Abstract
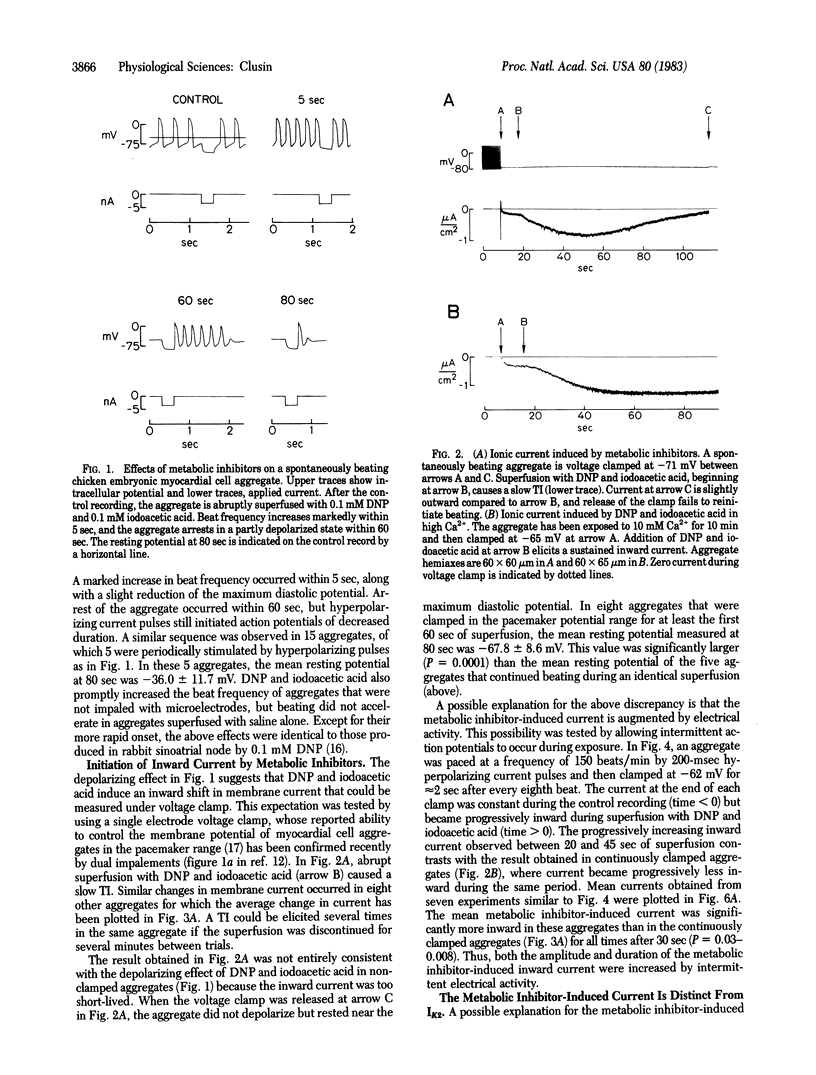
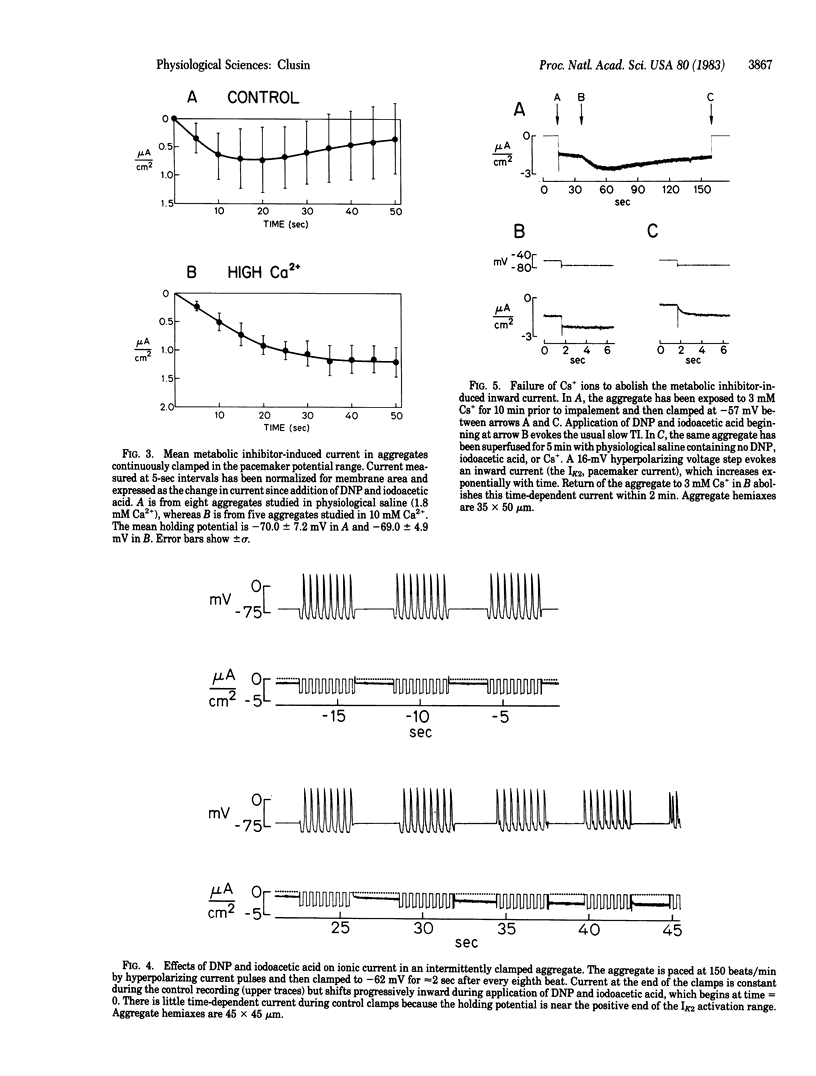
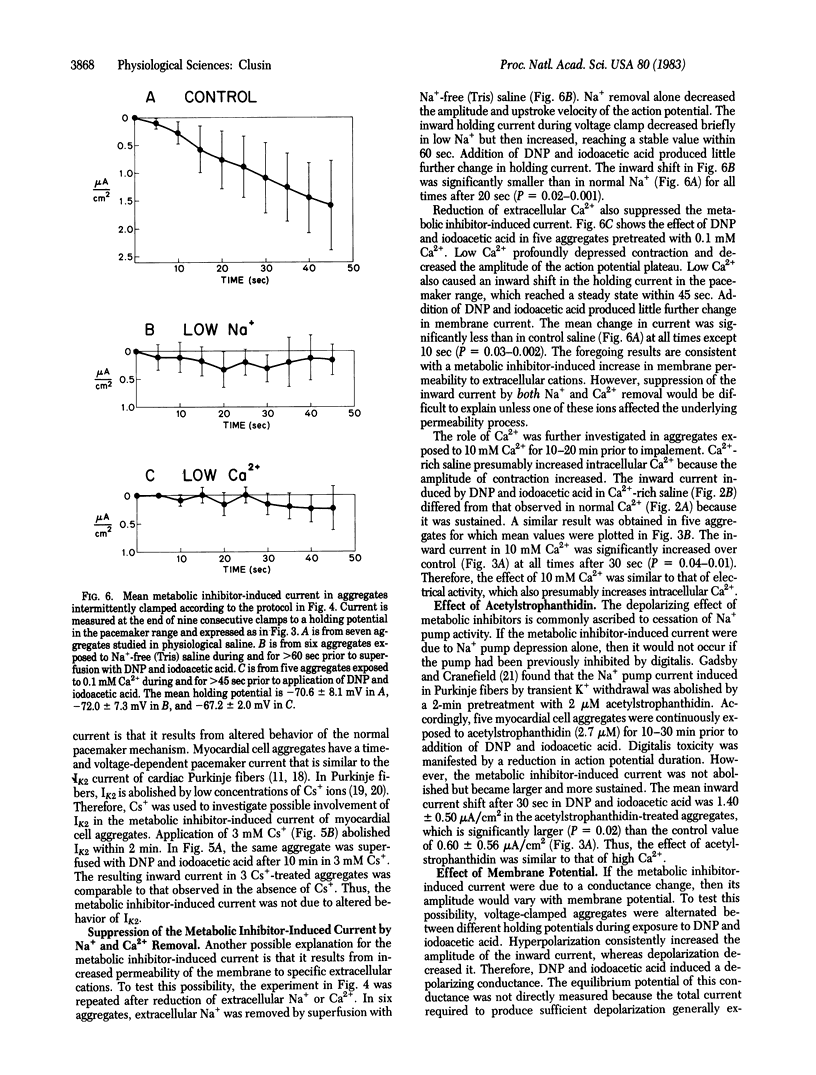
To elucidate the means by which metabolic inhibition depolarizes cardiac cells, spontaneously beating chicken embryonic myocardial cell aggregates were voltage clamped during superfusion with 2,4-dinitrophenol and iodoacetic acid. In aggregates continuously clamped in the pacemaker potential range, abrupt exposure to these metabolic inhibitors produced a slow transient inward current. This inward current was not due to an alteration of the pacemaker current, IK2, because it could still be elicited after IK2 was abolished by Cs+ ions. The inward current was increased by hyperpolarization and decreased by depolarization. It became larger and more sustained if intermittent action potentials were allowed during exposure or if the aggregates were pretreated with either 10 mM Ca2+ or 2.7 microM acetylstrophanthidin. The inward current was suppressed by removal of extracellular Na+ or Ca2+. These observations suggest that early depolarization of cultured cardiac cells by metabolic inhibitors involves some of the same mechanisms as the transient inward current of digitalis toxicity--specifically, an effect of intracellular Ca2+ ions on membrane permeability. Similar phenomena could occur during other forms of metabolic inhibition such as myocardial ischemia.
Full text
PDF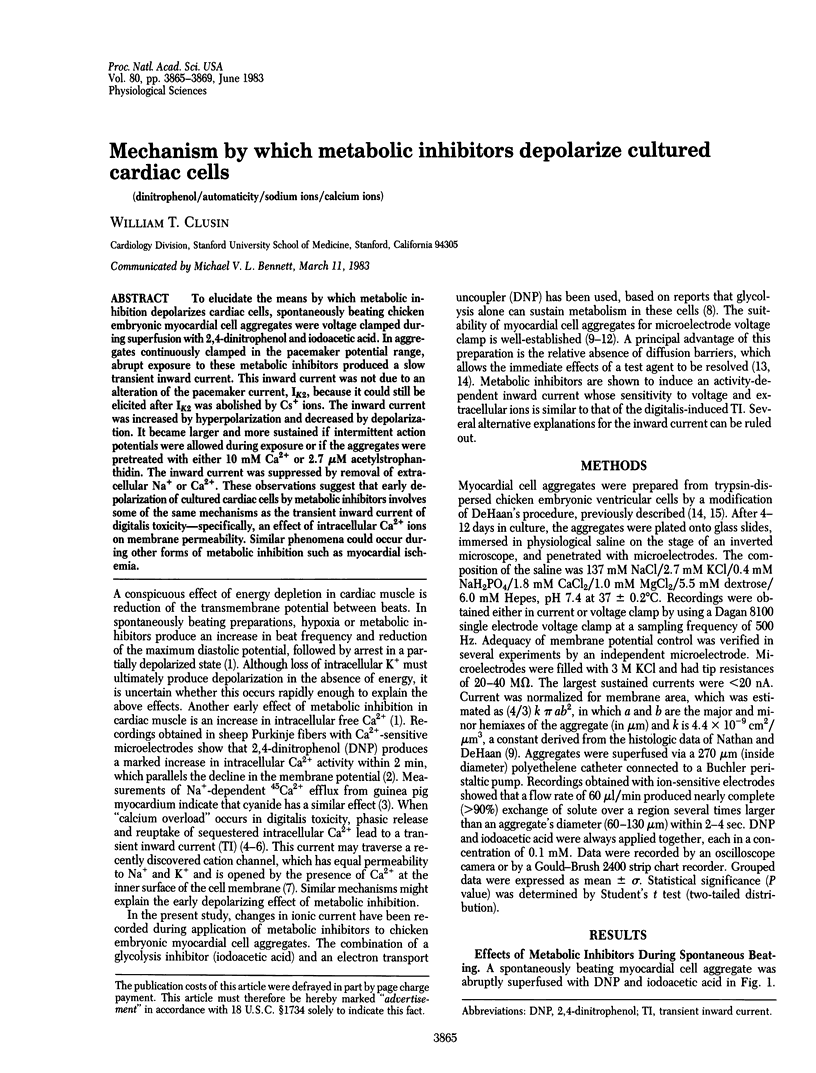

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry W. H., Pober J., Marsh J. D., Frankel S. R., Smith T. W. Effects of graded hypoxia on contraction of cultured chick embryo ventricular cells. Am J Physiol. 1980 Nov;239(5):H651–H657. doi: 10.1152/ajpheart.1980.239.5.H651. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac transmembrane potentials and metabolism. Circ Res. 1978 May;42(5):577–587. doi: 10.1161/01.res.42.5.577. [DOI] [PubMed] [Google Scholar]
- Clay J. R., Shrier A. Developmental changes in subthreshold pace-maker currents in chick embryonic heart cells. J Physiol. 1981 Mar;312:491–504. doi: 10.1113/jphysiol.1981.sp013640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T., Bristow M. R., Baim D. S., Schroeder J. S., Jaillon P., Brett P., Harrison D. C. The effects of diltiazem and reduced serum ionized calcium on ischemic ventricular fibrillation in the dog. Circ Res. 1982 Apr;50(4):518–526. doi: 10.1161/01.res.50.4.518. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Buchbinder M., Harrison D. C. Calcium overload, "injury" current, and early ischaemic cardiac arrhythmias--a direct connection. Lancet. 1983 Feb 5;1(8319):272–274. doi: 10.1016/s0140-6736(83)91688-4. [DOI] [PubMed] [Google Scholar]
- Clusin W. T. Caffeine induces a transient inward current in cultured cardiac cells. Nature. 1983 Jan 20;301(5897):248–250. doi: 10.1038/301248a0. [DOI] [PubMed] [Google Scholar]
- Clusin W. T. Correlation between relaxation and automaticity in embryonic heart cell aggregates. Proc Natl Acad Sci U S A. 1980 Jan;77(1):679–683. doi: 10.1073/pnas.77.1.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T., Hamilton W. E., Nelson D. V. The mechanical activity of chick embryonic myocardial cell aggregates. J Physiol. 1981 Nov;320:149–174. doi: 10.1113/jphysiol.1981.sp013941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- DE MELLO W. C. Metabolism and electrical activity of the heart: action of 2-4-dinitrophenol and ATP. Am J Physiol. 1959 Feb;196(2):377–380. doi: 10.1152/ajplegacy.1959.196.2.377. [DOI] [PubMed] [Google Scholar]
- Dahl G., Isenberg G. Decoupling of heart muscle cells: correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J Membr Biol. 1980 Mar 31;53(1):63–75. doi: 10.1007/BF01871173. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pace-maker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. J Physiol. 1982 Aug;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Direct measurement of changes in sodium pump current in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1783–1787. doi: 10.1073/pnas.76.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Smith I., Purves R. D. Synaptic delay in the heart: an ionophoretic study. J Physiol. 1978 Jun;279:31–54. doi: 10.1113/jphysiol.1978.sp012329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibers: cesium as a tool to block inward rectifying potassium currents. Pflugers Arch. 1976 Sep 30;365(2-3):99–106. doi: 10.1007/BF01067006. [DOI] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagueuzian H. S., Katzung B. G. Voltage-clamp studies of transient inward current and mechanical oscillations induced by ouabain in ferret papillary muscle. J Physiol. 1982 Jun;327:255–271. doi: 10.1113/jphysiol.1982.sp014230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung B. G., Hondeghem L. M., Grant A. O. Letter: Cardiac ventricular automaticity induced by current of injury. Pflugers Arch. 1975 Oct 28;360(2):193–197. doi: 10.1007/BF00580542. [DOI] [PubMed] [Google Scholar]
- Lantz R. C., Elsas L. J., DeHaan R. L. Ouabain-resistant hyperpolarization induced by insulin in aggregates of embryonic heart cells. Proc Natl Acad Sci U S A. 1980 May;77(5):3062–3066. doi: 10.1073/pnas.77.5.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. Voltage clamp analysis of embryonic heart cell aggregates. J Gen Physiol. 1979 Feb;73(2):175–198. doi: 10.1085/jgp.73.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier A., Clay J. R. Comparison of the pacemaker properties of chick embryonic atrial and ventricular heart cells. J Membr Biol. 1982;69(1):49–56. doi: 10.1007/BF01871241. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Mugelli A. An oscillatory current in sheep cardiac Purkinje fibers. Circ Res. 1981 May;48(5):618–631. doi: 10.1161/01.res.48.5.618. [DOI] [PubMed] [Google Scholar]


