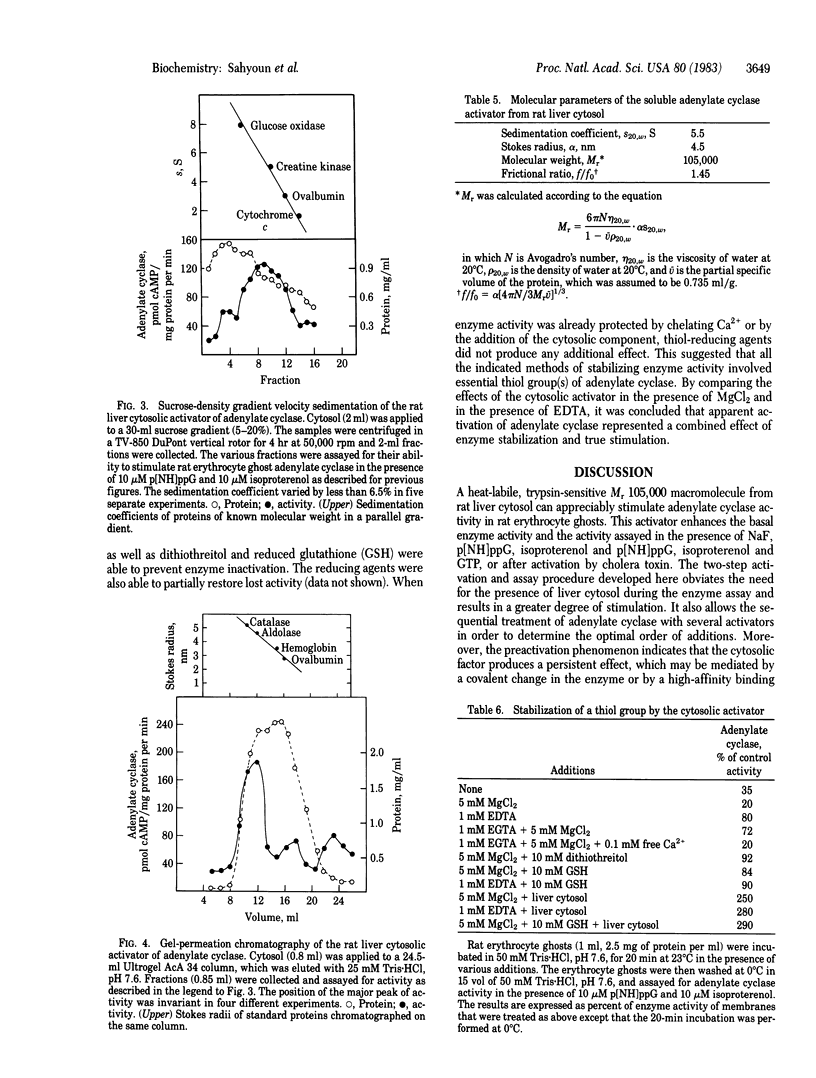
Rat liver and isolated hepatocytes contain high levels of a soluble adenylate cyclase stimulator, whereas rat erythrocytes lack this activity. Accordingly, a reconstitution system was developed with adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] from erythrocyte ghosts and the soluble activator from liver cytosol. Pretreatment of erythrocyte ghosts with the cytosolic factor resulted in a 5- to 15-fold activation of adenylate cyclase in the presence or absence of NaF, 5'-guanylyl imidodiphosphate, or isoproterenol and GTP. The sequence of addition of the cytosolic component and the other activators was critical in determining the maximal activity of the enzyme. The cytosolic factor appears to be a heat-labile Mr 105,000 protein, which activates adenylate cyclase in a saturable reaction involving binding of the protein to the erythrocyte ghosts. This molecular interaction was accompanied by stabilization of a labile thiol group that was essential for catalytic activity. The cytosolic component also unmasks latent adenylate cyclase activity in human erythrocyte ghosts and in cytoskeletal preparations from rat erythrocyte ghosts. These observations suggest that the cytosolic activator may also occur as a native, peripheral membrane component of adenylate cyclase systems and may be required for the expression and stabilization of catalytic activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnafous J. C., Dornand J., Mani J. C. Deactivation of lymphocyte adenylate cyclase by affinity chromatography on Con A-sepharose. Its reactivation by a cytosolic factor. FEBS Lett. 1979 Mar 1;99(1):152–156. doi: 10.1016/0014-5793(79)80268-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brodde O. E., Engel G., Hoyer D., Bock K. D., Weber F. The beta-adrenergic receptor in human lymphocytes: subclassification by the use of a new radio-ligand, (+/-)-125 Iodocyanopindolol. Life Sci. 1981 Nov 23;29(21):2189–2198. doi: 10.1016/0024-3205(81)90490-2. [DOI] [PubMed] [Google Scholar]
- Doberska C. A., Martin B. R. Activation of rat liver plasma membrane adenylate cyclase by a cytoplasmic protein factor. FEBS Lett. 1977 Oct 15;82(2):273–277. doi: 10.1016/0014-5793(77)80601-7. [DOI] [PubMed] [Google Scholar]
- Egan J. J., Majeska R. J., Rodan G. A. Adenylate cyclase enhancing factor from rat osteosarcoma cytosol. Biochem Biophys Res Commun. 1978 Jan 13;80(1):176–182. doi: 10.1016/0006-291x(78)91120-8. [DOI] [PubMed] [Google Scholar]
- Eng J., Lee L., Yalow R. S. Influence of the age of erythrocytes on their insulin receptors. Diabetes. 1980 Feb;29(2):164–166. doi: 10.2337/diab.29.2.164. [DOI] [PubMed] [Google Scholar]
- Hebdon M., Le Vine H., 3rd, Sahyoun N., Schmitges C. J., Cuatrecasas P. Properties of the interaction of fluoride- and guanylyl-5'-imidodiphosphate-regulatory proteins with adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3693–3697. doi: 10.1073/pnas.75.8.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. S., Kelly T. M., Piñeyro M. A., Gregerman R. I. Activation of epinephrine and glucagon-sensitive adenylate cyclases of rat liver by cytosol protein factors. Role in loss of enzyme activities during preparation of particulate fractions, quantitation and partial characterization. J Cyclic Nucleotide Res. 1978 Oct;4(5):389–407. [PubMed] [Google Scholar]
- Le Vine H., 3rd, Cuatrecasas P. Activation of pigeon erythrocyte adenylate cyclase by cholera toxin. Partial purification of an essential macromolecular factor from horse erythrocyte cytosol. Biochim Biophys Acta. 1981 Feb 5;672(3):248–261. doi: 10.1016/0304-4165(81)90291-9. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd, Sahyoun N. E., Cuatrecasas P. Properties of rat erythrocyte membrane cytoskeletal structures produced by digitonin extraction: digitonin-insoluble beta-adrenergic receptor, adenylate cyclase, and cholera toxin substrate. J Membr Biol. 1982;64(3):225–231. doi: 10.1007/BF01870889. [DOI] [PubMed] [Google Scholar]
- MacNeil S., Crawford A., Amirrasooli H., Johnson S., Pollock A., Ollis C., Tomlinson S. Stimulation of hormone-responsive adenylate cyclase activity by a factor present in the cell cytosol. Biochem J. 1980 May 15;188(2):393–400. doi: 10.1042/bj1880393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijjar M. S., Au A. W., Chaudhary K. C. Partial purification and properties of the cytoplasmic factors modulating adenylate cyclase activity in rat lung plasma membranes. Biochim Biophys Acta. 1981 Sep 18;677(1):153–159. doi: 10.1016/0304-4165(81)90157-4. [DOI] [PubMed] [Google Scholar]
- Nijjar M. S. Regulation of rat lung adenylate cyclase by cytoplasmic factor(s) during development. Biochim Biophys Acta. 1979 Apr 18;584(1):43–50. doi: 10.1016/0304-4165(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Pecker F., Hanoune J. Activation of epinephrine-sensitive adenylate cyclase in rat liver by cytosolic protein-nucleotide complex. J Biol Chem. 1977 Apr 25;252(8):2784–2786. [PubMed] [Google Scholar]
- Pecker F., Hanoune J. Uncoupled beta-adrenergic receptors and adenylate cyclase can be recoupled by a GTP-dependent cytosolic factor. FEBS Lett. 1977 Nov 1;83(1):93–98. doi: 10.1016/0014-5793(77)80649-2. [DOI] [PubMed] [Google Scholar]
- Rasenick M. M., Bitensky M. W. Partial purification and characterization of a macromolecule which enhances fluoride activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4628–4632. doi: 10.1073/pnas.77.8.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N. E., LeVine H., 3rd, Davis J., Hebdon G. M., Cuatrecasas P. Molecular complexes involved in the regulation of adenylate cyclase. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6158–6162. doi: 10.1073/pnas.78.10.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N. E., LeVine H., 3rd, Hebdon G. M., Khouri R. K., Cuatrecasas P. Evidence for cytoskeletal associations of the adenylate cyclase system obtained by differential extraction of rat erythrocyte ghosts. Biochem Biophys Res Commun. 1981 Aug 14;101(3):1003–1010. doi: 10.1016/0006-291x(81)91848-9. [DOI] [PubMed] [Google Scholar]
- Sahyoun N., Hollenberg M. D., Bennett V., Cuatrecasas P. Topographic separation of adenylate cyclase and hormone receptors in the plasma membrane of toad erythrocyte ghosts. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2860–2864. doi: 10.1073/pnas.74.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. B., Thompson W. J., Robison G. A. Epinephrine- and glucagon-stimulated cardiac adenylyl cyclase activity. Regulation by endogenous factors. Biochim Biophys Acta. 1977 Jun 23;498(1):10–20. doi: 10.1016/0304-4165(77)90082-4. [DOI] [PubMed] [Google Scholar]
- Shane E., Gammon D. E., Bilezikian J. P. A cellular activator of catecholamine-sensitive adenylate cyclase in rat reticulocytes and erythrocytes: changes during reticulocyte development and effects on the beta receptor. Arch Biochem Biophys. 1981 May;208(2):418–425. doi: 10.1016/0003-9861(81)90527-0. [DOI] [PubMed] [Google Scholar]
- Zahlten R. N., Rogoff T. M., Steer C. J. Isolated Kupffer cells, endothelial cells and hepatocytes as investigative tools for liver research. Fed Proc. 1981 Aug;40(10):2460–2468. [PubMed] [Google Scholar]


