Abstract
Emerging evidence indicates that cancer is primarily a metabolic disease involving disturbances in energy production through respiration and fermentation. The genomic instability observed in tumor cells and all other recognized hallmarks of cancer are considered downstream epiphenomena of the initial disturbance of cellular energy metabolism. The disturbances in tumor cell energy metabolism can be linked to abnormalities in the structure and function of the mitochondria. When viewed as a mitochondrial metabolic disease, the evolutionary theory of Lamarck can better explain cancer progression than can the evolutionary theory of Darwin. Cancer growth and progression can be managed following a whole body transition from fermentable metabolites, primarily glucose and glutamine, to respiratory metabolites, primarily ketone bodies. As each individual is a unique metabolic entity, personalization of metabolic therapy as a broad-based cancer treatment strategy will require fine-tuning to match the therapy to an individual’s unique physiology.
Introduction
Cancer is a disease involving multiple time- and space-dependent changes in the health status of cells and tissues that ultimately lead to malignant tumors. Neoplasia (abnormal cell growth) is the biological endpoint of the disease. Tumor cell invasion into surrounding tissues and their spread (metastasis) to distant organs is the primary cause of morbidity and mortality of most cancer patients (1–5). A major impediment in the effort to control cancer has been due in large part to the confusion surrounding the origin of the disease. Contradictions and paradoxes continue to plague the field (6–10). Much of the confusion surrounding cancer origin arises from the absence of a unifying theory that can integrate the many diverse observations on the nature of the disease. Without a clear understanding of how cancer arises, it becomes difficult to formulate a successful strategy for effective long-term management and prevention. The failure to clearly define the origin of cancer is responsible in large part for the failure to significantly reduce the death rate from the disease (2). Although cancer metabolism is receiving increased attention, cancer is generally considered a genetic disease (10,11). This general view is now under serious reevaluation (2,12). The information in this review comes in part from our previous articles and treatise on the subject (2,13–17).
Provocative question: does cancer arise from somatic mutations?
Most of those who conduct academic research on cancer would consider it a type of somatic genetic disease where damage to a cell’s nuclear DNA underlies the transformation of a normal cell into a potentially lethal cancer cell (7,10,11,18). Abnormalities in dominantly expressed oncogenes and in recessively expressed tumor suppressor genes have been the dogma driving the field for several decades (7,10). The discovery of millions of gene changes in different cancers has led to the perception that cancer is not a single disease, but is a collection of many different diseases (6,11,19,20). Consideration of cancer as a ‘disease complex’ rather than as a single disease has contributed to the notion that management of the various forms of the disease will require individual or ‘personalized’ drug therapies (2,21–23). Tailored therapies, unique to the genomic defects within individual tumors, are viewed as the future of cancer therapeutics (2,24). This therapeutic strategy would certainly be logical if the nuclear somatic mutations detected in tumors were the drivers of the disease. How certain are we that tumors arise from somatic mutations and that some of these mutations drive the disease? It would therefore be important to revisit the origin of the gene theory of cancer.
The gene theory of cancer originated with Theodor Boveri’s suggestion in 1914 that cancer could arise from defects in the segregation of chromosomes during cell division (18,25–29). As chromosomal instability in the form of aneuploidy (extra chromosomes, missing chromosomes or broken chromosomes) is present in many tumor tissues (21,30–32), it was logical to extend these observations to somatic mutations within individual genes including oncogenes and tumor suppressor genes (18,33–36). Boveri’s hypothesis on the role of chromosomes in the origin of malignancy was based primarily on his observations of chromosome behavior in nematodes (Ascaris) and sea urchins (Paracentrotus) and from his consideration of von Hansemann’s earlier observations of abnormal chromosome behavior in tumors (18,25,29). In contrast to Boveri’s view of aneuploidy as the origin of cancer, von Hansemann considered the abnormal chromosome behavior in tumors as an effect rather than as a cause of the disease (25). Although Boveri’s hypothesis emerged as the foundation for the somatic mutation theory of cancer, it appears that he never directly experimented on the disease (18,25,29). The reason for the near universal acceptance of Boveri’s hypothesis for the origin of cancer is not clear but might have been linked to his monumental achievement in showing that Gregor Mendel’s abstract heredity factors resided on chromosomes (29). Boveri’s cancer theory was also consistent with the gradual accumulation of evidence showing that DNA abnormalities are abundant in cancer cells.
In his 2002 review, Knudson stated that, ‘considerable evidence has been amassed in support of Boveri’s early hypothesis that cancer is a somatic genetic disease’ (37). The seeds of the somatic mutation theory of cancer might have been sowed even before the work of von Hansemann and Boveri. Virchow considered that cancer cells arose from other cancer cells (38). Robert Wagner provided a good overview of those early studies leading to the idea that somatic mutations give rise to cancer (38). It gradually became clear that almost every kind of genomic defect could be found in tumor cells whether or not the mutations were connected to carcinogenesis (10,11,18,26,31). The current somatic mutation theory involves a genomic landscape of incomprehensible complexity that also includes mysterious genomic ‘Dark Matter’ (2,10,11,19). Although massive evidence exists showing that genomic instability is present to some degree in all tumor cells, it is unclear how this phenotype relates to the origin of the disease. It appears that almost every neoplastic cell within a naturally arising human tumor is heterogeneous in containing a unique genetic architecture (31).
Inconsistencies with a nuclear gene origin of cancer
The distinguished British geneticist, C.D.Darlington (39), was one of the first to raise concerns regarding the nuclear genetic origin of cancer. Based on several inconsistencies in the association of mutagens with cancer, Darlington argued persuasively that nuclear genomic defects could not be the origin of cancer. Rather, he was convinced that cancer cells arose from defects in cytoplasmic elements, which he referred to as ‘plasmagenes’. Although Darlington did not specifically characterize the nature of the plasmagene, several characteristics of the plasmagenes suggested that they were mitochondria. It was unclear, however, if the radiation damage to the plasmagenes acted alone in causing cancer or also acted in conjunction with mutations in nuclear genes.
Inconsistencies regarding the somatic nuclear gene theory of cancer also come from nuclear/cytoplasmic transfer experiments between tumorigenic and non-tumorigenic cells. Several investigators showed that tumorigenicity is suppressed when cytoplasm from non-tumorigenic cells, containing normal mitochondria, is combined with nuclei from tumor cells (40–44). Moreover, the in vivo tumorigenicity of multiple human and animal tumor types is suppressed when the nucleus from the tumor cell is introduced into the cytoplasm of a non-tumorigenic cell (45–48). Tumors generally did not form despite the continued presence of the tumor-associated mutations. The nuclear gene mutations documented in mouse brain tumors and melanomas were also detected in the normal embryonic tissues of the mice derived from the tumor nuclei (47,48). Some embryos derived from tumor nuclei, which contained major chromosomal imbalances, proceeded through early development forming normal appearing tissues before dying. Despite the presence of tumor-associated aneuploidy and somatic mutations, tumors did not develop from these tumor-derived nuclei (49). Boveri also found that sea urchin embryos with chromosomal imbalances developed normally to gastrulation but then aborted (25,29). Hochedlinger et al. (48) showed that nuclei derived from melanoma cells were unable to direct complete mouse development due presumably to the chromosomal imbalances and irreversible tumor-associated mutations in the melanoma nucleus. Tumors did not arise in the embryos derived from the melanoma nuclei. These findings suggest that the nuclear genomic defects in these tumor cells have more to do with directing development than with causing tumors.
More recent mitochondrial transfer experiments support the general findings of the nuclear transfer experiments (50,51). The tumorigenic phenotype is suppressed when normal mitochondria are transferred to the tumor cell cytoplasm. On the other hand, the tumorigenic phenotype is enhanced when tumor mitochondria are transferred to a normal cell cytoplasm. These findings further suggest that tumorigenesis is dependent more on mitochondrial function than on the types of mutations in the nucleus.
In contrast to the suppressive effects of normal mitochondria on tumorigenicity, tumorigenicity is enhanced when nuclei of non-tumorigenic cells are combined with cytoplasm from tumor cells (52,53). These observations are consistent with the original view of Darlington that tumor cells arise from defects in the cytoplasm rather than from defects in the nucleus (39). Wallace et al. (53) also showed that introduction of mitochondrial DNA mutations into non-tumorigenic cybrids could reverse the anti-tumorigenic effect of normal mitochondria leading to the conclusion that cancer can be best defined as a type of mitochondrial disease. The nuclear transfer studies are summarized in Figure 1, highlighting the role of the mitochondria in suppressing tumorigenesis. These studies also raise questions regarding the role of somatic mutations as drivers of tumorigenesis. Further studies will be needed to determine whether tumors arise from defects in the nuclear genome alone or in the mitochondria alone, or require defects in both the mitochondria and the nuclear genome. Such studies will provide evidence for or against the nuclear gene driver hypothesis of cancer initiation.
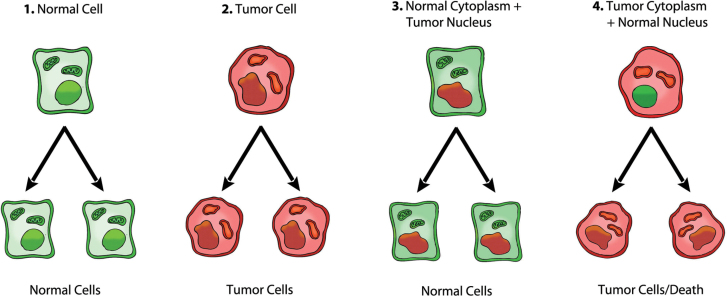
Fig. 1.
Role of the nucleus and mitochondria in the origin of tumors. This image summarizes the experimental evidence supporting a dominant role of the mitochondria in the origin of tumorigenesis as described previously (49). Normal cells are depicted in green with mitochondrial and nuclear morphology indicative of normal respiration and nuclear gene expression, respectively. Tumor cells are depicted in red with abnormal mitochondrial and nuclear morphology indicative of abnormal respiration and genomic instability. (1) Normal cells beget normal cells. (2) Tumor cells beget tumor cells. (3) Delivery of a tumor cell nucleus into a normal cell cytoplasm begets normal cells despite the persistence of tumor-associated genomic abnormalities. (4) Delivery of a normal cell nucleus into a tumor cell cytoplasm begets tumor cells or dead cells but not normal cells. The results suggest that tumors do not arise from nuclear genomic defects alone and that normal mitochondria can suppress tumorigenesis. Original diagram from Jeffrey Ling and Thomas N. Seyfried, with permission.
Respiratory insufficiency as the origin of cancer and the ‘Warburg effect’
Otto Warburg (54,55) first proposed that all cancers originate from dysfunctional cellular respiration. Warburg stated,
Just as there are many remote causes of plague, heat, insects, rats, but only one common cause, the plague bacillus, there are a great many remote causes of cancer-tar, rays, arsenic, pressure, urethane- but there is only one common cause into which all other causes of cancer merge, the irreversible injuring of respiration.
The key points of Warburg’s theory are (i) insufficient respiration initiates tumorigenesis and ultimately cancer, (ii) energy through glycolysis gradually compensates for insufficient energy through respiration, (iii) cancer cells continue to ferment lactate in the presence of oxygen and (iv) respiratory insufficiency eventually becomes irreversible (54–58). Efraim Racker (59) was the first to describe the increased aerobic glycolysis seen in cancer cells as the ‘Warburg effect’. Warburg, however, referred to the phenomenon in cancer cells as ‘aerobic fermentation’ to highlight the abnormal production of lactate in the presence of oxygen (54–58). As lactate production is widely recognized as an indicator of respiratory insufficiency in biological systems (60), Warburg also viewed the aerobic production of lactate in tumor cells as an indicator of respiratory insufficiency.
A deficiency in oxidative phosphorylation (OxPhos) energy is responsible for lactate production in most cases (61,62). For example, muscle cells significantly increase their metabolic rate during intense exercise and as a result oxygen becomes limiting. The oxygen deficiency causes a lack of energy through OxPhos prompting lactate production in an effort to provide compensatory energy from fermentation (glycolytic energy) (60). A competing argument would be that OxPhos is not insufficient during intense exercise and that aerobic fermentation is needed to provide more energy and growth metabolites in response to the increased work demand. This would be similar to the suggestion of Weinhouse and others for the increased aerobic glycolysis in tumor cells (63,64). Indeed, Kopennol et al. (64) suggest that the increased lactate production in tumor cells arises from damage to the regulation of glycolysis and not to insufficient respiration. However, the competing argument is inconsistent with the observation that the lactate made by muscle cells during intense exercise falls significantly after oxygen is restored to the muscle tissue. This would indicate that the lactate was made primarily because O2 was unavailable for robust OxPhos (65). In addition, oxygen deprivation or hypoxia causes all known cultured mammalian cells to increase lactate production (66–68). An increase in lactate is also seen in adequately oxygenated cells when respiration is inhibited either by respiratory poisons or null mutations in key respiratory enzymes (69–71). It is therefore clear from established bioenergetic principles that the excess lactate made by most mammalian cells is needed to sustain fermentation energy in order to compensate for insufficient energy from respiration. It is our view that tumor cells are not an exception to this general principle and that their lactate production results in part from insufficient respiratory activity. It is expected that an upregulation of glycolytic genes would be needed to facilitate compensatory energy production through glycolysis when cellular respiration is deficient for protracted periods (56). The reduction of pyruvate to lactate is needed to enhance the glycolytic pathway when respiration becomes insufficient.
It is important to recognize that pyruvate is produced through aerobic glycolysis in most normal cells of the body that use glucose for energy. The reduction of pyruvate to lactate distinguishes the tumor cells from most normal cells, which fully oxidize pyruvate to CO2 and water for adenosine triphosphate (ATP) production through the tricarboxylic acid (TCA) cycle and the electron transport chain (56). Aerobic glycolysis with lactate production can occur in normal retina though more ATP is produced through respiration than through glycolysis, as is the case in most respiring tissues (72). On the other hand, enhanced aerobic glycolysis without significant lactate production or energy through fermentation can occur in normal cardiac and brain tissues under conditions of increased activity (73–75). The slight transient increase in lactate production under these conditions is not associated with a significant increase in total energy production. As enhanced aerobic glycolysis does not produce significant lactate in normal cells under well-oxygenated conditions, a phenotype of enhanced aerobic glycolysis is therefore not synonymous with a Warburg effect.
Lactate will be produced in normal tissues under low oxygen conditions. Tumor cells also produce lactate under hypoxia through anaerobic glycolysis. Although many investigators of tumor cell energy metabolism use the term ‘aerobic glycolysis’ in referring to the Warburg effect, we consider the term ‘aerobic fermentation’ as a more accurate description of the Warburg effect since aerobic glycolysis occurs in most normal cells of the body. A key issue is whether the lactate produced in tumor cells under aerobic conditions results from insufficient respiration as Warburg proposed or is due to some other phenomenon. The origin of the Warburg effect is an issue of controversy that persists today despite Warburg’s data showing that it arose from insufficient respiration.
According to Warburg and Burk respiratory insufficiency together with lactate production are the key features of tumor cell energy metabolism (55,57,76,77). Respiratory insufficiency as the origin of tumorigenesis has remained controversial, however, due to observations of high oxygen consumption rates in many tumor cells (63,64,78–82). It is generally assumed that oxygen consumption rate is a good indicator of cellular respiration and OxPhos. Although low oxygen consumption rate seen together with high lactate production can be indicative of insufficient respiration, high oxygen consumption might not be indicative of sufficient respiration especially if lactate is also produced. It is now recognized from numerous studies that oxygen consumption rates are not always linked to a normally coupled oxidative phosphorylation (83–86). It can be difficult to determine the degree to which mitochondrial ATP production arises from coupled respiration or from TCA cycle substrate level phosphorylation (87–90). The origin of mitochondrial ATP production in tumor cells requires further clarification in light of these issues.
Mitochondrial structure is intimately connected to mitochondrial function. This fact cannot be overemphasized. We have reviewed substantial evidence of morphological, proteomic, and lipidomic abnormalities in mitochondria of numerous types of cancer cells (17,85,91). Tumor cells can have abnormalities in both the content and composition of their mitochondria. The work of Arismendi-Morillo and Oudard et al. showed that the ultrastructure of tumor tissue mitochondria differs markedly from the ultrastructure of normal tissue mitochondria (17,92–94). In contrast to normal mitochondria, which contain numerous cristae, mitochondria from tumor tissue samples showed swelling with partial or total cristolysis (Figure 2). Cristae contain the proteins of the respiratory complexes and play an essential structural role in facilitating energy production through OxPhos (95). The structural defects in human glioma mitochondria are also consistent with lipid biochemical defects in murine gliomas (96,97).
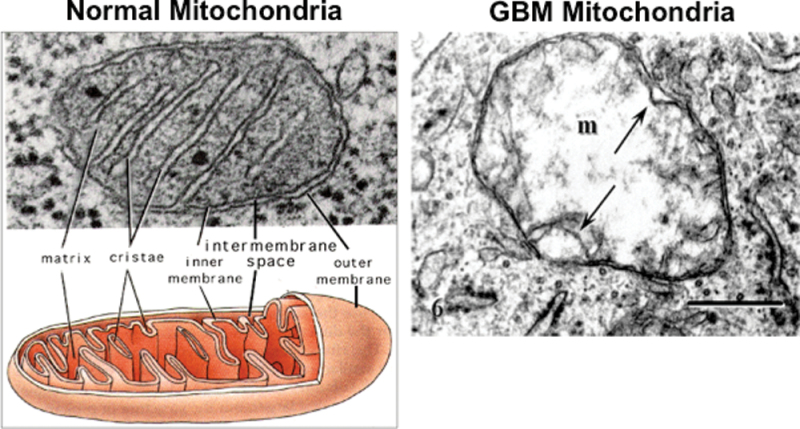
Fig. 2.
Typical ultrastructure of a normal mitochondrion and a mitochondrion from a human glioblastoma. Normal mitochondria contain elaborate cristae, which are extensions of the inner membrane and contain the protein complexes of the electron transport chain necessary for producing ATP through OxPhos. The mitochondrion from the glioblastoma (m) is enlarged and shows a near total breakdown of cristae (cristolysis) and an electron-lucent matrix. The absence of cristae in glioblastoma mitochondria indicates that OxPhos would be deficient. The arrow indicates an inner membrane fold. Bar: 0.33 μm. Method of staining: uranyl acetate/lead citrate. The glioblastoma multiforme mitochondrion was reprinted with permission from Journal of Electron Microscopy (94). The normal mitochondrion diagram was from http://academic.brooklyn.cuny.edu/biology/bio4fv/page/mito.htm.
More recent electron micrographic studies from Elliott et al. showed that mitochondria ultrastructure was abnormal to some degree in 778 patients with breast cancer (51). Remarkably, mitochondria were severely reduced in number or were undetectable in the tumor tissue from over 80% of the patients. These findings together with the evidence from the Pedersen (67) review would support Warburg’s central hypothesis that respiration is insufficient in tumor cells. It is obvious that mitochondrial function or OxPhos sufficiency cannot be normal in tumor cells that contain few if any mitochondria. Glycolysis and lactate fermentation would need to be upregulated in these tumor cells in order to compensate for the absence of OxPhos. Furthermore, the degree of malignancy in these breast tumors was correlated directly with the degree of mitochondrial structural abnormality (51). The high glycolytic activity and lactate production seen in the most malignant tumors were also linked to the mitochondrial structural abnormalities seen in the tumors (91,98–102). In contrast to inherited mitochondriopathies, where glycolysis might not compensate completely for mitochondrial energy failure, fermentation energy appears capable of compensating completely for the respiratory insufficiency in tumor cells (18,103). Further studies will be needed to distinguish the differences in glycolytic and respiratory energy metabolism in tumor cells and in cells with mitochondriopathies (18).
Pedersen (67) presented massive evidence showing that mitochondria in tumor cells are abnormal compared with mitochondria from normal cells. His review provides a comprehensive discussion of mitochondrial bioenergetics and dysfunction in cancer cells. It was clearly shown that the mitochondria of cancer cells contain numerous qualitative and quantitative abnormalities compared with mitochondria from tissue specific control cells. Summarized here are just a few of the conclusions from the Pedersen review. (i) Tumor mitochondria are abnormal in morphology and ultrastructure and respond differently to changes in growth media than mitochondria from normal cells. (ii) The protein and lipid composition of tumor mitochondria are markedly different from that of normal mitochondria. (iii) Proton leak and uncoupling is greater in tumor mitochondria than in normal mitochondria. (iv) Calcium regulation is impaired in tumor mitochondria. (v) Anion membrane transport systems are abnormal or dysregulated in mitochondria from many tumors. (vi) Defective shuttle systems are not responsible for elevated glucose fermentation in tumor cells. (vii) Pyruvate is not effectively oxidized in tumor mitochondria. (viii) Tumor mitochondria contain a surface-bound, fetal-like hexokinase. (ix) A deficiency in some aspect of respiration can account for excessive lactic acid production in tumor cells. Clearly, substantial evidence exists showing that mitochondrial structure, function and respiratory capacity is defective to some degree in all types of tumor cells. This information should be addressed in discussions of tumor cell energy metabolism.
Besides a generalized defect at the level of the mitochondrial electron transport chain in most tumor cells, numerous other mitochondrial abnormalities do exist that would diminish respiratory function (104,105). Interestingly, Warburg never stated that a generalized defect in electron transport was responsible for the origin of cancer despite suggestions from others (106,107). Rather, Warburg stated that insufficient respiration was responsible for aerobic fermentation and the origin of cancer (54,55,57,58,82). We know from the work of numerous investigators that electron transport may not be coupled to ATP synthesis in cancer cells (91,104). Any mitochondrial defect that would uncouple electron transport from OxPhos could reduce respiratory sufficiency and thus contribute to lactate formation or a Warburg effect.
Influence of unnatural growth environment on cellular energy metabolism
Much of the evidence arguing against Warburg’s central theory that respiratory insufficiency is the origin of the aerobic fermentation seen in cancer cells (Warburg effect) was derived from investigations of tumor cells grown in vitro (64,78,79,108–110). In contrast to the structural defects, reduced numbers or the absence of mitochondria observed in human cancerous tissues, such mitochondrial abnormalities are not generally seen in many human and animal tumor cells when they are grown in the in vitro environment. It is interesting that oxygen consumption rate can be similar or even greater in cultured tumor cells than in non-tumorigenic cells (83,86,111). The presence of mitochondria and robust oxygen consumption rates in tumor cells grown in vitro suggested to some that mitochondria are normal in tumor cells and that Warburg’s central theory was incorrect (64,81,109). As mentioned above, however, oxygen consumption rate is not always an indicator of coupled respiration. Some tumor cells consume oxygen while importing and hydrolyzing glycolytically derived ATP through the mitochondrial adenine nucleotide transporter 2 in order to maintain the proton motive gradient (112). We also showed that the growth of tumorigenic and non-tumorigenic cells in typical cell culture media changes the content and fatty acid composition of lipids especially cardiolipin, the signature phospholipid of the inner mitochondrial membrane that regulates OxPhos (96). No tumor cells have yet been described with a normal content and composition of cardiolipin (97,113,114). Cells cannot respire effectively if the content or composition of their cardiolipin is abnormal (97,115,116). This point cannot be overemphasized.
It is not clear why mitochondria might appear functionally normal in many types of cultured tumor cells but appear structurally abnormal when evaluated in the tumor cells of many primary malignant cancers. Cultured cell lines are usually derived from only a single cell or a few cells of a heterogeneous tumor. Is it possible that only those tumor cells with some level of mitochondrial function are capable of growing in vitro? Also the in vitro environment forces many cells into a state of aerobic fermentation whether or not they are tumorigenic. We showed that the typical culture environment produces immature cardiolipin in non-tumorigenic glial cells, which reduces the activity of mitochondrial respiratory chain complexes (96). Further studies are needed on the structure and function of mitochondria in tumor tissue and their derived cell lines.
Lactate production should be minimal in adequately oxygenated cells that have the capacity to respire normally. However, significant lactate production is often observed in proliferating non-tumorigenic cells grown in well-oxygenated cultures (96,103,117). It is not likely that the high aerobic fermentation seen in normal cells grown in culture is due to deregulated glycolysis, as suggested for tumor cells (64). Enhanced glycolysis in tumor cells cannot be considered only as deregulated but can also be considered as necessary to compensate for respiratory insufficiency.
Some investigators consider lactate production as necessary for normal cell proliferation (118,119). It is important to consider the differences in the metabolic requirements of tumorigenic and non-tumorigenic cells when grown in the in vivo and in vitro environments (117,120). In contrast to what is seen in cultured cells, no lactate production is seen in the rapidly growing embryonic chorion under aerobic conditions (57). Moreover, lactate production is minimal in rapidly growing hepatocytes during liver regeneration (121,122). Instead, regenerating liver cells use fatty acids rather than glucose to fuel proliferation. Fatty acid metabolism produces mostly water and CO2, but not lactate. In contrast to hepatomas, which have abnormal cardiolipin composition, the content and composition of cardiolipin is similar in resting liver cells and in proliferating liver cells during regeneration (123,124). These findings suggest that respiration can occur normally in rapidly proliferating liver cells during liver regeneration. Viewed together, these findings indicate that lactate production is not required for rapid cell proliferation in vivo. Tumor cells are an exception in this regard, as lactate production in these cells arises as a consequence of abnormal respiration, which can be linked to either the structural defects seen in tumor tissue mitochondria or to reduced number of mitochondria. If lactate production is not required for rapid cell growth, why are significant amounts of lactate produced in many types of rapidly growing tumorigenic and non-tumorigenic cells when grown in culture?
The ‘Crabtree effect’ can confound the interpretation of energy metabolism in cultured cells. The Crabtree effect involves a glucose-induced suppression of respiration leading to lactate production whether or not mitochondria are damaged (96,120,125,126). The Crabtree effect differs from the Warburg effect, which involves lactate production arising from insufficient respiration. In other words, the aerobic lactate produced under the Crabtree effect arises from a suppressed respiration rather than from insufficient respiration as occurs in the Warburg effect. It can be difficult to determine with certainty, however, whether the aerobic fermentation (aerobic glycolysis) observed in cultured cells arises from a Crabtree effect, a Warburg effect or some combination of these effects (126–128). We consider the Crabtree effect as an artifact of the in vitro environment that causes some non-tumorigenic mammalian cells to ferment lactate even in the presence of oxygen. It would therefore be important for investigators to exclude the influence of a Crabtree effect on the assessment of energy measurements in cultured cells. Although a Crabtree effect might suppress OxPhos, the TCA cycle should remain functional and produce ATP through substrate level phosphorylation (87–90). Under certain conditions (hypoxia), the tumor TCA cycle can work in both forward and reverse (reductive) directions (129,130). Although some tumor cells can have a functional TCA cycle linked to insufficient respiration, sufficient respiration is unlikely to occur without a functional TCA cycle. Support for this comes from findings that some rare cancers can arise from inherited mutations in TCA enzymes, e.g. fumarate hydratase and succinate dehydrogenase, which impede the TCA cycle (131,132). Based on the data presented over many years by numerous investigators, we consider that OxPhos is universally insufficient to some degree in all tumor cells. However, the Crabtree effect and the unnatural conditions of the in vitro environment can obscure this insufficiency. Although respiratory insufficiency might be more profound in some tumor cells than in others, most if not all tumor cells will express some degree of OxPhos insufficiency compared with appropriate controls matched for species, age and tissue type.
Besides the confounding influence of the in vitro environment on energy metabolism, abnormalities and misinformation can be obtained when human tumor cells are grown in non-syngeneic hosts (133). This is especially relevant with respect to the mouse xenograft models including the ‘patient-derived xenografts’. We found that human U87MG brain cancer cells express mouse carbohydrates on their surface when grown as a xenograft in immune deficient mice (134). Over 65% of the sialic acid composition on the U87MG tumor cells consisted of the nine-carbon sugar, N-glycolylneuraminic acid. Humans, however, are unable to synthesize N-glycolylneuraminic acid due to a mutation in the gene that encodes a common mammalian hydroxylase enzyme (134,135). The hydroxylase mutation occurred in the human genome sometime after our evolutionary split with the great apes (135). The acquisition of murine carbohydrates and lipids will likely occur in any human tumor cell grown in the body of a mouse or rat. N-glycolylneuraminic acid alters the characteristics of human embryonic stem cells when grown on non-human feeder cells (136). The influence of the murine host on gene expression in human tumor cells is a confounding variable that can create difficulties for data interpretation in tumor cells. Few investigators address these issues.
Expression of mouse carbohydrates and lipids on human tumor cells when grown as xenografts can alter gene expression patterns and growth behavior of the tumor cells, thus altering their response to changes in the microenvironment. It might be reasonable to view the human xenograft tumor models as a type of human-mouse centaur (133). In addition, the basal metabolic rate of the mouse is 7- to 8-fold greater than that of humans (137,138). The basal metabolic rate is the energy needed for the maintenance of all physiological processes under rest. Little attention is given to differences in metabolic rate when comparing metabolism among human and animal tumors (117). The difference in metabolic rate could cause the human tumor cells to grow slow or not at all in xenografts due to competition for energy metabolites with mouse host stromal cells that have a higher metabolic rate than the human tumor cells. This could account in part for the low incidence of systemic metastasis seen in xenograft models implanted with tumor cells taken from human metastases. Solid tumors that do not metastasize or are not invasive are generally considered benign (4). Further studies will be needed to determine if the human tumor cells that are selected to grow in the mouse have a metabolic rate more similar to that of the mouse than to that of the human.
Many human tumor cells or tissues are grown in mice that are Non-Obese Diabetic and have Severely Compromised Immuno-Deficiency (NOD-SCID) (139). These mice not only have a compromised innate and/or adaptive immune system but also express characteristics of both type-1 diabetes and type-2 diabetes (140). This is not a usual situation for most cancer patients. Despite some limited success, it is naive to assume that the growth behavior and response to therapies of human tumors grown as xenografts would be similar to the situation in the natural host. The evaluation of cancer drugs against tumor cells grown in unnatural environments together with the misunderstanding on the origin of cancer is responsible in large part for widespread failure in developing new cancer therapies (133). The use of syngeneic mouse tumor models will be more representative of the natural physiological state in humans than will the xenograft models.
Connecting the links from respiratory insufficiency to cancer origin
The path from normal cell physiology to malignant behavior, where all major cancer hallmarks are expressed, is depicted in Figure 3. Any unspecific condition that damages a cell’s respiratory capacity but is not severe enough to kill the cell can potentially initiate the path to a malignant cancer. Reduced respiratory capacity could arise from damage to any mitochondrial protein, lipid or mtDNA. Some of the many unspecific conditions that can diminish a cell’s respiratory capacity thus initiating carcinogenesis include inflammation, carcinogens, radiation (ionizing or ultraviolet), intermittent hypoxia, rare germline mutations, viral infections and age. The evidence supporting this statement also addresses Szent Giorgy’s ‘oncogenic paradox’, as was described in a recent treatise on the subject (141). The paradox addresses the difficulty in knowing how a plethora of disparate carcinogenic agents might produce cancer through a common mechanism. Some of the rare germline mutations that increase risk for cancer through an effect on cellular respiration include p53, BRACA1, RB and xeroderma pigmentosum (18). Cancer-causing viruses can be linked to mitochondrial dysfunction (18). If respiratory damage is acute, the cell will die. On the other hand, if damage is mild and protracted, the cell will elevate lactate or amino acid fermentation in order to compensate for insufficient OxPhos. Recent evidence also shows that mitochondrial dysfunction is the initial event in the path to tumorigenesis induced by the mutated Ras oncogene and is closely linked to the action of the BRAF oncogene (83,142,143). Cells will enter their default state of proliferation following loss of respiratory control (9,141). Several cancer hallmarks can be linked to the transition from quiescence to proliferation (Figure 3). Unbridled proliferation is linked to fermentation, which was the dominant form of energy metabolism during the oxygen deficient α period of earth’s history (144). OxPhos insufficiency in fusion hybrids between immune cells (mostly macrophages) and cancer stem cells can underlie the ability of tumor cells to intravasate the circulation locally and to extravasate the circulation at distant sites (145,146). As macrophages are already mesenchymal and naturally capable of systemic tissue dispersion, it is not necessary to explain the phenomenon of metastasis in terms of complicated gene-linked epithelial to mesenchymal and mesenchymal to epithelial transitions. Metastasis in our view would arise from the dysregulation of normal macrophage functions in fusion hybrids including intravasation and extravasation (146–148). All major hallmarks of cancer including genomic instability can be linked directly or indirectly to the respiratory dysfunction and the compensatory fermentation of the tumor cell.
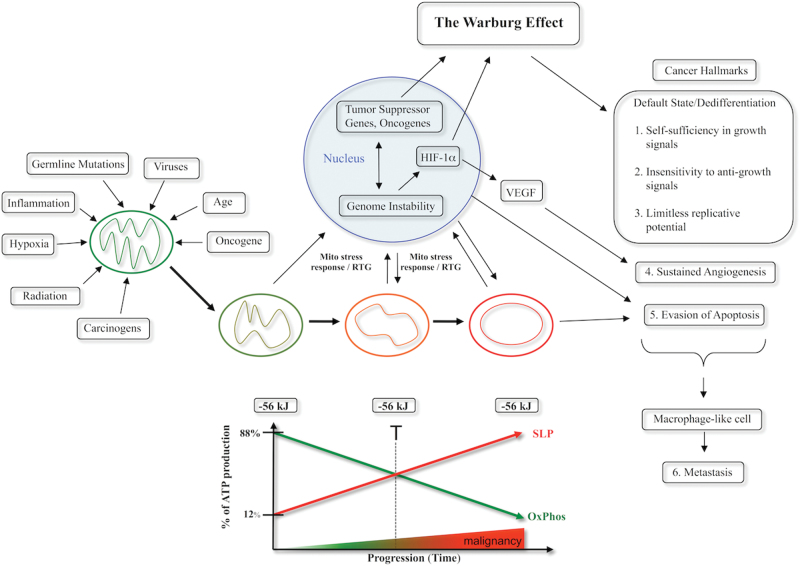
Fig. 3.
Mitochondrial respiratory dysfunction as the origin of cancer. Cancer can arise from any number of non-specific events that damage the respiratory capacity of cells over time. The path to carcinogenesis will occur only in those cells capable of enhancing energy production through fermentation (substrate level phosphorylation, SLP). Despite the shift from respiration to SLP the ΔG′ of ATP hydrolysis remains fairly constant at approximately −56 kJ indicating that the energy from SLP compensates for the reduced energy from OxPhos. The mitochondrial stress response or retrograde signaling will initiate oncogene upregulation and tumor suppressor gene inactivation that are necessary to maintain viability of incipient cancer cells when respiration becomes unable to maintain energy homeostasis. Genomic instability will arise as a secondary consequence of protracted mitochondrial stress from disturbances in the intracellular and extracellular microenvironment. Metastasis arises from respiratory damage in cells of myeloid/macrophage origin (146). The degree of malignancy is linked directly to the energy transition from OxPhos to SLP. This scenario links all major cancer hallmarks to an extrachromosomal respiratory dysfunction (141). The T signifies an arbitrary threshold when the shift from OxPhos to SLP might become irreversible. Reprinted with modifications from (17).
Are mutations in the P53 and the Ras genes primary or secondary causes of cancer?
Although germline or somatic mutations in the P53 tumor suppressor gene and somatic mutations the Ras oncogene occur frequently in many tumor cells and cancers (149,150), it is not clear if these genes or their products are primary or secondary causes of cancer. Hwang et al. showed that p53 regulates mitochondrial respiration through its transcriptional target gene Synthesis of Cytochrome c Oxidase 2 (SCO2) (151–153). In these studies the Warburg effect was linked directly to impaired respiration. Singh et al. showed that mitochondrial energy metabolism is impaired in human cancer cells containing defects in p53 (154). Huang et al. recently showed that the common K-RasG12V mutation causes a metabolic switch from OxPhos to glycolysis (Warburg effect) due to mitochondrial dysfunction (Figure 4). Lee et al. showed that transfection of human diploid cells with V12Ras significantly increased damaging oxygen species in mitochondria (155), whereas Weinberg et al. (156) showed that mitochondrial reactive oxygen species (ROS) generation and damage to complex III was essential for K-Ras-induced cell proliferation and tumorigenesis. Moreover, Yang et al. (157) showed that H-Ras transformation of mouse fibroblasts damaged respiration, thus forcing the cells into a glycolytic metabolism. This is notable since activated RAS has been proposed to induce MYC activity and to enhance non-hypoxic levels of HIF-1α (64). As MYC and HIF-1 drive glycolysis, their upregulation would be necessary to prevent senescence following respiratory impairment (18,158). As constitutive Ras activation is incompatible with prenatal development, a disruption of mitochondrial energy metabolism could underlie tumor formation in mice cloned from melanoma nuclei following the inadvertent expression of the Ras oncogene (48). Viewed collectively, these and other observations are consistent with Warburg’s theory and suggest that mutations in the P53 and Ras genes initiate cancer through their adverse effects on respiratory function. It will be up to each investigator to determine whether they consider these mutations as primary or secondary causes of cancer according to Warburg’s central theory (55,57,58). It is our view that all roads to the origin and progression of cancer pass through the mitochondria (Figure 3).
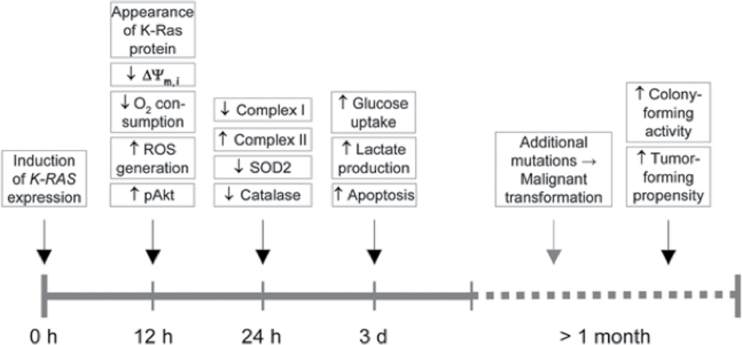
Fig. 4.
Timeline of events following expression of K-Ras. The time axis depicts the various events after stimulation of the K-RAS gene expression. The findings of Huang et al. indicate that mitochondria-linked changes are observed around the time of the increase in the K-Ras protein (142). This is then followed by other changes, such as alteration in cell metabolism. Gene mutations would form as a downstream epiphenomenon of altered metabolism. Malignant transformation, documented by the colony-forming activity of the cells and their propensity to form tumors ensue much later. According to these findings, the Warburg effect (aerobic fermentation) arises as a consequence of K-RAS-induced respiratory injury. This timeline is in general agreement with Warburg’s central theory and with other similar findings that respiratory disturbance is an initial event in K-RAS-induced tumorigenesis (237–239). The timeline will be greatly protracted in vivo as shown from the Roskelley et al. (240) experiments. Reprinted from Neuzil et al. (143) with permission.
Can tumor somatic mutations arise as a downstream epiphenomenon of abnormal energy metabolism?
How might protracted respiratory insufficiency cause somatic mutations and the nuclear genomic instability seen in tumor cells? The integrity of the nuclear genome is dependent to a large extent on the efficiency of mitochondrial respiratory function (159). Evidence indicates that a persistent retrograde response or mitochondrial stress response leads to abnormalities in DNA repair mechanisms and to the upregulation of fermentation pathways (50,160–164). Oncogene upregulation becomes essential for increased glucose and glutamine metabolism following respiratory impairment (83,165). The metabolic waste products of fermentation can destabilize the morphogenetic field of the tumor microenvironment thus contributing to inflammation, angiogenesis and progression (166–168). Normal mitochondrial function is necessary for maintaining intracellular calcium homeostasis, which is required for chromosomal integrity and the fidelity of cell division. Aneuploidy can arise during cell division from abnormalities in calcium homeostasis (159). In this general picture, the abnormal genomic landscape seen in tumor cells is considered a downstream epiphenomenon of dysfunctional respiration and protracted oncogene-driven fermentation. In other words, the somatic mutations arise as effects rather than as causes of tumorigenesis. The nuclear transfer experiments support this view (Figure 1). In light of this perspective, it would be important for those working in cancer genomics field to justify the logic of their experimental approach to the cancer problem (169,170).
Cancer progression is more consistent with Lamarckian than Darwinian evolution
When viewed as a mitochondrial metabolic disease cancer progression is more in line with the evolutionary theory of Lamarck than with the theory of Darwin (20). Many investigators in the cancer field have attempted to link the Darwinian theory of evolution to the phenomenon of tumor progression (171–174). The attempt to link cancer progression to Darwinian evolution is based largely on the view that nuclear somatic mutations are drivers of the disease. According to Lamarck’s theory, it is the environment that produces changes in biological structures (175). Through adaptation and differential use, these changes lead to modifications in the structures. The modifications of structures would then be passed on to successive generations as acquired traits. Lamarck’s evolutionary synthesis was based on his belief that the degree of use or disuse of biological structures shaped evolution along with the inheritance of acquired adaptability. Lamarck’s ideas could also accommodate a dominant role for epigenetics and horizontal gene transfer as factors that could facilitate tumor progression (176,177). In addition to nuclear epigenetic events involving acetylation and phosphorylations, mitochondria are also recognized as a powerful extra nuclear epigenetic system (159,178–180). Other epigenetic phenomena such as cytomegalovirus infection, cell fusion and horizontal gene transfer can also contribute to cancer progression and metastasis (147,159,181–184).
Considering the dynamic behavior of mitochondria involving regular fusions and fissions, abnormalities in mitochondrial structure can be rapidly disseminated throughout the cellular mitochondrial network and passed along to daughter cells somatically, through cytoplasmic inheritance (17,185). The capacity for mitochondrial respiratory function becomes progressively less with each cell division as adaptability to substrate level phosphorylation increases (Figure 3). The somatic progression of cancer would therefore embody the concept of the somatic inheritance of an acquired trait. The acquired trait in this case is alteration to mitochondrial structure. The most malignant cancer cells would sustain the near-complete replacement of their respiration with fermentation. This is obvious in those tumor cells with quantitative and qualitative abnormalities in their mitochondria (Figure 2). The somatic inheritance of mitochondrial dysfunction in tumor cells could contribute in part to the appearance of a clonal origin, but not directly involving the nuclear genome. However, the degree of nuclear genomic instability can be linked to mitochondrial dysfunction and both defects together can contribute to tumor progression. A Lamarckian view can account for the non-uniform accumulation of mutations and drug resistance seen during cancer progression. Drug resistance is linked to enhanced lactate fermentation, which is acquired during tumor progression (61,186). It is our opinion that the evolutionary concepts of Lamarck can better explain the phenomena of tumor progression than can the evolutionary concepts of Darwin. We encourage further research on this perspective of tumor progression.
Exploiting mitochondrial dysfunction for the metabolic management of cancer
If cancer is primarily a disease of energy metabolism, then rational strategies for cancer management should be found in those therapies that specifically target tumor cell energy metabolism. These therapeutic strategies should be applicable to the majority of cancers regardless of tissue origin, as nearly all cancers suffer from a common malady, i.e. insufficient respiration with compensatory fermentation (2,54,55,57). As glucose is the major fuel for tumor energy metabolism through lactate fermentation, the restriction of glucose becomes a prime target for management. However, most normal cells of the body also need glycolytic pathway products, such as pyruvate, for energy production through OxPhos. It therefore becomes important to protect normal cells from drugs or therapies that disrupt glycolytic pathways or cause systemic reduction of glucose. It is well known that ketones can replace glucose as an energy metabolite and can protect the brain from severe hypoglycemia (187–189). Hence, the shift in energy metabolism associated with a low carbohydrate, high-fat ketogenic diet administered in restricted amounts (KD-R) can protect normal cells from glycolytic inhibition and the brain from hypoglycemia.
When systemic glucose availability becomes limiting, most normal cells of the body will transition their energy metabolism to fats and ketone bodies. Ketone bodies are generated almost exclusively in liver hepatocytes largely from fatty acids of triglyceride origin during periods of fasting (187,190). There are no metabolic pathways described that can produce ketone bodies from carbohydrates despite suggestions to the contrary (191). A restriction of total caloric intake will facilitate a reduction in blood glucose and insulin levels and an elevation in ketone bodies (β-hydroxybutyrate and acetoacetate). Most tumor cells are unable to use ketone bodies for energy due to abnormalities in mitochondria structure or function (13,192). Ketone bodies can also be toxic to some cancer cells (193,194). Nutritional ketosis induces metabolic stress on tumor tissue that is selectively vulnerable to glucose deprivation (13). Hence, metabolic stress will be greater in tumor cells than in normal cells when the whole body is transitioned away from glucose and to ketone bodies for energy.
The metabolic shift from glucose metabolism to ketone body metabolism creates an anti-angiogenic, anti-inflammatory and pro-apoptotic environment within the tumor mass (192,195–199). The general concept of a survival advantage of tumor cells over normal cells occurs when fermentable fuels are abundant, but not when they are limited (20). Figure 5 illustrates the changes in whole body levels of blood glucose and ketone bodies (β-hydroxybutyrate) that will metabolically stress tumor cells while enhancing the metabolic efficiency of normal cells. This therapeutic strategy was illustrated previously in cancer patients and in preclinical models (200–205).
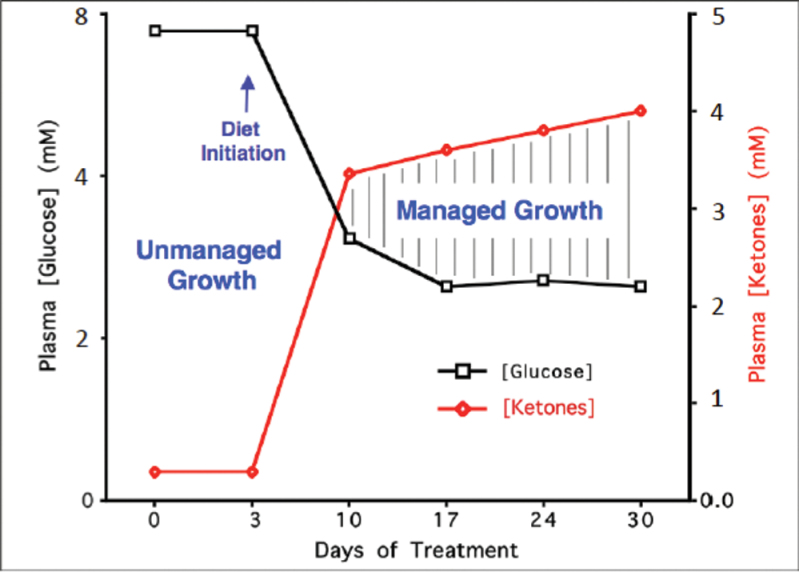
Fig. 5.
Relationship of circulating levels of glucose and ketones (β-hydroxybutyrate) to tumor management. The glucose and ketone values are within normal physiological ranges under fasting conditions in humans and will produce anti-angiogenic, anti-inflammatory and pro-apoptotic effects. We refer to this state as the zone of metabolic management. Metabolic stress will be greater in tumor cells than in normal cells when the whole body enters the metabolic zone. The values for blood glucose in mg/dl can be estimated by multiplying the mM values by 18. The glucose and ketone levels predicted for tumor management in human cancer patients are 3.1–3.8mM (55–65mg/dl) and 2.5–7.0mM, respectively. These ketone levels are well below the levels associated with ketoacidosis (blood ketone values greater than 15 mmol). Elevated ketones will protect the brain from hypoglycemia. Modified from a previous version (241).
Implications for novel therapeutics
Once the whole body enters the metabolic zone described in Figure 5, relatively low doses of a variety of drugs can be used to further target energy metabolism in any surviving tumor cells (192). It is interesting that the therapeutic success of imatinib (Gleevec) and trastuzumab (Herceptin) in managing BCR-ABL leukemia cells and ErbB2-positive breast cancers, respectively, is dependent on their ability to target signaling pathways linked to glucose metabolism (206,207). In contrast to these drugs, which target energy metabolism primarily in those individuals with mutations in specific receptors linked to the IGF-1/PI3K/Akt pathway, calorie-restricted KDs will target similar pathways in any cancer cell regardless of the mutations involved (197,208). Dietary energy reduction will simultaneously target multiple metabolic signaling pathways without causing adverse effects or toxicity (208). Non-toxic metabolic therapies might also be a preferable alternative to toxic immunotherapies for cancer management especially if both therapies target the same pathways. It must be emphasized that the therapeutic efficacy of the KD is strongly dependent on restricted intake, as consumption of the KD in unrestricted amounts can cause insulin insensitivity and glucose elevation despite the complete absence of carbohydrates in the diet (205). Elevated consumption of the KD is not often seen, however, as humans usually restrict intake due to the high fat content of the diet.
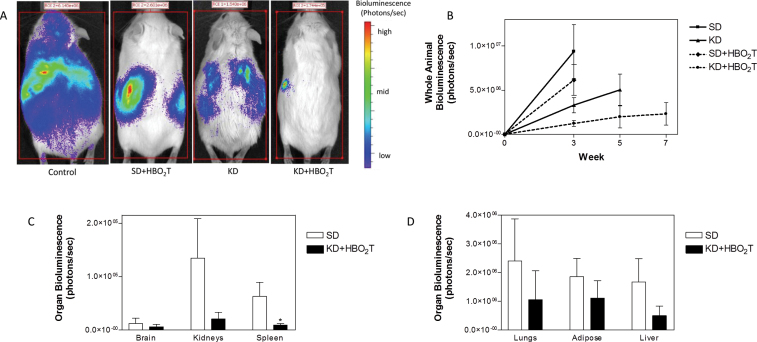
Poff et al. also recently showed a synergistic interaction between the KD and hyperbaric oxygen therapy (HBO2T) (Figure 6). The KD reduces glucose for glycolytic energy while also reducing NADPH levels for anti-oxidant potential through the pentose-phosphate pathway. HBO2T will increase ROS in the tumor cells, whereas the ketones will protect normal cells against ROS damage and from the potential for central nervous system oxygen toxicity (189,209). Glucose deprivation will enhance oxidative stress in tumor cells, whereas increased oxygen can reduce tumor cell proliferation (210,211). A dependency on glucose and an inability to use ketones for energy makes tumor cells selectively vulnerable to this therapy. Although this metabolic therapy is effective against those tumor cells that contain mitochondria, it remains to be determined if this therapy would be equally effective against those tumor cells containing few or no mitochondria (51). In contrast to radiation therapy, which also kills tumor cells through ROS production (212), the KD + HBO2T will kill tumor cells without causing toxic collateral damage to normal cells. Cancer patients and their oncologists should know about this. Some KDs might also enhance the therapeutic action of radiation therapy against brain and lung tumors (213,214). It will be important to compare and contrast the therapeutic efficacy of conventional radiation therapy with HBO2T when used with the KD-R. Although radiation is widely used as a cancer therapy, it should be recognized that radiation damages respiration in normal cells and can itself cause cancer (55). Radiation therapy for malignant brain cancer creates a necrotic microenvironment that can facilitate recurrence and progression through enhanced glucose and glutamine metabolism (13,215).
Fig. 6.
The KD and HBO2T are synergistic in reducing systemic metastatic cancer in the syngeneic VM mouse model. VM-M3/Fluc tumor cells were implanted subcutaneously and systemic organ metastasis was evaluated ex vivo using bioluminescent imaging as described previously (242,243). Tumor growth was slower in mice fed the KD than in mice fed a standard high carbohydrate diet. (A) Representative animals from each treatment group demonstrating tumor bioluminescence at day 21 after tumor cell inoculation. Treated animals showed less bioluminescence than controls with KD + HBO2T mice exhibiting a profound decrease in tumor bioluminescence compared with all groups. (B) Total body bioluminescence was measured weekly as a measure of tumor size; error bars represent standard error of the mean. KD + HBO2T mice exhibited significantly less tumor bioluminescence than control animals at week 3 (P < 0.01; two-tailed student’s t-test) and an overall trend of notably slower tumor growth than controls and other treated animals throughout the study. (C and D) Day 21 organ bioluminescence of standard high carbohydrate diet and KD + HBO2T animals (N = 8) demonstrated a trend of reduced metastatic tumor burden in animals receiving the combined therapy. Spleen bioluminescence was significantly decreased in KD + HBO2T mice (*P < 0.02; two-tailed student’s t-test). Reprinted with permission from Poff et al. (243).
Besides drugs that target glucose, drugs that target glutamine can also be effective in killing systemic metastatic cancer cells (192,216,217). Many metastatic cancers express multiple characteristics of macrophages (146,218). Glutamine is a major fuel of macrophages and other cells of the immune system (146,219). As glutamine is the most abundant amino acid in the body and is used in multiple metabolic reactions, targeting glutamine without toxicity might be more difficult than targeting glucose (220,221). Although glutamine interacts synergistically with glucose to drive energy metabolism in cultured tumor cells, there are reports suggesting that glutamine can have chemo-preventive effects (222). Further studies are needed to evaluate the role of glutamine as a facilitator of tumor energy metabolism in vivo.
The novelty of the metabolic approach to cancer management involves the implementation of a synergistic combination of nutritional ketosis, cancer metabolic drugs and HBO2T. This therapeutic approach would be similar to the ‘Press-Pulse’ scenario for the mass extinction of organisms in ecological communities (223,224). The KD-R would act as a sustained ‘Press’, whereas HBO2T and metabolic drugs would act as a ‘Pulse’ for the mass elimination of tumor cells in the body. Some of the cancer metabolic drugs could include 2-deoxyglucose, 3-bromopyruvate and dichloroacetate (56,120,225–227). This therapeutic strategy produces a shift in metabolic physiology that will not only kill tumor cells but also enhance the general health and metabolic efficiency of normal cells, and consequently the whole body (189,209). We view this therapeutic approach as a type of ‘mitochondrial enhancement therapy’ (192). As we consider OxPhos insufficiency with compensatory fermentation as the origin of cancer, enhanced OxPhos efficiency would be anti-carcinogenic.
Many cancers are infected with human cytomegalovirus, which acts as an oncomodulator of tumor progression (228). Products of the virus can damage mitochondria in the infected tumor cells, thus contributing to a further dependence on glucose and glutamine for energy metabolism (18,229–231). The virus often infects cells of monocyte/macrophage origin, which are considered the origin of many metastatic cancers (145,146,232,233). We predict that the KD-R used together with anti-viral therapy will also be an effective Press-Pulse strategy for reducing progression of those cancers infected with human cytomegalovirus (234).
Advanced metastatic cancers can become manageable when their access to fermentable fuels becomes restricted. The metabolic shift associated with the KD-R involves ‘keto-adaptation’. However, the adaptation to this new metabolic state can be challenging for some people. The administration of ketone esters could conceivably enable patients to circumvent the dietary restriction generally required for sustained nutritional ketosis. Ketone ester-induced ketosis would make sustained hypoglycemia more tolerable and thus assist in metabolic management of cancer (235,236). As each person is a unique metabolic entity, personalization of metabolic therapy as a broad-based cancer treatment strategy will require fine-tuning based on an understanding of individual human physiology. Also, personalized molecular therapies developed through the genome projects could be useful in targeting and killing those tumor cells that might survive the non-toxic whole body metabolic therapy. The number of molecular targets should be less in a few survivor cells of a small tumor than in a heterogeneous cell population of a large tumor. We would therefore consider personalized molecular therapy as a final strategy rather than as an initial strategy for cancer management. Non-toxic metabolic therapy should become the future of cancer treatment if the goal is to manage the disease without harming the patient. Although it will be important for researchers to elucidate the mechanistic minutia responsible for the therapeutic benefits, this should not impede an immediate application of this therapeutic strategy for cancer management or prevention.
Funding
National Institutes of Health (HD-39722, NS-55195, CA-102135); American Institute of Cancer Research; the Boston College Expense Fund (to T.N.S.); Scivation and Office of Naval Research (to D.P.D.).
Acknowledgements
We thank Miriam Kalamian for helpful comments.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ATP
adenosine triphosphate
- HBO2T
hyperbaric oxygen therapy
- KD
ketogenic diet
- OxPhos
oxidative phosphorylation
- ROS
reactive oxygen species
- SLP
substrate level phosphorylation
- TCA
tricarboxylic acid.
References
- 1. Sporn M.B. (1996). The war on cancer. Lancet, 347, 1377–1381 [DOI] [PubMed] [Google Scholar]
- 2. Seyfried T.N. (2012). Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 3. Fidler I.J. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer, 3, 453–458 [DOI] [PubMed] [Google Scholar]
- 4. Lazebnik Y. (2010). What are the hallmarks of cancer? Nat. Rev. Cancer, 10, 232–233 [DOI] [PubMed] [Google Scholar]
- 5. Tarin D. (2011). Cell and tissue interactions in carcinogenesis and metastasis and their clinical significance. Semin. Cancer Biol., 21, 72–82 [DOI] [PubMed] [Google Scholar]
- 6. Seyfried T.N. (2012). Confusion surrounds the origin of cancer. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 15–29 [Google Scholar]
- 7. Hanahan D., et al. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 8. Baker S.G., et al. (2007). Paradoxes in carcinogenesis: new opportunities for research directions. BMC Cancer, 7, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soto A.M., et al. (2004). The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays, 26, 1097–1107 [DOI] [PubMed] [Google Scholar]
- 10. Vogelstein B., et al. (2013). Cancer genome landscapes. Science, 339, 1546–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexandrov L.B., et al. (2013). Signatures of mutational processes in human cancer. Nature, 500, 415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soto A.M., et al. (2012). Is systems biology a promising approach to resolve controversies in cancer research? Cancer Cell Int., 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seyfried T.N., et al. (2012). Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer? Epilepsy Res., 100, 310–326 [DOI] [PubMed] [Google Scholar]
- 14. Seyfried T.N. (2013). Cancer as a metabolic disease: implications for novel therapeutics. Amer. Assoc. Cancer Res. Education Book, 2013, 31–36 [Google Scholar]
- 15. Seyfried T.N., et al. (2011). Metabolic management of brain cancer. Biochim. Biophys. Acta, 1807, 577–594 [DOI] [PubMed] [Google Scholar]
- 16. Seyfried T.N., et al. (2005). Targeting energy metabolism in brain cancer: review and hypothesis. Nutr. Metab. (Lond)., 2, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seyfried T.N., et al. (2010). Cancer as a metabolic disease. Nutr. Metab. (Lond)., 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seyfried T.N. (2012). Genes, respiration, viruses, and cancer. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 145–176 [Google Scholar]
- 19. Stratton M.R. (2011). Exploring the genomes of cancer cells: progress and promise. Science, 331, 1553–1558 [DOI] [PubMed] [Google Scholar]
- 20. Seyfried T.N. (2012). Nothing in cancer biology makes sense except in the light of evolution. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 261–275 [Google Scholar]
- 21. Nowell P.C. (1976). The clonal evolution of tumor cell populations. Science, 194, 23–28 [DOI] [PubMed] [Google Scholar]
- 22. Fojo T., et al. (2010). Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clin. Cancer Res., 16, 5972–5980 [DOI] [PubMed] [Google Scholar]
- 23. Rosell R., et al. (2009). Customized treatment in non-small-cell lung cancer based on EGFR mutations and BRCA1 mRNA expression. PLoS One, 4, e5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McLeod H.L. (2013). Cancer pharmacogenomics: early promise, but concerted effort needed. Science, 339, 1563–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hardy P.A., et al . (2005) Reappraisal of the Hansemann-Boveri hypothesis on the origin of tumors. Cell Biol. Int., 29, 983–992 [DOI] [PubMed] [Google Scholar]
- 26. Gibbs W.W. (2003). Untangling the roots of cancer. Sci. Am., 289, 56–65 [DOI] [PubMed] [Google Scholar]
- 27. Hameroff S.R. (2004). A new theory of the origin of cancer: quantum coherent entanglement, centrioles, mitosis, and differentiation. Biosystems., 77, 119–136 [DOI] [PubMed] [Google Scholar]
- 28. Manchester K. (1997). The quest by three giants of science for an understanding of cancer. Endeavour, 21, 72–76 [DOI] [PubMed] [Google Scholar]
- 29. Wolf U. (1974). Theodor Boveri and his book, on the problem of the origin of malignant tumors. In German J. (ed.) Chromosomes and Cancer, John Wiley & Sons, New York, pp. 1–20 [Google Scholar]
- 30. Duesberg P., et al. (2000). Aneuploidy, the somatic mutation that makes cancer a species of its own. Cell Motil. Cytoskeleton, 47, 81–107 [DOI] [PubMed] [Google Scholar]
- 31. Salk J.J., et al. (2010). Mutational heterogeneity in human cancers: origin and consequences. Annu. Rev. Pathol., 5, 51–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cairns J. (1981). The origin of human cancers. Nature, 289, 353–357 [DOI] [PubMed] [Google Scholar]
- 33. Loeb L.A. (2001). A mutator phenotype in cancer. Cancer Res., 61, 3230–3239 [PubMed] [Google Scholar]
- 34. Whitman R.C. (1919). Somatic mutations as a factor in the production of cancer; a critical review of von Hansemanns’s theory of anaplasia in light of modern knowledge of genetics. J. Cancer Res., 4, 181–202 [Google Scholar]
- 35. Nigro J.M., et al. (1989). Mutations in the p53 gene occur in diverse human tumour types. Nature, 342, 705–708 [DOI] [PubMed] [Google Scholar]
- 36. Fearon E.R., et al. (1990). A genetic model for colorectal tumorigenesis. Cell, 61, 759–767 [DOI] [PubMed] [Google Scholar]
- 37. Knudson A.G. (2002). Cancer genetics. Am. J. Med. Genet., 111, 96–102 [DOI] [PubMed] [Google Scholar]
- 38. Wagner R.P. (1999). Anecdotal, historical and critical commentaries on genetics. Rudolph Virchow and the genetic basis of somatic ecology. Genetics, 151, 917–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Darlington C.D. (1948) The plasmagene theory of the origin of cancer. Br. J. Cancer, 2, 118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koura M., et al. (1982). Suppression of tumorigenicity in interspecific reconstituted cells and cybrids. Gann, 73, 574–580 [PubMed] [Google Scholar]
- 41. Israel B.A., et al. (1987). Cytoplasmic suppression of malignancy. In Vitro Cell. Dev. Biol., 23, 627–632 [DOI] [PubMed] [Google Scholar]
- 42. Shay J.W., et al. (1988). Cytoplasmic suppression of tumorigenicity in reconstructed mouse cells. Cancer Res., 48, 830–833 [PubMed] [Google Scholar]
- 43. Howell A.N., et al. (1978). Tumorigenicity and its suppression in cybrids of mouse and Chinese hamster cell lines. Proc. Natl Acad. Sci. U. S. A., 75, 2358–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jonasson J., et al. (1977). The analysis of malignancy by cell fusion. VIII. Evidence for the intervention of an extra-chromosomal element. J. Cell Sci., 24, 255–263 [DOI] [PubMed] [Google Scholar]
- 45. McKinnell R.G., et al. (1969). Transplantation of pluripotential nuclei from triploid frog tumors. Science, 165, 394–396 [DOI] [PubMed] [Google Scholar]
- 46. Mintz B., et al. (1975). Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl Acad. Sci. U. S. A., 72, 3585–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li L., et al. (2003). Mouse embryos cloned from brain tumors. Cancer Res., 63, 2733–2736 [PubMed] [Google Scholar]
- 48. Hochedlinger K., et al. (2004). Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev., 18, 1875–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seyfried T.N. (2012). Mitochondria: the ultimate tumor suppressor. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 195–205 [Google Scholar]
- 50. Kaipparettu B.A., et al. (2013). Crosstalk from non-cancerous mitochondria can inhibit tumor properties of metastatic cells by suppressing oncogenic pathways. PLoS One, 8, e61747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elliott R.L., et al . (2012) Mitochondria organelle transplantation: introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast Cancer Res. Treat., 136, 347–354 [DOI] [PubMed] [Google Scholar]
- 52. Israel B.A., et al. (1988). Cytoplasmic mediation of malignancy. In Vitro Cell. Dev. Biol., 24, 487–490 [DOI] [PubMed] [Google Scholar]
- 53. Petros J.A., et al. (2005). mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl Acad. Sci. U. S. A., 102, 719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Warburg O. (1931). The Metabolism of Tumours. Richard R. Smith, New York [Google Scholar]
- 55. Warburg O. (1956). On the origin of cancer cells. Science, 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 56. Pedersen P.L. (2007). Warburg, me and hexokinase 2: multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J. Bioenerg. Biomembr., 39, 211–222 [DOI] [PubMed] [Google Scholar]
- 57. Warburg O. (1956). On respiratory impairment in cancer cells. Science, 124, 269–270 [PubMed] [Google Scholar]
- 58. Warburg O. (1969). Revidsed Lindau lectures: the prime cause of cancer and prevention - Parts 1 & 2. In Burk D. (ed.) Meeting of the Nobel-Laureates. K.Triltsch, Lindau, Lake Constance, Germany [Google Scholar]
- 59. Racker E. (1972) Bioenergetics and the problem of tumor growth. Am. Sci., 60, 56–63 [PubMed] [Google Scholar]
- 60. Nelson D.L., et al. (2008) Lehninger Principles of Biochemistry W. H. Freeman, New York [Google Scholar]
- 61. Cuezva J.M., et al. (2002). The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res., 62, 6674–6681 [PubMed] [Google Scholar]
- 62. Acebo P., et al. (2009). Cancer abolishes the tissue type-specific differences in the phenotype of energetic metabolism. Transl. Oncol., 2, 138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weinhouse S. (1956). On respiratory impairment in cancer cells. Science, 124, 267–269 [DOI] [PubMed] [Google Scholar]
- 64. Koppenol W.H., et al. (2011). Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer, 11, 325–337 [DOI] [PubMed] [Google Scholar]
- 65. Stellingwerff T., et al . (2006) Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. Am. J. Physiol. Endocrinol. Metab., 290, E1180–E1190 [DOI] [PubMed] [Google Scholar]
- 66. Gatenby R.A., et al. (2004). Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer, 4, 891–899 [DOI] [PubMed] [Google Scholar]
- 67. Pedersen P.L. (1978). Tumor mitochondria and the bioenergetics of cancer cells. Prog. Exp. Tumor Res., 22, 190–274 [DOI] [PubMed] [Google Scholar]
- 68. Chevrollier A., et al. (2005) ANT2 expression under hypoxic conditions produces opposite cell-cycle behavior in 143B and HepG2 cancer cells. Mol. Carcinog., 42, 1–8 [DOI] [PubMed] [Google Scholar]
- 69. Wijburg F.A., et al. (1989) Studies on the formation of lactate and pyruvate from glucose in cultured skin fibroblasts: implications for detection of respiratory chain defects. Biochem. Int., 19, 563–570 [PubMed] [Google Scholar]
- 70. Tiefenthaler M., et al. (2001) Increased lactate production follows loss of mitochondrial membrane potential during apoptosis of human leukaemia cells. Br. J. Haematol., 114, 574–580 [DOI] [PubMed] [Google Scholar]
- 71. Donnelly M., et al. (1976). Energy metabolism in respiration-deficient and wild type Chinese hamster fibroblasts in culture. J. Cell. Physiol., 89, 39–51 [DOI] [PubMed] [Google Scholar]
- 72. Fiske B.P., et al. (2012) Seeing the Warburg effect in the developing retina. Nat. Cell Biol., 14, 790–791 [DOI] [PubMed] [Google Scholar]
- 73. Prichard J., et al. (1991) Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc. Natl Acad. Sci. U. S. A., 88, 5829–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fox P.T., et al. (1988). Nonoxidative glucose consumption during focal physiologic neural activity. Science, 241, 462–464 [DOI] [PubMed] [Google Scholar]
- 75. Krasnow N., et al. (1962) Myocardial lactate and pyruvate metabolism. J. Clin. Invest., 41, 2075–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Burk D., et al. (1956). On respiratory impairment in cancer cells. Science, 124, 270–272 [PubMed] [Google Scholar]
- 77. Burk D., et al. (1967). On the significance of glucolysis for cancer growth, with special reference to Morris rat hepatomas. J. Natl Cancer Inst., 38, 839–863 [PubMed] [Google Scholar]
- 78. Vaupel P., et al. (2012) Availability, not respiratory capacity governs oxygen consumption of solid tumors. Int. J. Biochem. Cell Biol., 44, 1477–1481 [DOI] [PubMed] [Google Scholar]
- 79. Moreno-Sánchez R., et al. (2007). Energy metabolism in tumor cells. FEBS J., 274, 1393–1418 [DOI] [PubMed] [Google Scholar]
- 80. Haq R., et al. (2013) Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell, 23, 302–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Weinhouse S. (1976). The Warburg hypothesis fifty years later. Z. Krebsforsch. Klin. Onkol. Cancer Res. Clin. Oncol., 87, 115–126 [DOI] [PubMed] [Google Scholar]
- 82. Ferreira L.M. (2010). Cancer metabolism: the Warburg effect today. Exp. Mol. Pathol., 89, 372–380 [DOI] [PubMed] [Google Scholar]
- 83. Hall A., et al. (2013). Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget, 4, 584–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hochachka P.W., et al. (2002). Biochemical Adaptation: Mechanism and Process in Physiological Evolution. Oxford Press, New York [Google Scholar]
- 85. Seyfried T.N. (2012). Is respiration normal in cancer cells? In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 119–132 [Google Scholar]
- 86. Ramanathan A., et al. (2005). Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc. Natl Acad. Sci. U. S. A., 102, 5992–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Seyfried T.N. (2012). Is mitochondrial glutamine fermentation a missing link in the metabolic theory of cancer? In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 133–144 [Google Scholar]
- 88. Chinopoulos C., et al. (2010) Forward operation of adenine nucleotide translocase during F0F1-ATPase reversal: critical role of matrix substrate-level phosphorylation. FASEB J., 24, 2405–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Phillips D., et al. (2009). Succinyl-CoA synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry, 48, 7140–7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schwimmer C., et al. (2005). Increasing mitochondrial substrate-level phosphorylation can rescue respiratory growth of an ATP synthase-deficient yeast. J. Biol. Chem., 280, 30751–30759 [DOI] [PubMed] [Google Scholar]
- 91. Seyfried T.N. (2012). Respiratory dysfunction in cancer cells. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 73–105 [Google Scholar]
- 92. Arismendi-Morillo G. (2011) Electron microscopy morphology of the mitochondrial network in gliomas and their vascular microenvironment. Biochim. Biophys. Acta, 1807, 602–608 [DOI] [PubMed] [Google Scholar]
- 93. Arismendi-Morillo G. (2009). Electron microscopy morphology of the mitochondrial network in human cancer. Int. J. Biochem. Cell Biol., 41, 2062–2068 [DOI] [PubMed] [Google Scholar]
- 94. Arismendi-Morillo G.J., et al. (2008). Ultrastructural mitochondrial pathology in human astrocytic tumors: potentials implications pro-therapeutics strategies. J. Electron Microsc. (Tokyo)., 57, 33–39 [DOI] [PubMed] [Google Scholar]
- 95. Galluzzi L., et al. (2010) Mitochondrial gateways to cancer. Mol. Aspects Med., 31, 1–20 [DOI] [PubMed] [Google Scholar]
- 96. Kiebish M.A., et al. (2009). In vitro growth environment produces lipidomic and electron transport chain abnormalities in mitochondria from non-tumorigenic astrocytes and brain tumours. ASN Neuro, 1, e00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kiebish M.A., et al. (2008). Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res., 49, 2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gonzalez M.J., et al. (2012) The bio-energetic theory of carcinogenesis. Med. Hypotheses, 79, 433–439 [DOI] [PubMed] [Google Scholar]
- 99. Benard G., et al. (2008) Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid. Redox Signal., 10, 1313–1342 [DOI] [PubMed] [Google Scholar]
- 100. Shapovalov Y., et al. (2011) Mitochondrial dysfunction in cancer cells due to aberrant mitochondrial replication. J. Biol. Chem., 286, 22331–22338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Alirol E., et al. (2006). Mitochondria and cancer: is there a morphological connection? Oncogene, 25, 4706–4716 [DOI] [PubMed] [Google Scholar]
- 102. Oudard S., et al. (1997). Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res., 17, 1903–1911 [PubMed] [Google Scholar]
- 103. Wu S.B., et al. (2012). AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: implication of the cell survival in mitochondrial diseases. Biochim. Biophys. Acta, 1822, 233–247 [DOI] [PubMed] [Google Scholar]
- 104. Fulda S., et al. (2010) Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov., 9, 447–464 [DOI] [PubMed] [Google Scholar]
- 105. Shapovalov Y., et al. (2011) Mitochondrial dysfunction in cancer cells due to aberrant mitochondrial replication. J. Biol. Chem., 286, 22331–22338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chance B., et al. (1959). Spectroscopic evidence of metabolic control. Science, 129, 700–708 [DOI] [PubMed] [Google Scholar]
- 107. Colowick S.P. (1961). The status of Warburg’s theory of glycolysis and respiration in tumors. Quart. Rev. Biol., 36, 273–276 [Google Scholar]
- 108. Cairns R.A., et al. (2011) Regulation of cancer cell metabolism. Nat. Rev. Cancer, 11, 85–95 [DOI] [PubMed] [Google Scholar]
- 109. Ward P.S., et al. (2012). Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell, 21, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Vander Heiden M.G., et al. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rossignol R., et al. (2004). Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res., 64, 985–993 [DOI] [PubMed] [Google Scholar]
- 112. Chevrollier A., et al. (2011) Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim. Biophys. Acta, 1807, 562–567 [DOI] [PubMed] [Google Scholar]
- 113. Jahnke V.E., et al. (2010). Evidence for mitochondrial respiratory deficiency in rat rhabdomyosarcoma cells. PLoS One, 5, e8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schild L., et al. (2012) Composition of molecular cardiolipin species correlates with proliferation of lymphocytes. Exp. Biol. Med. (Maywood)., 237, 372–379 [DOI] [PubMed] [Google Scholar]
- 115. Kocherginsky N. (2009). Acidic lipids, H(+)-ATPases, and mechanism of oxidative phosphorylation. Physico-chemical ideas 30 years after P. Mitchell’s Nobel Prize award. Prog. Biophys. Mol. Biol., 99, 20–41 [DOI] [PubMed] [Google Scholar]
- 116. Claypool S.M., et al. (2012) The complexity of cardiolipin in health and disease. Trends Biochem. Sci., 37, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zu X.L., et al. (2004). Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun., 313, 459–465 [DOI] [PubMed] [Google Scholar]
- 118. Wang T., et al. (1976). Aerobic glycolysis during lymphocyte proliferation. Nature, 261, 702–705 [DOI] [PubMed] [Google Scholar]
- 119. Lunt S.Y., et al. (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol., 27, 441–464 [DOI] [PubMed] [Google Scholar]
- 120. Papandreou I., et al. (2011) Anticancer drugs that target metabolism: is dichloroacetate the new paradigm? Int. J. Cancer, 128, 1001–1008 [DOI] [PubMed] [Google Scholar]
- 121. Burk D., et al. (1941). Metabolism of butter yellow rat liver cancers. Canc. Res., 1, 733–734 [Google Scholar]
- 122. Simek J., et al. (1965). Effect of glucose administered in vivo or in vitro on the respiratory quotient of rat liver tissue after partial hepatectomy. Nature, 207, 761–762 [DOI] [PubMed] [Google Scholar]
- 123. Diatlovitskaia E.V., et al. (1972). [Cardiolipins of mitochondria and microsomes of Jensen’s sarcoma]. Dokl. Akad. Nauk SSSR, 206, 737–739 [PubMed] [Google Scholar]
- 124. Diatlovitskaia E.V., et al. (1976). [Positional distribution of fatty acids in the phospholipids of regenerating rat liver]. Biokhimiia., 41, 538–542 [PubMed] [Google Scholar]
- 125. Crabtree H.G. (1929). Observations on the carbohydrate metabolism of tumours. Biochem. J., 23, 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Redman E.K., et al. (2013) Role of p90(RSK) in regulating the Crabtree effect: implications for cancer. Biochem. Soc. Trans., 41, 124–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Díaz-Ruiz R., et al. (2008) Mitochondrial oxidative phosphorylation is regulated by fructose 1,6-bisphosphate. A possible role in Crabtree effect induction? J. Biol. Chem., 283, 26948–26955 [DOI] [PubMed] [Google Scholar]
- 128. Diaz-Ruiz R., et al. (2011) The Warburg and Crabtree effects: on the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim. Biophys. Acta, 1807, 568–576 [DOI] [PubMed] [Google Scholar]
- 129. Holleran A.L., et al. (1995) Glutamine metabolism in AS-30D hepatoma cells. Evidence for its conversion into lipids via reductive carboxylation. Mol. Cell. Biochem., 152, 95–101 [DOI] [PubMed] [Google Scholar]
- 130. Fendt S.M., et al. (2013) Reductive glutamine metabolism is a function of the α-ketoglutarate to citrate ratio in cells. Nat. Commun., 4, 2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pollard P.J., et al. (2003). The TCA cycle and tumorigenesis: the examples of fumarate hydratase and succinate dehydrogenase. Ann. Med., 35, 632–639 [DOI] [PubMed] [Google Scholar]
- 132. Gottlieb E., et al. (2005). Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer, 5, 857–866 [DOI] [PubMed] [Google Scholar]
- 133. Seyfried T.N. (2012). Cancer models. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 31–46 [Google Scholar]
- 134. Ecsedy J.A., et al. (1999). Expression of mouse sialic acid on gangliosides of a human glioma grown as a xenograft in SCID mice. J. Neurochem., 73, 254–259 [DOI] [PubMed] [Google Scholar]
- 135. Chou H.H., et al. (1998). A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl Acad. Sci. U. S. A., 95, 11751–11756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Martin M.J., et al. (2005). Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med., 11, 228–232 [DOI] [PubMed] [Google Scholar]
- 137. Davies B., et al. (1993) Physiological parameters in laboratory animals and humans. Pharm. Res., 10, 1093–1095 [DOI] [PubMed] [Google Scholar]
- 138. Mahoney L.B., et al. (2006). Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis., 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tentler J.J., et al. (2012) Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol., 9, 338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chaparro R.J., et al. (2006). Nonobese diabetic mice express aspects of both type 1 and type 2 diabetes. Proc. Natl Acad. Sci. U. S. A., 103, 12475–12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Seyfried T.N. (2012). Mitochondrial respiratory dysfunction and the extrachromosomal origin of cancer. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 253–259 [Google Scholar]
- 142. Hu Y., et al. (2012). K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res., 22, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Neuzil J., et al. (2012) K-Ras and mitochondria: dangerous liaisons. Cell Res., 22, 285–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Szent-Györgyi A. (1977). The living state and cancer. Proc. Natl Acad. Sci. U. S. A., 74, 2844–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pawelek J.M., et al. (2008). The cancer cell–leukocyte fusion theory of metastasis. Adv. Cancer Res., 101, 397–444 [DOI] [PubMed] [Google Scholar]
- 146. Seyfried T.N., et al. (2013) On the origin of cancer metastasis. Crit. Rev. Oncog., 18, 43–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Powell A.E., et al. (2011). Fusion between Intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res., 71, 1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pawelek J.M. (2005). Tumour-cell fusion as a source of myeloid traits in cancer. Lancet Oncol., 6, 988–993 [DOI] [PubMed] [Google Scholar]
- 149. Malkin D., et al. (1990). Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science, 250, 1233–1238 [DOI] [PubMed] [Google Scholar]
- 150. Funes J.M., et al. (2007). Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc. Natl Acad. Sci. U. S. A., 104, 6223–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sung H.J., et al. (2011). Mitochondrial respiration protects against oxygen-associated DNA damage. Nat Commun, 1, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lago C.U., et al. (2011) p53, aerobic metabolism, and cancer. Antioxid. Redox Signal., 15, 1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Matoba S., et al. (2006). p53 regulates mitochondrial respiration. Science, 312, 1650–1653 [DOI] [PubMed] [Google Scholar]
- 154. Zhou S., et al. (2003). Mitochondrial impairment in p53-deficient human cancer cells. Mutagenesis, 18, 287–292 [DOI] [PubMed] [Google Scholar]
- 155. Lee A.C., et al. (1999). Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem., 274, 7936–7940 [DOI] [PubMed] [Google Scholar]
- 156. Weinberg F., et al. (2010). Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. U. S. A., 107, 8788–8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yang D., et al. (2010). Impairment of mitochondrial respiration in mouse fibroblasts by oncogenic H-RAS(Q61L). Cancer Biol. Ther., 9, 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Moiseeva O., et al. (2009). Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol. Cell. Biol., 29, 4495–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Seyfried T.N. (2012). Respiratory insufficiency, the retrograde response, and the origin of cancer. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 177–194 [Google Scholar]
- 160. Guha M., et al. (2013) Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion, 13, 577–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Singh K.K., et al. (2005). Inter-genomic cross talk between mitochondria and the nucleus plays an important role in tumorigenesis. Gene, 354, 140–146 [DOI] [PubMed] [Google Scholar]
- 162. Jazwinski S.M. (2005). The retrograde response links metabolism with stress responses, chromatin-dependent gene activation, and genome stability in yeast aging. Gene, 354, 22–27 [DOI] [PubMed] [Google Scholar]
- 163. Nargund A.M., et al. (2012). Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science, 337, 587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Al Mamun A.A., et al. (2012). Identity and function of a large gene network underlying mutagenic repair of DNA breaks. Science, 338, 1344–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Dang C.V. (2010). Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle, 9, 3884–3886 [DOI] [PubMed] [Google Scholar]
- 166. Bissell M.J., et al. (2011). Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med., 17, 320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gatenby R.A., et al. (2007). Glycolysis in cancer: a potential target for therapy. Int. J. Biochem. Cell Biol., 39, 1358–1366 [DOI] [PubMed] [Google Scholar]
- 168. Husain Z., et al. (2013) Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J. Immunol., 191, 1486–1495 [DOI] [PubMed] [Google Scholar]
- 169. Liu E.T. (2013). Grappling with cancer. Science, 339, 1493. [DOI] [PubMed] [Google Scholar]
- 170. Pennisi E. (2013). Steering cancer genomics into the fast lane. Science, 339, 1540–1542 [DOI] [PubMed] [Google Scholar]
- 171. Stratton M.R., et al. (2009). The cancer genome. Nature, 458, 719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Crespi B., et al. (2005). Evolutionary biology of cancer. Trends Ecol. Evol., 20, 545–552 [DOI] [PubMed] [Google Scholar]
- 173. Merlo L.M., et al. (2006). Cancer as an evolutionary and ecological process. Nat. Rev. Cancer, 6, 924–935 [DOI] [PubMed] [Google Scholar]
- 174. Davies P.C., et al. (2011). Cancer tumors as Metazoa 1.0: tapping genes of ancient ancestors. Phys. Biol., 8, 015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mayer E. (1982). Evolution before Darwin. In The Growth of Biological Thought: Diversity, Evolution, and Inheritance. Belknap Harvard, Cambridge, MA, pp. 343–362 [Google Scholar]
- 176. Handel A.E., et al. (2010). Is Lamarckian evolution relevant to medicine? BMC Med. Genet., 11, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Koonin E.V., et al. (2009). Is evolution Darwinian or/and Lamarckian? Biol. Direct, 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Smiraglia D.J., et al. (2008). A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol. Ther., 7, 1182–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Minocherhomji S., et al. (2012) Mitochondrial regulation of epigenetics and its role in human diseases. Epigenetics, 7, 326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Feinberg A.P., et al. (2004). The history of cancer epigenetics. Nat. Rev. Cancer, 4, 143–153 [DOI] [PubMed] [Google Scholar]
- 181. Pawelek J.M. (2000). Tumour cell hybridization and metastasis revisited. Melanoma Res., 10, 507–514 [DOI] [PubMed] [Google Scholar]
- 182. Huysentruyt L.C., et al. (2010). Perspectives on the mesenchymal origin of metastatic cancer. Cancer Metastasis Rev., 29, 695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Holmgren L., et al. (1999). Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood, 93, 3956–3963 [PubMed] [Google Scholar]
- 184. Dziurzynski K., et al. ; HCMV and Gliomas Symposium (2012) Consensus on the role of human cytomegalovirus in glioblastoma. Neuro. Oncol., 14, 246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Detmer S.A., et al. (2007). Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol., 8, 870–879 [DOI] [PubMed] [Google Scholar]
- 186. Xu R.H., et al. (2005). Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res., 65, 613–621 [PubMed] [Google Scholar]
- 187. VanItallie T.B., et al. (2003). Ketones: metabolism’s ugly duckling. Nutr. Rev., 61, 327–341 [DOI] [PubMed] [Google Scholar]
- 188. Drenick E.J., et al. (1972) Resistance to symptomatic insulin reactions after fasting. J. Clin. Invest., 51, 2757–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Veech R.L. (2004). The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins. Leukot. Essent. Fatty Acids, 70, 309–319 [DOI] [PubMed] [Google Scholar]
- 190. Krebs H.A., et al. (1971). The role of ketone bodies in caloric homeostasis. Adv. Enzyme Reg., 9, 387–409 [Google Scholar]
- 191. Bonuccelli G., et al. (2010). Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle, 9, 3506–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Seyfried T.N. (2012). Metabolic management of cancer. In Cancer As a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. John Wiley & Sons, Hoboken, NJ, pp. 291–354 [Google Scholar]
- 193. Maurer G.D., et al. (2011). Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer, 11, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Skinner R., et al. (2009). Ketone bodies inhibit the viability of human neuroblastoma cells. J. Pediatr. Surg., 44, 212–6; discussion 216 [DOI] [PubMed] [Google Scholar]
- 195. Seyfried T.N., et al. (2003). Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br. J. Cancer, 89, 1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Jiang Y.S., et al. (2013) Caloric restriction reduces edema and prolongs survival in a mouse glioma model. J. Neurooncol., 114, 25–32 [DOI] [PubMed] [Google Scholar]
- 197. Mukherjee P., et al. (2004). Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin. Cancer Res., 10, 5622–5629 [DOI] [PubMed] [Google Scholar]
- 198. Mukherjee P., et al. (2002). Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br. J. Cancer, 86, 1615–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Mulrooney T.J., et al. (2011). Influence of caloric restriction on constitutive expression of NF-κB in an experimental mouse astrocytoma. PLoS One, 6, e18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Simone B.A., et al. (2013). Selectively starving cancer cells through dietary manipulation: methods and clinical implications. Future Oncol., 9, 959–976 [DOI] [PubMed] [Google Scholar]
- 201. Stafford P., et al. (2010). The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr. Metab. (Lond)., 7, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Fine E.J., et al. (2012). Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition, 28, 1028–1035 [DOI] [PubMed] [Google Scholar]
- 203. Zuccoli G., et al. (2010). Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr. Metab. (Lond)., 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Nebeling L.C., et al. (1995). Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J. Am. Coll. Nutr., 14, 202–208 [DOI] [PubMed] [Google Scholar]
- 205. Zhou W., et al. (2007). The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. (Lond)., 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Gottschalk S., et al. (2004) Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin. Cancer Res., 10, 6661–6668 [DOI] [PubMed] [Google Scholar]
- 207. Zhao Y., et al. (2011). Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res., 71, 4585–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Marsh J., et al. (2008). Akt-dependent proapoptotic effects of dietary restriction on late-stage management of a phosphatase and tensin homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma. Clin. Cancer Res., 14, 7751–7762 [DOI] [PubMed] [Google Scholar]
- 209. Veech R.L., et al. (2001). Ketone bodies, potential therapeutic uses. IUBMB Life, 51, 241–247 [DOI] [PubMed] [Google Scholar]
- 210. Chen Y., et al. (2009). Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS One, 4, e7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Spitz D.R., et al. (2000). Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann. N. Y. Acad. Sci., 899, 349–362 [DOI] [PubMed] [Google Scholar]
- 212. Harrison L., et al. (2004). Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist, 9 (suppl. 5), 31–40 [DOI] [PubMed] [Google Scholar]
- 213. Allen B.G., et al. (2013) Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin. Cancer Res., 19, 3905–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Abdelwahab M.G., et al. (2012). The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One, 7, e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Seyfried T.N., et al. (2010). Does the existing standard of care increase glioblastoma energy metabolism? Lancet Oncol., 11, 811–813 [DOI] [PubMed] [Google Scholar]
- 216. Yuneva M. (2008). Finding an “Achilles’ heel” of cancer: the role of glucose and glutamine metabolism in the survival of transformed cells. Cell Cycle, 7, 2083–2089 [DOI] [PubMed] [Google Scholar]
- 217. DeBerardinis R.J., et al. (2010). Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene, 29, 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lazova R., et al. (2013). A melanoma brain metastasis with a donor-patient hybrid genome following bone marrow transplantation: first evidence for fusion in human cancer. PLoS One, 8, e66731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Newsholme P. (2001). Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr., 131(suppl. 9), 2515S–2522S; discussion 2523S. [DOI] [PubMed] [Google Scholar]
- 220. Mates J.M., et al. (2012). Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Current Mol. Med., 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 221. Shelton L.M., et al. (2010). Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int. J. Cancer, 127, 2478–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Lim V., et al. (2009). Glutamine prevents DMBA-induced squamous cell cancer. Oral Oncol., 45, 148–155 [DOI] [PubMed] [Google Scholar]
- 223. Vincent M.D. (2011) Cancer: beyond speciation. Adv. Cancer Res., 112, 283–350 [DOI] [PubMed] [Google Scholar]
- 224. Arens N.C., et al. (2008). Press-pulse: a general theory of mass extinction? Paleobiology, 34, 456–471 [Google Scholar]
- 225. Ko Y.H., et al. (2004). Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem. Biophys. Res. Commun., 324, 269–275 [DOI] [PubMed] [Google Scholar]
- 226. Bonnet S., et al. (2007). A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell, 11, 37–51 [DOI] [PubMed] [Google Scholar]
- 227. Marsh J., et al. (2008). Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-D-glucose and the restricted ketogenic diet. Nutr. Metab. (Lond)., 5, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Michaelis M., et al. (2009). The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia, 11, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Yu Y., et al. (2013) Genome-wide identification and characterization of polygalacturonase genes in Cucumis sativus and Citrullus lanatus. Plant Physiol. Biochem., 74C, 263–275 [DOI] [PubMed] [Google Scholar]
- 230. Bozidis P., et al. (2010) Trafficking of UL37 proteins into mitochondrion-associated membranes during permissive human cytomegalovirus infection. J. Virol., 84, 7898–7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Williamson C.D., et al. (2009) Access of viral proteins to mitochondria via mitochondria-associated membranes. Rev. Med. Virol., 19, 147–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Dziurzynski K., et al. (2011) Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clin. Cancer Res., 17, 4642–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Munzarová M., et al. (1991). Do some malignant melanoma cells share antigens with the myeloid monocyte lineage? Neoplasma, 38, 401–405 [PubMed] [Google Scholar]
- 234. Söderberg-Nauclér C., et al. (2013) Survival in patients with glioblastoma receiving valganciclovir. N. Engl. J. Med., 369, 985–986 [DOI] [PubMed] [Google Scholar]
- 235. Clarke K., et al. (2012) Kinetics, safety and tolerability of ®-3-hydroxybutyl ®-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol., 63, 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. D’Agostino D.P., et al. (2013) Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol., 304, R829–R836 [DOI] [PubMed] [Google Scholar]
- 237. Lu W., et al. (2012). Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol., 10, e1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. de Groof A.J., et al. (2009). Increased OXPHOS activity precedes rise in glycolytic rate in H-RasV12/E1A transformed fibroblasts that develop a Warburg phenotype. Mol. Cancer, 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Baracca A., et al. (2010) Mitochondrial Complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim. Biophys. Acta, 1797, 314–323 [DOI] [PubMed] [Google Scholar]
- 240. Roskelley R.C., et al. (1943). Studies in cancer. VII. Enzyme deficiency in human and experimental cancer. J. Clin. Invest., 22, 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Seyfried T.N., et al. (2008). Targeting energy metabolism in brain cancer with calorically restricted ketogenic diets. Epilepsia, 49 Suppl 8, 114–116 [DOI] [PubMed] [Google Scholar]
- 242. Huysentruyt L.C., et al. (2008). Metastatic cancer cells with macrophage properties: evidence from a new murine tumor model. Int. J. Cancer, 123, 73–84 [DOI] [PubMed] [Google Scholar]
- 243. Poff A.M., et al. (2013). The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS One, 8, e65522. [DOI] [PMC free article] [PubMed] [Google Scholar]