Abstract
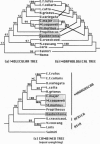
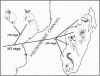
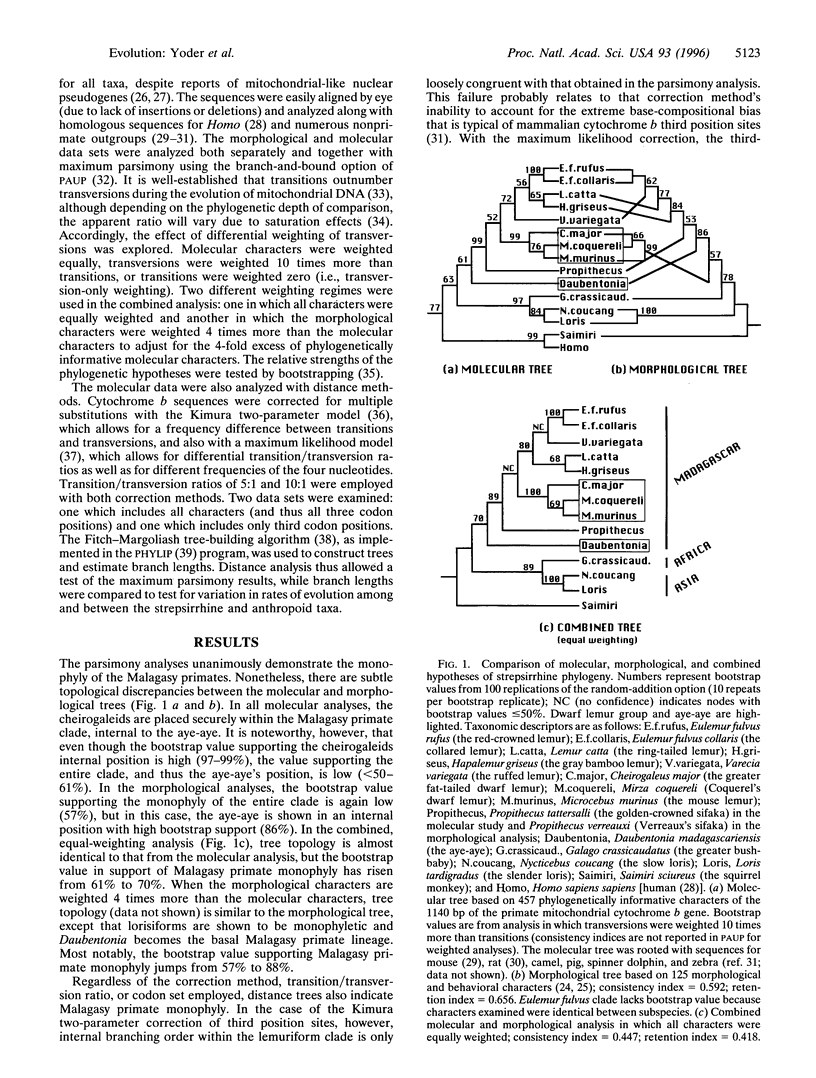
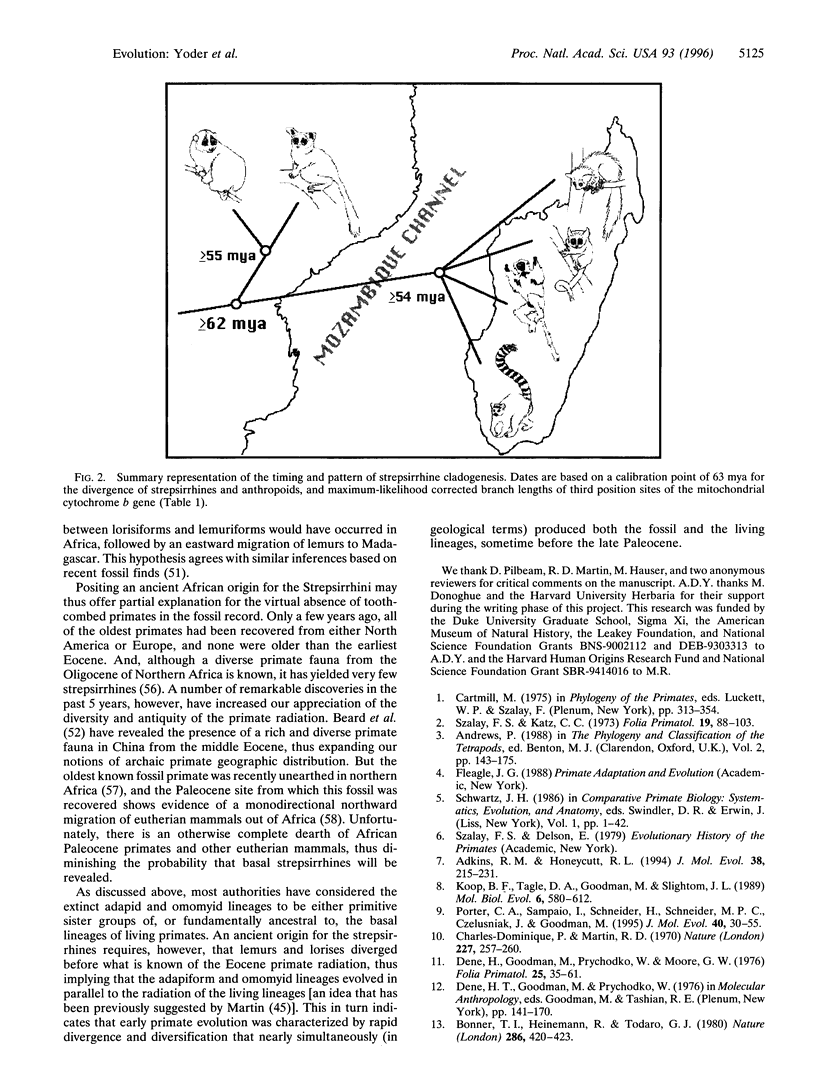
We report new evidence that bears decisively on a long-standing controversy in primate systematics. DNA sequence data for the complete cytochrome b gene, combined with an expanded morphological data set, confirm the results of a previous study and again indicate that all extant Malagasy lemurs originated from a single common ancestor. These results, as well as those from other genetic studies, call for a revision of primate classifications in which the dwarf and mouse lemurs are placed within the Afro-Asian lorisiforms. The phylogenetic results, in agreement with paleocontinental data, indicate an African origin for the common ancestor of lemurs and lorises (the Strepsirrhini). The molecular data further suggest the surprising conclusion that lemurs began evolving independently by the early Eocene at the latest. This indicates that the Malagasy primate lineage is more ancient than generally thought and places the split between the two strepsirrhine lineages well before the appearance of known Eocene fossil primates. We conclude that primate origins were marked by rapid speciation and diversification sometime before the late Paleocene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins R. M., Honeycutt R. L. Evolution of the primate cytochrome c oxidase subunit II gene. J Mol Evol. 1994 Mar;38(3):215–231. doi: 10.1007/BF00176084. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bailey W. J., Fitch D. H., Tagle D. A., Czelusniak J., Slightom J. L., Goodman M. Molecular evolution of the psi eta-globin gene locus: gibbon phylogeny and the hominoid slowdown. Mol Biol Evol. 1991 Mar;8(2):155–184. doi: 10.1093/oxfordjournals.molbev.a040641. [DOI] [PubMed] [Google Scholar]
- Beard K. C., Dagosto M., Gebo D. L., Godinot M. Interrelationships among primate higher taxa. Nature. 1988 Feb 25;331(6158):712–714. doi: 10.1038/331712a0. [DOI] [PubMed] [Google Scholar]
- Beard K. C., Qi T., Dawson M. R., Wang B., Li C. A diverse new primate fauna from middle Eocene fissure-fillings in southeastern China. Nature. 1994 Apr 14;368(6472):604–609. doi: 10.1038/368604a0. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Heinemann R., Todaro G. J. Evolution of DNA sequences has been retarded in Malagasy primates. Nature. 1980 Jul 24;286(5771):420–423. doi: 10.1038/286420a0. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Charles-Dominique P., Martin R. D. Evolution of lorises and lemurs. Nature. 1970 Jul 18;227(5255):257–260. doi: 10.1038/227257a0. [DOI] [PubMed] [Google Scholar]
- Collura R. V., Stewart C. B. Insertions and duplications of mtDNA in the nuclear genomes of Old World monkeys and hominoids. Nature. 1995 Nov 30;378(6556):485–489. doi: 10.1038/378485a0. [DOI] [PubMed] [Google Scholar]
- Dene H., Goodman M., Prychodko W., Moore G. W. Immunodiffusion systematics of the primates. III. The strepsirhini. Folia Primatol (Basel) 1976;25(1):35–61. doi: 10.1159/000155706. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989 Jun;28(6):497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Gingerich P. D. Primate evolution. African dawn for primates. Nature. 1990 Aug 2;346(6283):411–411. doi: 10.1038/346411a0. [DOI] [PubMed] [Google Scholar]
- Irwin D. M., Kocher T. D., Wilson A. C. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991 Feb;32(2):128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Koop B. F., Tagle D. A., Goodman M., Slightom J. L. A molecular view of primate phylogeny and important systematic and evolutionary questions. Mol Biol Evol. 1989 Nov;6(6):580–612. doi: 10.1093/oxfordjournals.molbev.a040574. [DOI] [PubMed] [Google Scholar]
- Martin R. D. Primate origins: plugging the gaps. Nature. 1993 May 20;363(6426):223–234. doi: 10.1038/363223a0. [DOI] [PubMed] [Google Scholar]
- Porter C. A., Sampaio I., Schneider H., Schneider M. P., Czelusniak J., Goodman M. Evidence on primate phylogeny from epsilon-globin gene sequences and flanking regions. J Mol Evol. 1995 Jan;40(1):30–55. doi: 10.1007/BF00166594. [DOI] [PubMed] [Google Scholar]
- Rabinowitz P. D., Coffin M. F., Falvey D. The separation of madagascar and Africa. Science. 1983 Apr 1;220(4592):67–69. doi: 10.1126/science.220.4592.67. [DOI] [PubMed] [Google Scholar]
- Rumpler Y., Warter S., Petter J. J., Albignac R., Dutrillaux B. Chromosomal evolution of Malagasy lemurs. XI. Phylogenetic position of Daubentonia madagascariensis. Folia Primatol (Basel) 1988;50(1-2):124–129. doi: 10.1159/000156336. [DOI] [PubMed] [Google Scholar]
- Ruvolo M., Zehr S., von Dornum M., Pan D., Chang B., Lin J. Mitochondrial COII sequences and modern human origins. Mol Biol Evol. 1993 Nov;10(6):1115–1135. doi: 10.1093/oxfordjournals.molbev.a040068. [DOI] [PubMed] [Google Scholar]
- Simons E. L., Rasmussen D. T. A remarkable cranium of Plesiopithecus teras (Primates, Prosimii) from the Eocene of Egypt. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9946–9950. doi: 10.1073/pnas.91.21.9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay F. S., Katz C. C. Phylogeny of lemurs, galagos and lorises. Folia Primatol (Basel) 1973;19(2):88–103. doi: 10.1159/000155535. [DOI] [PubMed] [Google Scholar]
- Yoder A. D. Relative position of the Cheirogaleidae in strepsirhine phylogeny: a comparison of morphological and molecular methods and results. Am J Phys Anthropol. 1994 May;94(1):25–46. doi: 10.1002/ajpa.1330940104. [DOI] [PubMed] [Google Scholar]
- Zischler H., Geisert H., von Haeseler A., Päbo S. A nuclear 'fossil' of the mitochondrial D-loop and the origin of modern humans. Nature. 1995 Nov 30;378(6556):489–492. doi: 10.1038/378489a0. [DOI] [PubMed] [Google Scholar]