Abstract
Background:
T-cell interferon-γ release assays (IGRAs) are used in the diagnosis of Mycobacterium tuberculosis infection and could be useful biomarkers of response to treatment of latent TB infection for clinical trials, infection control units, and TB programs.
Methods:
This investigation was a prospective, controlled substudy of IGRA responses in 82 healthy South African adults with HIV seronegative and positive tuberculin skin test results randomly assigned to treatment with 6 months of daily isoniazid preventive therapy (IPT) or observation before Bacillus Calmette-Guérin revaccination in a clinical trial. QuantiFERON-TB Gold In-Tube (QFT-GIT) assay was used to measure interferon-γ (IFN-γ) response to mycobacterial antigens at baseline and after IPT or observation.
Results:
IFN-γ levels declined between baseline and the end of IPT (signed rank test P ≤ .0001) and between baseline and a similar period of observation without IPT (signed rank test P = .03). The rate of decrease in IFN-γ responses over time did not differ between the groups (Mann-Whitney-Wilcoxon test P = .31). QFT-GIT test results in two subjects (5%) in the IPT group and two subjects (5%) in the observation group reverted from positive to negative during follow-up. No significant difference was found between the groups with respect to baseline positivity or the proportion of patients whose tests reverted to negative.
Conclusions:
IPT had no effect on changes in QFT-GIT readouts during short-term follow-up of adults with positive tuberculin skin tests in a high TB incidence setting. QFT-GIT is unlikely to be a useful biomarker of response to treatment of latent TB infection.
Trial registry:
ClinicalTrials.gov; No.: NCT01119521; URL: www.clinicaltrials.gov
T-cell interferon-γ release assays (IGRAs), such as the QuantiFERON-TB Gold In-Tube (QFT-GIT) (Cellestis Inc), are new, widely recommended alternative1 or additional2 tests in the diagnosis of latent TB infection (LTBI). Their specificity, particularly in people who received Bacillus Calmette-Guérin (BCG) vaccination in infancy, has been reported to be greater than that of the tuberculin skin test (TST).3 It has been suggested that effector T-cell responses after short-term incubation with Mycobacterium tuberculosis (MTB)-specific antigens used in available IGRAs are correlated with antigenic and bacillary burden and, hence, may be a marker of response to treatment.4,5 Several studies have reported decreases in MTB-specific T-cell response and MTB bacillary load during anti-TB treatment4 or treatment of LTBI6; however, the results have been inconsistent. Some studies showed a decline in interferon-γ (IFN-γ) levels after treatment, whereas others showed no change or increased levels of IFN-γ.5,7‐11 More limited data are available on T-cell response and IGRA after treatment of LTBI, although there is substantial interest in identifying a biomarker of response to treatment of LTBI.
We recently completed a phase 1 randomized clinical trial in healthy South African adults with positive TST and HIV-seronegative results to study whether isonicotinylhydrazine (INH) treatment of LTBI before revaccination with BCG changed the magnitude of MTB-specific immune responses. Prior MTB infection may prevent optimal immune responses to live TB vaccines by inhibiting macrophage function,12 preventing phagolysosomal fusion,13‐16 and decreasing major histocompatibility complex class I and II presentation to T cells.17‐19 Preclearance of latent tubercle bacilli by isoniazid preventive therapy (IPT) might result in a better immunologic response following revaccination with BCG or a new TB vaccine.20 Healthy volunteers with HIV-seronegative tests were randomly assigned to receive 6 months of either isoniazid for treatment of LTBI or observation before BCG revaccination. Active TB was excluded before enrollment. IGRA (QFT-GIT) was done at baseline, after completion of 6 months of IPT or observation, and after BCG revaccination. The aim of this substudy was to examine QFT-GIT responses before and after IPT in healthy patients with positive TST results living in a high TB incidence area to assess whether QFT-GIT is a useful biomarker of response to treatment of LTBI.
Materials and Methods
We compared QFT-GIT results at baseline and after 6 months of daily IPT (isoniazid 5 mg/kg/d; maximum, 300 mg/d) or 6 months of observation in healthy adults with positive TST results enrolled in a phase 1 randomized clinical trial to assess the safety and immunogenicity of BCG revaccination after IPT or observation. Healthy adults aged 18 to 40 years with HIV-seronegative test results, a BCG scar, and a TST ≥ 15 mm induration at 48 to 72 h after placement (2 tuberculin units purified protein derivative [PPD] [PPD-RT23; Statens Serum Institut]) were randomly assigned to receive either IPT (180 doses within no more than 7 months) or observation for an equivalent time period before BCG revaccination. TST was performed by trained personnel following standard procedures, including intradermal injection of PPD-RT23 into the volar aspect of the left forearm and measurement of the transverse diameter of induration 48 to 72 h after injection. Subjects assigned to the observation before BCG revaccination group subsequently received IPT beginning 6 months after revaccination. IPT administration was supervised by trained study field workers.
Adherence to IPT was monitored by treatment supervisors and study staff. The number of INH tablets was reconciled and a 1-month supply dispensed at scheduled monthly clinic visits during IPT. Treatment cards were issued to subjects, with daily entries for medication taken initialed and dated by the subject and his or her treatment supervisor. Treatment supervisors were nominated by subjects and were most often responsible members of their household. Supervisors were trained by study field staff. At the monthly clinic visits during IPT, study staff reviewed the treatment card and reconciled counts of returned empty and unopened study medication containers. Missed doses and other discrepancies were noted and discussed with the subject. As a further measure to monitor adherence, INH metabolite testing (BBL Taxo INH test strips; BD) was done on urine samples provided by the subject during scheduled visits at months 1, 3, and 5 of IPT and on two unscheduled spot urine samples collected by field workers at home visits during the first and second 3 months of IPT. Eighty-seven percent of all urine INH metabolite tests performed during the study were positive results.
Healthy volunteers from the Worcester area near Cape Town, South Africa, were recruited for the parent trial. This rural town (population, 92,000) has a smear-positive TB incidence of 815 per 100,000 population per year and an all-forms TB incidence of 1,030 per 100,000 population per year (Regional Health Department, unpublished data, Western Cape Province, South Africa, 2010). In an earlier study, 52% of healthy adults without HIV in this area had a positive TST result ≥ 15 mm.21 Active TB in all subjects was excluded at baseline by history and physical examination, posteroanterior chest radiography, sputum smear and culture, and other testing if indicated.
QFT-GIT, an in vitro T-cell IGRA using the mycobacterial antigens early secreted antigenic target (ESAT)-6, culture filtrate protein (CFP)-10, and TB 7.7(p4), was done according to the manufacturer’s instructions22 for each subject at baseline, after completion of IPT or observation, and after BCG revaccination. Testing was completed at the quality-controlled, Good Laboratory Practice-certified laboratory of the South African Tuberculosis Vaccine Initiative, Cape Town, South Africa. Laboratory staff performing QFT-GIT assays were blinded to each subject’s group (IPT or observation). QFT-GIT data were analyzed with the manufacturer’s software-generated standard curves, pass-fail criteria, and definitions. Test results were determined positive (IFN-γ for TB antigens minus negative control ≥ 0.35 IU/mL), negative, or indeterminate (qualitative test result) and presented as the IFN-γ concentration in International Units per milliliter (quantitative result). No indeterminate values were observed for any subject. QFT-GIT conversion was defined as a change from a negative IFN-γ result (< 0.35 IU/mL) to a positive result. QFT-GIT reversion was defined as a change from a positive (≥ 0.35 IU/mL) to a negative (< 0.35 IU/mL) result.11 Because QFT-GIT cannot accurately measure IFN-γ concentrations > 10 IU/mL, IFN-γ levels > 10 IU/mL were assigned a value of 10 IU/mL for analysis.11,22 QFT-GIT responses before and after IPT or observation were the focus of the present analysis. QFT-GIT response after BCG revaccination was a secondary immunogenicity end point for the parent trial and will be reported separately.
The study was conducted in accordance with the amended Declaration of Helsinki. The trial protocol was approved by the Medicines Control Council of South Africa, the Human Research Ethics Committee of the University of Cape Town (387/2008), and the University Hospitals Case Medical Center institutional review board. Written informed consent was obtained from all subjects.
Mann-Whitney-Wilcoxon and t tests were used for between-group comparisons of mean ranks and mean IFN-γ values, respectively. The Wilcoxon signed rank test was used to assess significant changes in paired IFN-γ levels over time. Group proportions were compared by Fisher exact test. A two-tailed P < .05 was considered significant. Analyses were done with SAS 9.3 (SAS Institute Inc) statistical software.
Results
Study Population
Of 253 subjects screened for the parent phase 1 clinical trial, 85 were eligible, of whom 82 were assigned to IPT or observation before BCG revaccination by central, permuted block randomization after review of all screening data. Forty-two subjects were assigned to the IPT arm and 40 to the observation arm. Four subjects (three in the IPT group and one in the observation group) without a follow-up QTF-GIT test were excluded, leaving 39 subjects in each arm of the analysis.
Baseline clinical and sociodemographic characteristics are shown in Table 1. Seventy percent of the subjects were women. The median age was 26.6 years. The median baseline TST induration was 19 mm. No significant differences in baseline characteristics were observed between study groups. Active TB did not develop in any subject during the 22.5 months of scheduled study follow-up. Adherence to treatment was satisfactory. Ninety percent of subjects in the IPT group received 180 doses of INH within the parent trial protocol-specified 7-month period. Four subjects (10%) received < 6 months of IPT (range, 61-106 days). The baseline QFT-GIT blood draw was done within a median of 9 days (interquartile range, 8-11 days; range, 6-59 days) after the TST was placed.
Table 1.
—Characteristics of the Study Subjects
| Characteristic | IPT Group (n = 39) | Observation Group (n = 39) | All Subjects (N = 78) |
| Age, y | 26.3 ± 6.3 | 26.9 ± 6.3 | 26.6 ± 6.4 |
| Male sex | 12 (31) | 13 (33) | 25 (32) |
| Weight, kg | 69.8 ± 16.1 | 70.8 ± 17.1 | 70.3 ± 16.5 |
| BMI, kg/m2 | 26.0 ± 6.6 | 26.1 ± 6.3 | 26.1 ± 6.4 |
| Hemoglobin, g/dL | 14.2 ± 1.2 | 14.1 ± 1.3 | 14.2 ± 1.3 |
| Tuberculin skin test, mm induration | 19 (17-23) | 19 (17-22) | 19 (17-22) |
| Baseline QFT-GIT, IU/mL | 10.0 (5.8-10.0) | 10.0 (1.9-10.0) | 10.0 (2.5-10.0) |
| QFT-GIT at end of IPT or observation, IU/mL | 5.2 (1.7-10.0) | 4.9 (1.1-10.0) | 5.1 (1.4-10.0) |
Data are presented as mean ± SD, No. (%), or median (interquartile range). IPT = isoniazid preventive therapy; QFT-GIT = QuantiFERON-TB Gold In-Tube.
Quantitative QFT-GIT Results
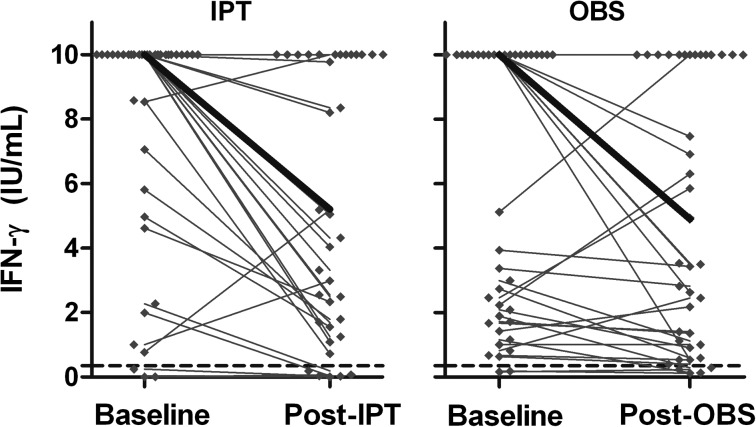
The median baseline QFT-GIT result was 10 IU/mL. As shown in Figure 1 and Table 1, IFN-γ levels declined between baseline and the end of IPT (Wilcoxon signed rank test P ≤ .0001) and observation (Wilcoxon signed rank test P = .03). The rate of decrease in IFN-γ responses over time did not differ between the groups (Mann-Whitney-Wilcoxon test P = .31).
Figure 1.
Change in IFN-γ level (antigen-stimulated value − unstimulated value) before and after IPT or OBS. IFN-γ concentrations from each subject are shown (♦). Median levels are indicated by the heavy lines. The QuantiFERON-TB Gold In-Tube diagnostic cutoff level for a positive test is shown by the dashed lines at 0.35 IU/mL. IFN-γ = interferon-γ; IPT = isoniazid preventive therapy; OBS = observation.
Qualitative QFT-GIT Results
At baseline, 36 of 39 subjects (92%) who received IPT and 37 of 39 subjects (95%) who were observed had positive QFT-GIT results. QFT-GIT results reverted from positive to negative in two subjects (5%) in the IPT group and in two subjects (5%) in the observation group. Five subjects had negative QFT-GIT results at baseline, which remained negative. There were no significant differences (Fisher exact test P = 1.0) between the groups with respect to baseline positivity or the proportion of subjects whose results reverted to negative.
Discussion
To our knowledge, there currently are no well-qualified or validated biomarkers of response to TB treatment or treatment of LTBI. Earlier studies suggested that IGRA results may correlate with MTB bacillary load and might be a potential surrogate marker of response to therapy. Several studies have shown decreases in IGRA or reversion to negative results after treatment of active TB5,23 or LTBI.6,11 Most of these reports are from small cohorts and lacked proper controls. In the present substudy from a randomized clinical trial of adults with positive TST results from a high TB incidence setting and randomly allocated to either IPT or observation before BCG revaccination, we found no differences in decline in QFT-GIT or reversion to negative results after IPT or observation alone. The results in this South African population confirm those from a recent randomized placebo-controlled trial in household contacts of patients with TB in the Gambia, which examined whether an IFN-γ enzyme-linked immunospot (ELISPOT) assay using ESAT-6 and CFP-10 antigens was a useful biomarker of treatment efficacy in LTBI.24 In the Gambia trial, which also enrolled subjects with positive TST results (median, 15.5 mm induration), the proportion of subjects with a positive ELISPOT assay and the number of median spot-forming units decreased over time during IPT and follow-up but did not differ between those treated with IPT and those receiving placebo.
Earlier uncontrolled studies reported that results in 8% to 25% of subjects receiving 6 months of INH for the treatment of LTBI reverted to negative.25‐27 The similar results of the present study in South Africa and the recent trial in the Gambia of two different IGRAs [QFT-GIT using an IFN-γ enzyme-linked immunosorbent assay using ESAT-6, CFP-10, and TB 7.7(p4) antigens and an IFN-γ ELISPOT assay using ESAT-6 and CFP-10 antigens, respectively] and randomly allocated control groups in comparable high TB incidence settings cast further doubt on the utility of IGRAs as biomarkers of response to treatment of LTBI.
The present study has several limitations. The parent clinical trial enrolled subjects with TST ≥ 15 mm to increase the likelihood that they were truly latently infected with MTB. We performed baseline QFT-GIT testing at a median of 9 days after TST. Some studies have reported increased positive IFN-γ responses after TST,28,29 whereas others have not.30,31 Leyten and colleagues32 reported no evidence of a boosting of QFT-GIT responses in subjects undergoing TST 3 days before IGRA. Although we cannot exclude the possibility that baseline QFT-GIT responses were boosted by TST, any such effect would have applied equally to subjects receiving IPT or observation alone. We assigned a value of 10 IU/mL to IFN-γ concentrations exceeding 10 IU/mL because assay standards lose linearity and predictive value outside this range.11,22,32 Performing the same analysis with original values or a subset of values within this range yielded trends similar to what is reported in the present study. Furthermore, assigning arbitrary diagnostic cutoffs is not fully consistent with the diagnostic spectrum of TB disease, latent infection, and clearance,33 especially because QFT-GIT conversion and reversion rates did not differ between the two groups. Diluting plasma samples to be within the linear range of QFT-GIT is complex owing to the difficulty of estimating appropriate dilution factors without a prior knowledge of the range of concentrations. Importantly, IFN-γ release does not distinguish between active infection and cleared infection because antigen-specific memory T cells induced by prior exposure to MTB may persist.34 These factors collectively provide evidence that IGRA performs poorly as a biomarker of infection clearance following IPT.
The persistently elevated IFN-γ responses seen in the present subjects may be due to persistent infection with latent bacilli or to reinfection with ongoing stimulation and chronic activation of effector T cells producing IFN-γ. IFN-γ responses might decline in settings with less TB transmission and little reinfection during or following IPT. We repeated QFT-GIT shortly after completion of IPT; hence, it is possible that IFN-γ responses may not have returned to normal at this time.26 Repeating the test at additional time points to better characterize the kinetics of the response might be informative.35 Exposure to environmental mycobacteria might also contribute to persistent IFN-γ responses to some mycobacterial antigens. The antigens used in the QFT-GIT assay [ESAT-6, CFP-10, and TB 7.7(p4)] were selected because they are more specific to MTB than PPD and are not shared by BCG vaccine strains and most nontuberculous mycobacteria. Other strengths of the study are the use of standardized procedures for TST and QFT-GIT testing, careful exclusion of active TB at baseline, and administration of IPT and careful follow-up in a clinical trial setting.
Active TB did not develop in any of the subjects during follow-up, and we cannot comment on QFT-GIT responses and protective efficacy against the development of TB. Additional studies with later IGRA measurements to better characterize the kinetics of IFN-γ responses after treatment of LTBI and long-term follow-up for protection of TB in high and low TB transmission settings will be required to answer these questions.
In conclusion, biomarkers of response to treatment of LTBI and protection against the development of active TB would be valuable for TB control programs, infection control services, and clinical trialists. The present results and those of other prospective studies in high-TB-burden settings suggest that in vitro IGRAs to mycobacterial antigens are unlikely to be useful for this purpose.
Acknowledgments
Author contributions: Dr Johnson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Johnson: contributed to the study design, data interpretation and analysis, and drafting and approval of the manuscript.
Dr Geldenhuys: contributed to the study design, direction of the fieldwork, data collection and analysis, and approval of the manuscript.
Ms Thiel: contributed to the study design, data analysis, and drafting and approval of the manuscript.
Ms Toefy: contributed to the study design, QuantiFERON Gold In-Tube assays, data analysis, and approval of the manuscript.
Dr Suliman: contributed to the study design, data analysis, and writing and approval of the manuscript.
Ms Pienaar: contributed to the study design, data collection and analysis, and approval of the manuscript.
Dr Chheng: contributed to the study design, data analysis, and approval of the manuscript.
Dr Scriba: contributed to the study design, supervision of the QuantiFERON Gold In-Tube assays, data analysis, and writing and approval of the manuscript.
Dr Boom: contributed to the study design, data analysis, and approval of the manuscript.
Dr Hanekom: contributed to the study design, data analysis, and approval of the manuscript.
Dr Hatherill: contributed to the study design, supervision of the field trial and data collection, data analysis, and writing and approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor was not involved in the study design, data analysis, or writing, revision, and submission of this manuscript.
Other contributions: We thank the study subjects for their participation in the study. We also thank the staff and field-workers of the South African Tuberculosis Vaccine Initiative, Worcester and Cape Town, South Africa, for diligent follow-up during the trial. We thank Charles Bark, MD, for critical review of the manuscript.
Abbreviations
- BCG
Bacillus Calmette-Guérin
- CFP
culture filtrate protein
- ELISPOT
enzyme-linked immunospot
- ESAT
early secreted antigenic target
- IFN-γ
interferon-γ
- IGRA
interferon-γ release assay
- INH
isonicotinylhydrazine
- IPT
isoniazid preventive therapy
- LTBI
latent TB infection
- MTB
Mycobacterium tuberculosis
- PPD
purified protein derivative
- QFT-GIT
QuantiFERON-TB Gold In-Tube
- TST
tuberculin skin test
Footnotes
Funding/Support: This work was supported by the Tuberculosis Research Unit, Case Western Reserve University, established with funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Department of Health and Human Services [N01-AI-95383 and HHSN266200700022C/N01-AI-70022].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25 [PubMed] [Google Scholar]
- 2. National Collaborating Centre for Chronic Conditions; Centre for Clinical Practice at NICE. Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Clinical Guidelines, No. 117. London, England: National Institute for Health and Clinical Excellence; 2011.
- 3.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149(3):177-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004;38(5):754-756 [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro S, Dooley K, Hackman J, et al. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC Infect Dis. 2009;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, Wang YT. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am J Respir Crit Care Med. 2007;175(3):282-287 [DOI] [PubMed] [Google Scholar]
- 7.Chiappini E, Bonsignori F, Mangone G, et al. Serial T-SPOT.TB and Quantiferon-TB-Gold In-Tube assays to monitor response to antitubercular treatment in Italian children with active or latent tuberculosis infection. Pediatr Infect Dis J. 2012;31(9):974-977 [DOI] [PubMed] [Google Scholar]
- 8.Chiappini E, Fossi F, Bonsignori F, Sollai S, Galli L, de Martino M. Utility of interferon-γ release assay results to monitor anti-tubercular treatment in adults and children. Clin Ther. 2012;34(5):1041-1048 [DOI] [PubMed] [Google Scholar]
- 9.Dheda K, Pooran A, Pai M, et al. Interpretation of Mycobacterium tuberculosis antigen-specific IFN-gamma release assays (T-SPOT.TB) and factors that may modulate test results. J Infect. 2007;55(2):169-173 [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Lee CT, Yim JJ. Serial interferon-gamma release assays during treatment of active tuberculosis in young adults. BMC Infect Dis. 2010;10:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SW, Lee SH, Yim JJ. Serial interferon-gamma release assays after chemoprophylaxis in a tuberculosis outbreak cohort. Infection. 2012;40(4):431-435 [DOI] [PubMed] [Google Scholar]
- 12.Hougardy JM, Place S, Hildebrand M, et al. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med. 2007;176(4):409-416 [DOI] [PubMed] [Google Scholar]
- 13.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134(3):713-740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102(11):4033-4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walburger A, Koul A, Ferrari G, et al. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304(5678):1800-1804 [DOI] [PubMed] [Google Scholar]
- 16.Xu S, Cooper A, Sturgill-Koszycki S, et al. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153(6):2568-2578 [PubMed] [Google Scholar]
- 17.Fulton SA, Reba SM, Pai RK, et al. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect Immun. 2004;72(4):2101-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect Immun. 2003;71(8):4487-4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandra L, Noss E, Boom WH, Harding CV. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J Exp Med. 2001;194(10):1421-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson KA, Kon OM, Newton SM, et al. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis. 2006;193(3):354-359 [DOI] [PubMed] [Google Scholar]
- 21.Mahomed H, Hughes EJ, Hawkridge T, et al. Comparison of mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int J Tuberc Lung Dis. 2006;10(3):310-316 [PubMed] [Google Scholar]
- 22.QuantiFERON-TB Gold [package insert]. Valencia, CA: Cellestis Inc; 2013. Cellestis website. http://www.cellestis.com/IRM/content/PI/us.pdf. Accessed May 6, 2013
- 23.Adetifa IM, Ota MO, Walther B, et al. Decay kinetics of an interferon gamma release assay with anti-tuberculosis therapy in newly diagnosed tuberculosis cases. PLoS ONE. 2010;5(9):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adetifa IM, Ota MO, Jeffries DJ, et al. Interferon-γ ELISPOT as a biomarker of treatment efficacy in latent tuberculosis infection: a clinical trial. Am J Respir Crit Care Med. 2013;187(4):439-445 [DOI] [PubMed] [Google Scholar]
- 25.Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361(9364):1168-1173 [DOI] [PubMed] [Google Scholar]
- 26.Higuchi K, Harada N, Mori T. Effect of prophylaxis on responses for ESAT-6/CFP-10 in whole blood IFN-g test. Eur Respir J. 2005;26(suppl 49):22s [Google Scholar]
- 27.Higuchi K, Okada K, Harada N, Mori T. Effects of prophylaxis on QuantiFERON TB-2G responses among children [in Japanese]. Kekkaku. 2008;83(9):603-609 [PubMed] [Google Scholar]
- 28.Baker CA, Thomas W, Stauffer WM, Peterson PK, Tsukayama DT. Serial testing of refugees for latent tuberculosis using the QuantiFERON-gold in-tube: effects of an antecedent tuberculin skin test. Am J Trop Med Hyg. 2009;80(4):628-633 [PubMed] [Google Scholar]
- 29.Park JS, Lee JS, Kim MY, et al. Monthly follow-ups of interferon-γ release assays among health-care workers in contact with patients with TB. Chest. 2012;142(6):1461-1468 [DOI] [PubMed] [Google Scholar]
- 30.Richeldi L, Bergamini BM, Vaienti F. Prior tuberculin skin testing does not boost QuantiFERON-TB results in paediatric contacts. Eur Respir J. 2008;32(2):524-525 [DOI] [PubMed] [Google Scholar]
- 31.Richeldi L, Ewer K, Losi M, Roversi P, Fabbri LM, Lalvani A. Repeated tuberculin testing does not induce false positive ELISPOT results. Thorax. 2006;61(2):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyten EM, Prins C, Bossink AW, et al. Effect of tuberculin skin testing on a Mycobacterium tuberculosis-specific interferon-gamma assay. Eur Respir J. 2007;29(6):1212-1216 [DOI] [PubMed] [Google Scholar]
- 33.Barry CE, III, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borgström E, Andersen P, Andersson L, et al. Detection of proliferative responses to ESAT-6 and CFP-10 by FASCIA assay for diagnosis of Mycobacterium tuberculosis infection. J Immunol Methods. 2011;370(1-2):55-64 [DOI] [PubMed] [Google Scholar]
- 35.van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med. 2009;180(1):49-58 [DOI] [PubMed] [Google Scholar]