Summary
We explored the mechanism of the AhR agonist, BNF, antitumor effects in human breast cancer cells and found that BNF inhibits breast cancer growth through the AKT pathway.
Abstract
Beta-naphthoflavone (BNF, DB06732) is an agonist of aryl hydrocarbon receptor (AhR) and a putative chemotherapeutic agent that has antitumor activity against mammary carcinomas in vivo. However, the mechanism by which BNF exerts this antitumor effect remains unclear. Thus, we explored mechanisms of BNF’s antitumor effects in human breast cancer cells. This study showed that BNF suppressed cell proliferation and induced cell cycle arrest in the G0/G1 phase with downregulation of cyclin D1/D3 and CDK4 and upregulation of p21Cip1/Waf1, leading to a senescence-like phenotype in estrogen receptor (ER)-positive MCF-7 cells, but not in ER-negative MDA-MB-231 cells. In addition, BNF inhibited PI3K/AKT signaling, and the PI3K inhibitor, LY294,002, exhibited the same inhibitory effects on cyclinD1/D3, CDK4 and the cell cycle as BNF. Interestingly, BNF activated mitogen-activated protein kinase-extracellular signal-regulated kinase (MAPK-ERK) signaling, and more notably, MEK inhibitor PD98059 significantly blocked the BNF-induced cell cycle arrest and upregulation of p21Cip1/Waf1. Furthermore, specific ERα and AhR siRNA studies indicate that ERα is required in BNF-induced p21Cip1/Waf1 expression, and BNF-mediated cell cycle arrest and modulation of AKT and ERK signaling is AhR-dependent. Taken together, AhR-dependent inhibition of the PI3K/AKT pathway, activation of MAPK/ERK and modulation of ERα is a novel mechanism underlying BNF-mediated antitumor effects in breast cancer, which may represent a promising strategy to be exploited in future clinical trials.
Introduction
Beta-naphthoflavone (BNF, DB06732), also called 5,6-benzoflavone, is a synthetically derived flavonoid and a known agonist of aryl hydrocarbon receptor (AhR), through which it induces cytochrome P450 1A (CYP1A) expression (1). The therapeutic potential of flavonoids has been touted for treatment of medical problems ranging from cardiovascular disease (2) to cancer (3). BNF also exhibits potent antitumor activity against mammary carcinomas in vivo (4–6); however, its mechanism of action remains unclear.
AhR is a ligand-activated transcription factor that mediates the effects of many environmental contaminants through inducing CYP1A (7). Ligand binding to AhR prompts nuclear translocation and subsequent heterodimerization with aryl hydrocarbon nuclear translocator (ARNT), as well as with transcriptional co-activators or co-repressors (7). The activated AhR complex binds to specific DNA sequences, termed dioxin-response elements and regulates transcription of genes. AhR has long been investigated for its role in mediating dioxin toxicity (8). However, recent studies have also explored other intriguing biological roles of AhR, including regulation of development, immunity, circadian rhythm and cancer biology (7,9,10).
The role of AhR in breast cancer biology has been extensively investigated, and notably increasing evidence indicates that the ultimate response of breast cancer to AhR is dependent upon estrogen receptor (ER) status, ligand presence and cell type (11–13). Deregulation of ER expression is crucial in the development of breast cancer, and estrogen-mediated ER alpha (ERα) activation promotes breast cancer growth (14). Under certain circumstances, agonist-activated AhR prompts ERα protein ubiquitination and degradation, and upregulation of enzymes that metabolize estrogen, which synergistically inhibits estrogen-induced ER-positive breast cancer cell proliferation (7,13). In contrast, other agonists activate AhR and subsequently inhibit ER-negative breast cancer cell proliferation or cell cycle progression independent of ERα (11,15). Clearly then, AhR has effects on breast cancer cell proliferation that are both dependent and independent of its crosstalk with ERα. In addition, some AhR agonists can also directly bind to ER and regulate breast cancer cell proliferation independent of AhR (16). Taken together, these data suggest that mechanisms underlying the effects of AhR agonists on breast cancer are very complex.
In the present study, we explored the effects and molecular mechanisms of an AhR agonist, BNF, on ER-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Our results show that BNF inhibits the proliferation of MCF-7 cells, but not MDA-MB-231 cells through a novel mechanism in which BNF induces G0/G1 phase arrest and senescence through AhR-mediated inhibition of PI3K/AKT signaling, consequently downregulation of cyclin D1/D3 and CDK4, as well as activation of the mitogen-activated protein kinase-extracellular signal-regulated kinase (MAPK/ERK) causing ERα-dependent upregulation of p21Cip1/Waf1.
Materials and methods
Reagents
BNF (DB06732), LY294,002, PD98059 and MG132 from Sigma–Aldrich (St Louis, MO) were dissolved in dimethyl sulfoxide (DMSO) as stock solutions, diluted in culture medium and added to cells at a final DMSO concentration of 0.1%. The following primary antibodies were used for immunoblot: ERα, p53 and CYP1A1 from Santa Cruz Biotechnology (Santa Cruz, CA); AhR from Abcam; β-Actin from Sigma–Aldrich; poly(ADP-ribose) polymerase (PARP), p-ERK1/2, ERK1/2, p-AKT pathway antibody and cell cycle regulation sampler kit were purchased from Cell Signaling Technology (Beverly, MA). Other chemicals and biochemistry regents were obtained from Sigma–Aldrich unless otherwise mentioned.
Cell culture and siRNA transfection
Two human breast cancer cell lines MCF-7 (ER-positive) and MDA-MB-231(ER-negative) (ATCC) were cultured and treated in DMEM Reduced Serum medium (HyClone) with 7.5% bovine growth serum (HyClone) and penicillin/streptomycin/amphotericin (MP Biomedicals) at 37°С in a humidified, 5% CO2 atmosphere. Cultures were treated with BNF or an equal volume of the DMSO vehicle (0.1% of the total volume). Negative, AhR and ERα siRNAs (40nM, Life Technologies) were delivered into cells (2.5×105) in 6-well plates using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s recommendations. The sequences used were AhR siRNA sense 5′GCA UGA UAG UUU UCC GGC U dTdT and AhR siRNA antisense 5′AGC CGG AAA ACU AUC AUG C dCdA; ERα siRNA sense 5′GAU GAA AGG UGG GAU ACG A dTdT3′ and ERα siRNA antisense 5′UCG UAU CCC ACC UUU CAU C dTdT3′ (17).
Immunoblot analysis
Cells were washed with cold phosphate-buffered saline and homogenized in lysis buffer (Roche); total protein (80–100 μg) was separated using 10 or 14% sodium dodecyl sulfate–polyacrylamide gel electrophoresis depending on protein size, followed by electrophoretic transfer to nitrocellulose membranes (Bio-Rad). The transblotted membrane was blocked with Tris-buffered saline containing 0.05% Tween 20 (TBST) containing 5% non-fat milk for 60min at room temperature, and then washed three times with TBST. The membrane was incubated with a primary antibody at an appropriate dilution according to the manufacturer’s recommendations in TBST at 4°С overnight, and then washed three times with TBST. The membrane was probed with anti-mouse, anti-rabbit or anti-goat secondary antibody (LI-COR Bioscience) for 60min at room temperature and washed three times with TBST. Images were taken by LI-COR imaging system (LI-COR Bioscience).
MTT colorimetric assay
Cells were seeded onto 96-well tissue culture plates at a density of 5 × 103 per well and incubated overnight. After 24, 48 and 72 h exposures with 10 or 100 µM BNF, MTT (Research Products International) was added to each well (final concentration, 2.5mg/ml) and incubated for 3h to allow metabolism of MTT by mitochondrial dehydrogenase to an insoluble formazan product. The medium was aspirated and formazan was solubilized by the addition of 100 µl of DMSO. Cell viability was determined by absorbance at 570nm on a universal microplate reader.
Cell cycle analysis
The MCF-7 and MDA-MB-231 cell lines were plated in 60mm dishes at a density of 5 × 105 cells. After treatment with 10 µM BNF for 48h, the cells were trypsinized and washed once with cold phosphate-buffered saline. Cells were then fixed in cold 70% ethanol overnight at 4°С for cell cycle analysis. Before the assay, Rnase A (final concentration, 0.2–0.5mg/ml) digestion was performed at 37°С for 1h. The cells were stained with propidium iodide (final concentration, 100 µg/ml) and kept in the dark at 4°С until analysis. Flow cytometry was performed in the Core Lab at South Illinois University School of Medicine.
RNA isolation, reverse transcription and real-time quantitative PCR
MCF-7 and MDA-MB-231 cells were plated in a 6-well plate at a density of 2.5 × 105 cells, grown overnight and transfected with siRNA and/or treated with 10 µM BNF or DMSO for 36–48h. Total RNA was extracted using TRIzol (Life Technologies) according to the manufacturer’s recommendations. Following cDNA synthesis (Promega protocol), 5 μl cDNA (1:5 dilution) was used for quantitative polymerase chain reaction (qPCR) using SYBR green (Quanta Biosciences) in a Smart Cycler rapid thermal cycler (Cepheid). Each assay included a no reverse transcriptase negative control. β-Actin was used for normalization. The primers used were 5′CTG GAG ACT CTC AGG GTC GAA3′ and 5′GGA TTA GGG CTT CCT CTT GGA 3′for p21waf1/cip1 (18), 5′CTT CCG ACA CTC TTC CTT CG3′ and 5′GGT TGA TCT GCC ACT GGT TT3′for CYP1A1, and 5′ATC TGG CAC CAC ACC TTC TA3′ and 5′GTA CAT GGC TGG GGT GTT GAA G3′for β-Actin (19). ‘Relative standard curves were created to evaluate primer’s amplification efficiency.’ Relative expression was assessed by the comparative CT method and normalized by β-Actin. ‘PCR product specificity from each primer pair was confirmed using melting curve analysis and subsequent agarose gel electrophoresis.’
Senescence-associated β-galactosidase activity
Cells growing on 6-well tissue culture plates were fixed with 2% formaldehyde and 0.2% glutaraldehyde. Senescence-associated β-galactosidase (SAβ-gal) activity was detected following the Senescence β–galactosidase Staining Kit (Cell Signaling Technology). Cells were photographed and scored under an inverted microscope. SAβ-gal-positive rate (SAβ-gal+) (%) was calculated as follows: (number of SAβ-gal staining-positive cells/number of total cells) × 100.
Apoptosis detection
Apoptosis was evaluated by PARP cleavage and DNA fragmentation because both of them are key features of apoptosis. PARP cleavage was detected by western blot as described above; DNA fragmentation was examined as addressed previously (20). In brief, cells growing on 60 mm cell culture dishes were harvested and lysed in a lysis buffer including 0.5% Triton X-100, 5mM Tris-HCl (pH 7.4) and 5mM EDTA for 20min on ice. Supernatants were collected by centrifugation at 10 000g for 15min, and incubated with 100 µg/ml of proteinase K (Applied Biosystems) and 0.5% sodium dodecyl sulfate (final concentration) for 2h at 56oC. DNA was extracted with phenol-chloroform and precipitated with ethanol. DNA pellets were dissolved in 20mM Tris-HCl (pH 7.4) with 50 µg/ml of RNase A and incubated at 37oC for 1h. DNA products were then separated on 2% agarose gel.
Statistics
Statistical analysis was performed using Student’s t-test or one-way ANOVA with Tukey’s post hoc comparison. The P values were considered statistically significant at P < 0.05. All data are means ± SEM for at least three separate experiments.
Results
BNF induces CYP1A1 differently in MCF-7 versus MDA-MB-231 cells
To study the effect of BNF on breast cancer cells, we used two typical human breast cancer cell lines, MCF-7 and MDA-MB-231 cells. Both cell lines express AhR protein (Supplementary Figure S1A, available at Carcinogenesis Online), and the AhR level is higher in MDA-MB-231 cells than in MCF-7 cells. We evaluated the AhR target gene CYP1A1 after BNF treatment in these cell lines; in MCF-7 cells both CYP1A1 protein and mRNA were increased after 10 µM BNF treatment (Supplementary Figure S1B and C, available at Carcinogenesis Online). In MDA-MD-231 cells, however, CYP1A1 mRNA was only weakly upregulated and CYP1A1 protein was almost unchanged (Supplementary Figure S1B and C, available at Carcinogenesis Online), consistent with a previous report (21).
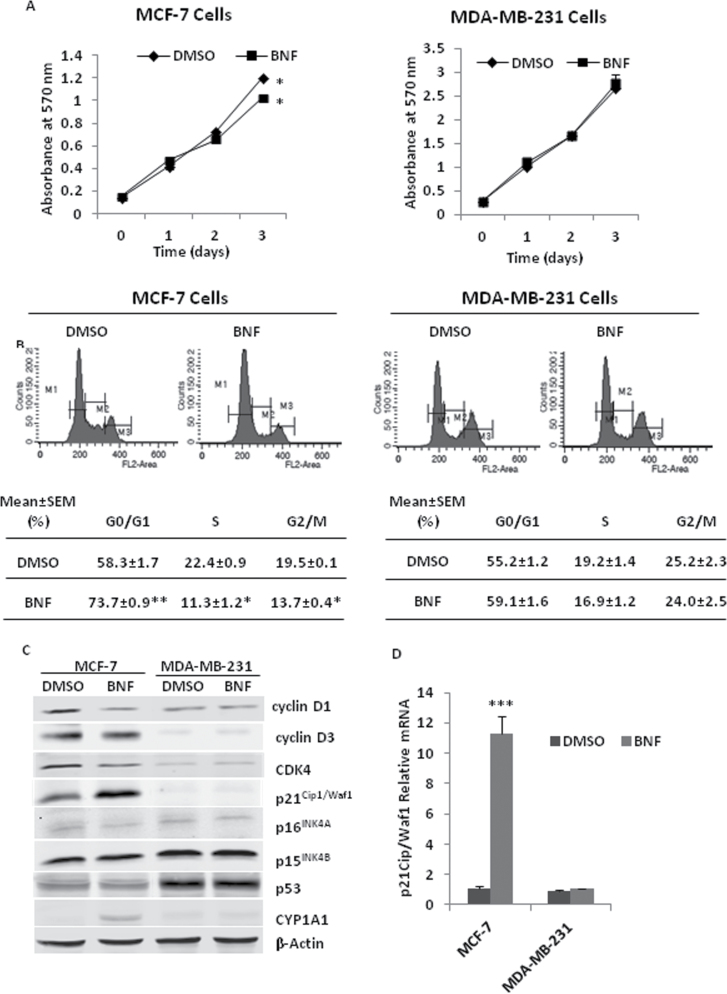
BNF inhibits cell proliferation, induces cell cycle arrest and senescence and regulates cell cycle proteins in MCF-7 cells
We examined the effects of BNF on cell proliferation in both MCF-7 and MDA-MB-231 cell lines. BNF (10 µM) inhibited MCF-7 proliferation at 72 h, but had no effect on MDA-MB-231 cells (Figure 1). Increasing the BNF dose to 100 µM nearly completely abolished MCF-7 cell proliferation, but still had no effect on MDA-MB-231 cells (Supplementary Figure S2, available at Carcinogenesis Online).
Fig. 1.
BNF inhibits cell proliferation, induces cell cycle arrest and cell senescence, and regulates the expression of cell cycle proteins MCF-7 cells. (A) 5×103 MCF-7 and MDA-MB-231 cells were seeded in 96-well plates. After an overnight culture, cells were treated with 10 µM BNF for 0, 1, 2 or 3 days. Cell proliferation was assessed by MTT. (B) Distribution of cell cycle in MCF-7 and MDA-MB-231 cells following treatment with 10 µM BNF for 48h. Upper panel, representative cell cycle distribution; Lower panel, quantitation of cell cycle distribution. (C) Expression levels of cell cycle proteins were detected by western blot in 10 µM BNF-treated MCF-7 and MDA-MB-231 cells for 48h. (D) The transcription level of p21Cip1/Waf1 was tested by qPCR in 10 µM BNF-treated MCF-7 and MDA-MB-231 cells. (E and F) BNF regulates the expression of cell cycle proteins by dose dependence (E) and time dependence (F) in MCF-7 cells. (G) Cell senescence was examined by SAβ-gal activity analysis. Right panel, representative senescent morphology; Left panel, quantitation of SAβ-gal activity, and SAβ-gal-positive rate (SAβ-gal+) (%) is calculated as follows: (number of SAβ-gal staining-positive cells/number of total cells) × 100. The data represent the mean ± SEM from three to five separate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001, versus DMSO-treated cells.
To assess the mechanism responsible for the growth-inhibitory activity of BNF, cell apoptosis detection and flow cytometric analysis of the cell cycle were performed in both MCF-7 and MDA-MB-231 cells. BNF (10 µM for 48h) did not induce cell apoptosis by DNA ladder analysis and cleaved PARP detection (data not shown). However, BNF treatment (10 µM for 48h) inhibited a cell cycle progression in MCF-7 cells, but not in MDA-MB-231 cells, which was reflected by a distinct increase of the percentage of cells in the G0/G1 phase and a significant reduction in the S and G2/M phase (Figure 1B).
Cell cycle progression through G1/S phase is tightly regulated by the interplay between Cyclin D, CDK4/6 and their inhibitors, p21Cip1/Waf1, p16INK4A and p15INK4B. Thus, we examined the effects of BNF on expression of these cell cycle proteins. Consistent with its effects on cell cycle arrest, BNF (10 µM) reduced cyclin D1 and CDK4, slightly reduced cyclin D3 and distinctly increased p21Cip1/Waf1 protein and mRNA levels in MCF-7 cells (Figure 1C and D) in a time- and dose-dependent manner (Figure 1E and F). BNF induced more p21Cip1/Waf1 mRNA than protein in 48 h of treatment (Figure 1C and D) possibly because mRNA was not fully translated into protein in short time, and more p21Cip1/Waf1 mRNA was translated into protein in 72 h (Figure 1F). The upstream regulator of p21Cip1/Waf1, the p53 tumor suppressor (22), however, was not changed in BNF-treated MCF-7 cells with functional p53 or MDA-MB-231 cells with p53 mutation (Figure 1C), suggesting that BNF-induced p21Cip1/Waf1 increase may be p53-independent.
p21Cip1/Waf1-mediated cell cycle arrest is accompanied by phenotypic features of senescence (23,24). Cell senescence assay detected a large flat morphology and SAβ-gal activity (Figure 1G), an indicator of cell senescence (25), in MCF-7 cells exposed to BNF for 3 days.
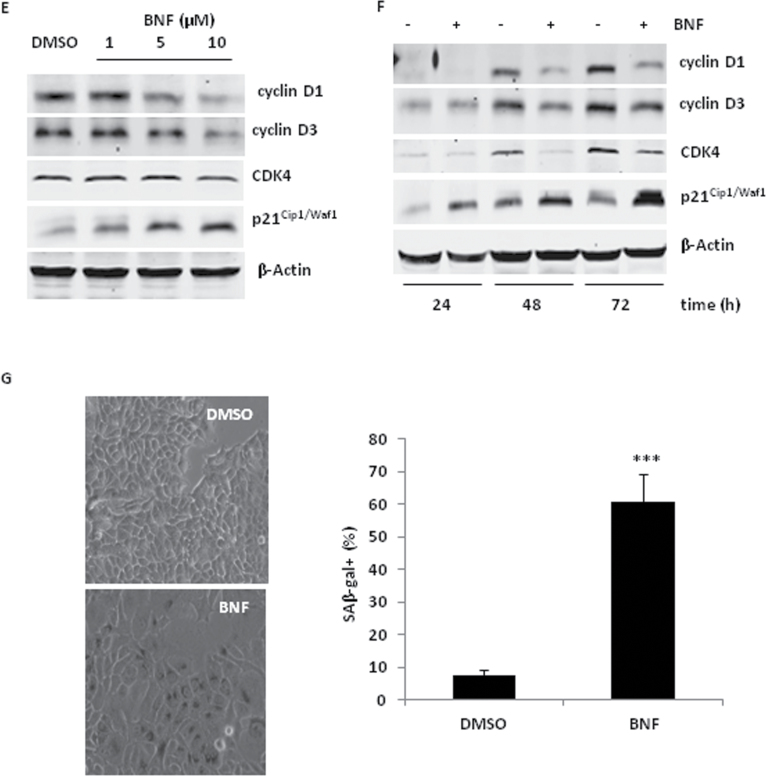
BNF regulates the PI3K/AKT pathway and MAPK signaling
The phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) is critical in breast cancer development and therapy due to a frequent mutation in this pathway that causes hyperactivation and promotes a cell cycle progression (26–28). Although the MAPK-ERK pathway is generally associated with cell proliferation (29), increasing evidence indicates that prolonged hyperactivation of ERK leads to p21Cip1/Waf1 upregulation, cell cycle arrest and cell senescence (23,30).
Considering the importance of PI3K/AKT pathway and MAPK signaling in cell cycle regulation, we examined the effects of BNF on PI3K/AKT and ERK signaling. MCF-7 and MDA-MB-231cells were exposed to 10 µM BNF for 48 h. BNF significantly reduced phosphorylated AKT protein (Ser473 and Thr308) and increased phosphorylated ERK protein (Figure 2A) in MCF-7 cells. Accordingly, phosphorylation of PI3K/AKT pathway proteins, p-PDK1 (Ser241) and p-GSK-3β (ser9), and p-c-Raf (Ser259) were also significantly reduced (Figure 2A). The effects of BNF on PI3K/AKT and ERK in MCF-7 cells were dose- and time-dependent (Figure 2B and C).
Fig. 2.
BNF regulates the PI3K/AKT pathway and MAPK signaling. Western blot analysis of the PI3K/AKT pathway-regulated proteins and phosphorylated ERK in (A) MCF-7 and MDA-MB-231 cells treated with 10 µM BNF, (B) MCF-7 cells treated with different concentration of BNF and (C) MCF-7 cells treated with 10 µM BNF for different time.
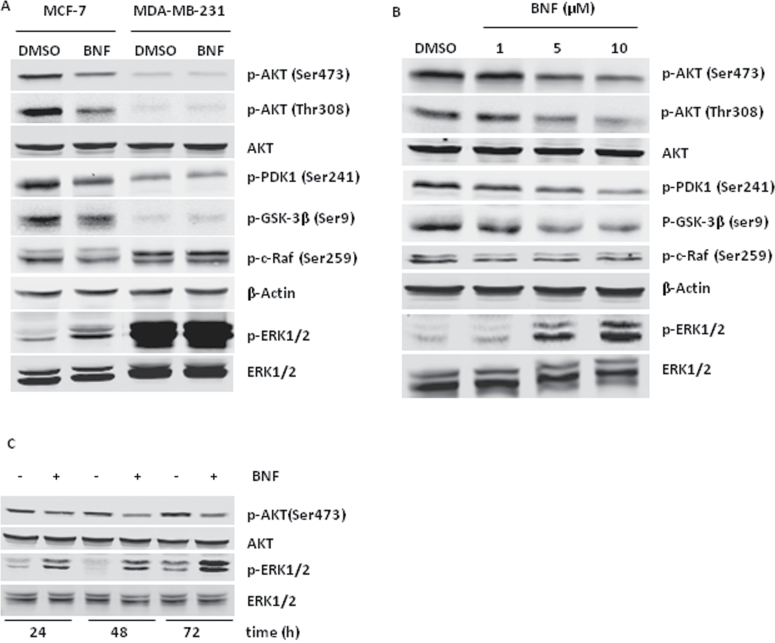
BNF inhibits cyclinD1/D3 and CDK4 and induces p21Cip1/Waf1 by modulating PI3K/AKT and MAPK signaling respectively
To determine if the inhibition of PI3K/AKT pathway and/or the activation of MAPK signaling are responsible for the change of cell cycle regulating proteins, we used two chemical inhibitors of the PI3K and MAPK kinase, LY294,002 and PD98059, respectively. Increasing concentrations of LY294002 (24 h) resulted in dose-dependent downregulation of p-AKT, cyclinD1/D3 and CDK4, but did not change p21Cip1/Waf1 expression, ERK activation or the basal CYP1A1 protein level (Figure 3A). These data demonstrate that the PI3K/AKT pathway positively regulates the expression of cyclinD1/D3 and CDK4, but is not related to the increasing of p21Cip1/Waf1 expression or ERK activation in BNF-treated MCF-7 cells. Similar to BNF, LY294002 treatment also resulted in a cell cycle arrest in G0/G1 (Figure 3B).
Fig. 3.
BNF inhibits the expression of cyclinD1/D3 and CDK4 and induces p21Cip1/Waf1 by modulating PI3K/AKT and MAPK signaling respectively. For A and B, MCF-7 cells were treated with PI3K inhibitor LY294, 002 for 48h. (A) Western blot analysis of the cell cycle proteins and the PI3K/AKT and ERK signaling proteins. (B) Flow cytometric analysis of cell cycle arrest. Upper panel, representative cell cycle distribution; Lower panel, quantitation of cell cycle distribution. For C, D and E, MCF-7 cells were preincubated with MEK inhibitor PD98059 (10 µM) for 1h and then treated with 10 µM BNF for 48h. (C) Western blot analysis of the cell cycle proteins, AKT and ERK phosporylation and (D) qPCR analysis of p21Cip1/Waf1 mRNA level. (E) Flow cytometric analysis of cell cycle arrest. Left panel, representative cell cycle distribution; Right panel, quantitation of cell cycle distribution. The data represent the mean ± SEM from four separate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001, versus DMSO-treated cells; $, P < 0.05 and $$, P < 0.01, versus PD98059-treated cells; #, P < 0.05, ##, P < 0.01 and ###, P < 0.001, versus BNF-treated cells.
Activation of MAPK signaling can induce p21Cip1/Waf1 expression and cell cycle arrest/cell senescence (30). To determine if the MEK/ERK pathway is involved in BNF-induced p21Cip1/Waf1 transcriptional upregulation, we exposed MCF-7 cells to the MEK inhibitor PD98059 (10 µM). Preincubation with PD98059 significantly reduced the expression of p21 Cip1/Waf1 protein and mRNA induced by BNF (Figure 3C and D). Furthermore, PD98059 pre-incubation significantly reversed BNF-induced cell cycle arrest (Figure 3E), but did not affect AKT phosphorylation, CYP1A1 induction or expression of cyclinD1/D3 and CDK4 regulated by BNF (Figure 3C).
ERα is required for BNF-induced p21Cip1/Waf1 expression
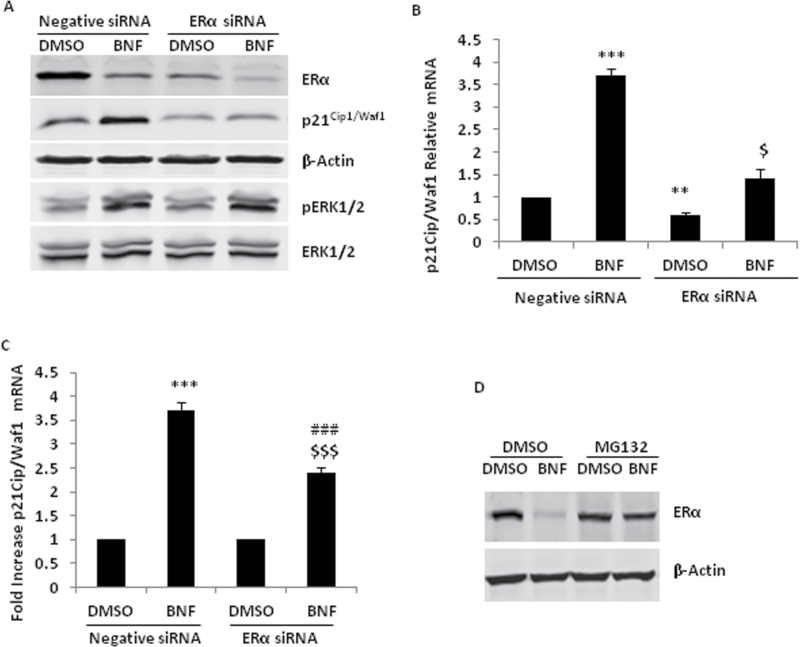
BNF had different effects on p21Cip1/Waf1 induction in ER-positive MCF-7 and ER-negative MDA-MB-231 cells. Previous studies have shown that ERα increases p21Cip1/Waf1 protein and mRNA expression through proteinprotein interactions and transcriptional functions of the receptor in MCF-7 cells (31,32). To investigate whether ERα is important for BNF-induced p21Cip1/Waf1 expression in MCF-7 cells, we used specific siRNA to knockdown ERα expression. RNAi-mediated knockdown of endogenous ERα significantly inhibited the basal levels of p21Cip1/Waf1, and BNF still induced a little p21Cip1/Waf1 (Figure 4A and B). To allow better comparison of the induction effect of BNF on p21Cip1/Waf1 between negative and ERα siRNA groups, we calculated the fold increase of p21Cip1/Waf1 mRNA relative to DMSO treatment. BNF-induced p21Cip1/Waf1 expression is significantly inhibited after ERα knockdown (Figure 4A and C). Concomitantly, BNF significantly reduced ERα protein (Figure 4A). Studies show that agonist-dependent activation of AhR initiates proteasomal degradation of ERα protein (33). To determine if the BNF-mediated reduction of ERα protein is due to protein degradation, we used the proteosome inhibitor, MG132. MG132 blocked the decrease of ERα protein mediated by BNF. (Figure 4D). ERα knockdown did not affect the phosphorylation of ERK1/2 induced by BNF (Figure 4A), implying that ERα may be directly involved in BNF-induced p21Cip1/Waf1 expression.
Fig. 4.
ERα is required in the regulation of BNF-mediated p21Cip1/Waf1 expression. For A, B and C, MCF-7 cells were transfected with 40nM negative or ERα siRNA for 36h, then treated with 10 µM BNF for 36h in A and B, (A) Western blot analysis of p21Cip1/Waf1 protein ERK phosphorylation, (B) qPCR analysis of p21Cip1/Waf1 mRNA. (C) qPCR analysis of p21Cip1/Waf1 mRNA in BNF-treated MCF-7 cells between negative siRNA and ERα siRNA group. (D) Western blot analysis of ERα protein in MCF-7 cells pretreated with 10 µM MG132 for 1h and then treated with 10 µM BNF or 10nM TCDD for 24h. **P < 0.01 and ***P < 0.001, versus DMSO-treated cells with negative siRNA; $, P < 0.05 and $$$, P < 0.001 versus DMSO-treated cells with ERα siRNA; ###, P < 0.001, versus BNF-treated cells with negative siRNA.
BNF induces cell cycle arrest through AhR
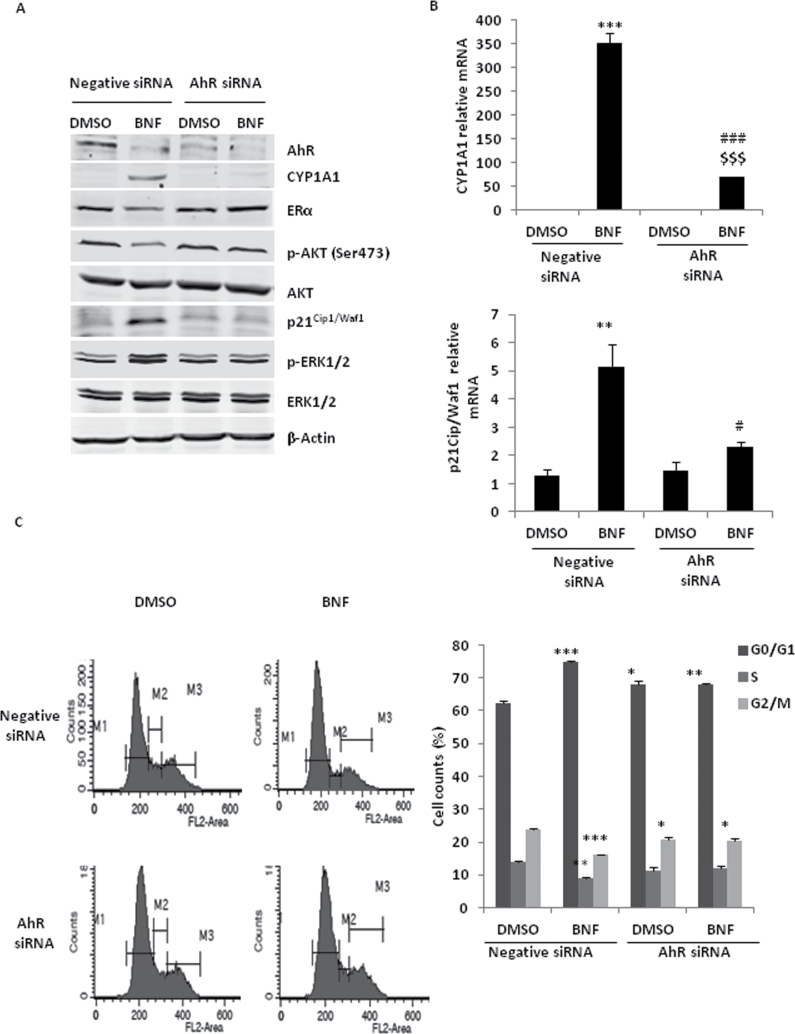
To determine if the effect of BNF on MCF-7 cells is dependent on AhR, we used specific AhR siRNA to knockdown AhR protein. RNAi-mediated knockdown of endogenous AhR significantly inhibited BNF-induced CYP1A1 protein and mRNA (Figure 5A and B). Furthermore, AhR knockdown significantly inhibited BNF-induced phosphorylation of ERK and p21Cip1/Waf1 increase (Figure 5A and B). AhR knockdown also blocked the effect of BNF on inhibition of AKT phosphorylation and ERα degradation (Figure 5A). Although AhR knockdown itself slightly resulted in a cell cycle arrest in G0/G1 phase (Figure 5C), consistent with other studies that suggest the AhR endogenously facilitate cell cycle progression through G1 in the absence of an exogenous ligand (15,34), BNF did not further induce cell cycle arrest in G0/G1 phase when AhR was knocked down (Figure 5C).
Fig. 5.
The effect of BNF on MCF-7 cell cycle depends on AhR. For A, B and C, MCF-7 cells were transfected with 40nM negative or AhR siRNA for 36h, then treated with 10 µM BNF for 36h. (A) Western blot analysis of the phosphorylation of AKT and ERK1/2, p21Cip1/Waf1, CYP1A1 and ERα protein. (B) qPCR analysis of p21Cip1/Waf1 and CYP1A1 mRNA. (C) Flow cytometric analysis of cell cycle arrest. *P < 0.05, **P < 0.01 and ***P < 0.001, versus DMSO-treated cells with negative siRNA; $$$, P < 0.001, versus DMSO-treated cells with AhR siRNA #, P <0.05 and ###, P < 0.001, versus BNF-treated cells with negative siRNA.
Discussion
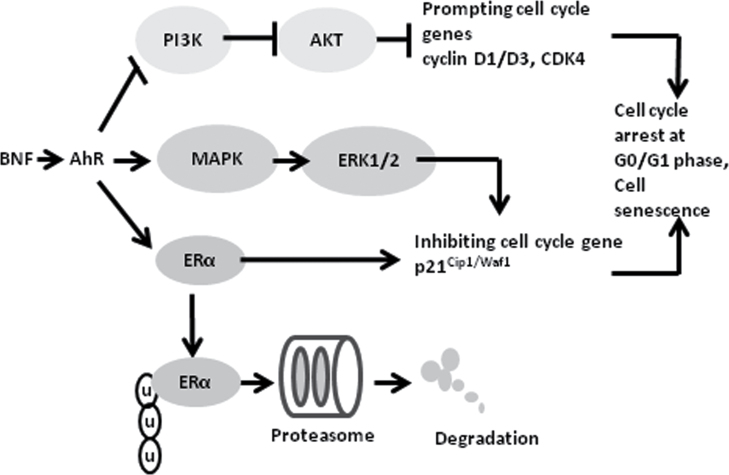
In the present study, we demonstrated a strong antitumor effect of BNF on human ER-positive breast carcinoma cells (MCF-7) through inducing cell cycle arrest and cell senescence (Figure 1). BNF inhibited PI3K/AKT activation, leading to decreased levels of the cell cycle promoters, cyclinD1/D3 and CDK4, and activated MAPK/ERK signaling. Furthermore, BNF increased the cell cycle inhibitor, p21Cip1/Waf1, an effect dependent upon ERK activation and ERα. Collectively, these events resulted in cell cycle arrest and cell senescence. Importantly, the effects of BNF on MCF-7 cells are dependent upon expression of the AhR (Figure 6). The modulation of cell cycle proteins by AhR-mediated PI3K/AKT, MAPK/ERK and ERα signaling is a possible molecular mechanism underlying the effects of BNF on MCF-7 breast cancer cells (Figure 6).
Fig. 6.
Hypothetical model for BNF-induced cell cycle arrest and senescence. BNF induces MCF-7 cells accumulation in G0/G1 phase of the cell cycle and cell senescence by activating AhR. BNF reduces cyclinD1/D4 and CDK4 through inhibiting PI3K/AKT pathway, and increases p21Cip1/Waf1 through activating MAPK/ERK signaling in the presence of ERα. Meanwhile, BNF also causes ERα degradation through ubiquitination-proteasome pathway.
Two key classes of regulatory molecules control cell cycle progression, i.e., positive regulators, cyclins and cyclin-dependent kinases (CDKs) prompt cells to progress through the cell cycle; negative regulators, cyclin-dependent kinase inhibitors (p21Cip1/Waf1, p27 Kip1, and so on) cause cell cycle arrest. The balance between the activation of cyclin D/CDK4 complex and the binding of p21Cip1/Waf1 and p27Kip1 to cyclin/CDK complexes determines the progression from G1 to S phase (35). Many studies have shown that AhR affects cell cycle progression through multiple mechanisms that are ligand and cell-context dependent (36). AhR can affect the cell cycle through regulating the expression of p21Cip1/Waf1, p27Kip1 and cyclin G2 (37,38) or interacting with retinoblastoma protein (pRb), E2F and CDK4 (15,39). Our results provide a novel mechanism for AhR-mediated cell cycle arrest in BNF-treated MCF-7 cells by negatively regulating the PI3K/AKT pathway and simultaneous activation of ERK signaling, which may lead to a decrease in cyclinD1/D3 and CDK4 proteins and increases p21Cip1/Waf1 expression, respectively.
The relevance of the PI3K/AKT pathway in breast cancer is becoming increasingly recognized; hyperactivation and aberrations of this pathway are major causes of breast cancer metastasis, poor survival rates and therapeutic resistance (28); in addition, drugs targeting PI3K/AKT pathway are in clinical development for breast cancer treatment (26). AKT regulates the cell cycle by preventing GSK-3β mediated phosphorylation and degradation of cyclin D protein (27) and by negatively regulating the CDK inhibitors p21Cip1/Waf1 (23). To confirm PI3K/AKT-dependent regulation of cell cycle proteins in this study, we evaluated the effect of LY294,002, a pharmacologic PI3K inhibitor on MCF-7 cells. Increasing concentrations of LY294,002 inhibited AKT signaling and resulted in downregulation of cyclinD1/D3 and CDK4, and cell cycle arrest (Figure 3A and B). However, unlike BNF, LY294,002 had no effect on ERK1/2 phosphorylation or p21Cip1/Waf1 expression (Figure 3A), implying that BNF-mediated PI3K/AKT inhibition may only be responsible for decreasing of cyclinD1/D3 and CDK4, but not responsible for increasing of p21Cip1/Waf1 expression. Although one study shows that LY294,002 is an AhR antagonist (40), LY294,002 did not affect the basal CYP1A1 protein expression (Figure 3A) in this study.
Activation of the MAPK pathway is generally associated with increased cell proliferation (29), but increasing studies have shown that the prolonged MAPK/ERK activation can induce p21Cip1/Waf1 expression, leading to cell cycle arrest and cell senescence (23,30). AhR-MAPK crosstalk has been reported; those studies indicate that TCDD activates ERK via an AhR-dependent or AhR-independent pathway, but TCDD-stimulated ERK appears only to affect AhR activity, with no effects on the ERK-mediated downstream transcriptional activity (41). In contrast, our study shows that BNF induced, in a dose- and time-dependent fashion, ERK phosphorylation that is dependent on AhR (Figures 2 and 5). Furthermore, BNF induced p21waf1/cip1 expression and cell cycle arrest by activating ERK (Figure 3C−E). Although one study has indicated that PD98059 may be a potent AhR antagonist (42), our data clearly indicate that PD98059 does not inhibit BNF-induced CYP1A1 expression (Figure 3C), thereby excluding PD98059 as an AhR ligand.
By using AhR siRNA, we demonstrated that BNF-mediated regulation of PI3K/AKT and ERK signaling is dependent on AhR (Figure 5). Furthermore, our data showed that BNF induced very high expression of CYP1A1 mRNA and protein in MCF-7 cells, but only very weak CYP1A1 mRNA induction in MDA-MB-231 cells (Supplementary Figure S1, available at Carcinogenesis Online), which is consistent with previous studies that demonstrate responsiveness of MCF-7 but not MDA-MB-231 cells to TCDD (21), and that the biological activity of AhR in MDA-MB-231 cells is altered due to expression of a variant ARNT protein (43). In addition, MDA-MB-231 cells showed inherently different characteristics in both pathways; AKT activation is very weak and ERK is hyperactivated compared to MCF-7 cells (Figure 2A). Our data, together with the findings reported by others provide good explanations for why BNF effects differ in MCF-7 compared to MDA-MB-231 cells.
PI3K/AKT and Raf-MAPK-ERK mediate conflicting cellular responses including proliferation, apoptosis, cell cycle arrest and senescence; these pathways functionally synergize or antagonize each other depending on the extent and duration of the external stimulus and on cell type (23,44). Given this and our data, BNF-activated AhR may switch the balance toward MAPK/ERK activation, inducing p21Cip1/Waf1, whereas PI3K/AKT–mediated cell cycle progression signal are suppressed, synergistically leading to cell cycle arrest and cell senescence. This model is completely different from previous reports that PI3K pathway inhibition results in compensatory ERK activation (45) because our study showed that PI3K inhibitor, LY294,002 had no effect on basic ERK phosphorylation (Figure 3A). The exact mechanism of BNF-activated, AhR-mediated modulation of both pathways remains unclear. Currently, there are almost no clear reports about the regulation of AhR on PI3K/AKT and MAPK/ERK pathways. Numerous studies have shown that the effect of AhR on cell cycle progression is ligand and cell-context dependent, implying that AhR activation is necessary but not sufficient for ligand-mediated cell cycle regulation. Many studies have shown the importance of CYP1 in cancer prevention (46,47). For example, some antitumor regents selectively inhibit breast cancer cell proliferation through AhR/CYP1A1-mediated drug metabolism (48,49). AhR ligands have an array of different chemical structures that may produce different metabolic products after induction of P450 metabolizing enzymes. Reactive metabolites may then act through different mechanisms downstream and/or independent of activating AhR. In this context, it is conceivable that BNF activates AhR to induce CYP enzymes that feed back to metabolize BNF into active compounds that may regulate PI3K/AKT and MAPK-ERK signal. Different breast cancer cell lines may have different biological characteristics, like PI3K/AKT mutations and Ras/Raf/ERK mutations, which provide incentive for examining cell types in cancer patients and providing individualized treatment in the clinic. Considering the endogenous biological differences in breast cancer cell lines, future studies are designed to thoroughly investigate and compare the effects of BNF and other AhR agonists on breast cancer in vitro with multiple cell lines and in vivo with tumor models Our objective is to gain insight into crosstalk among the AhR response, ERα expression, agonists and cell types in breast cancer. p21Cip1/Waf1 is usually activated by p53 triggered by DNA damage. Here, BNF did not change p53 protein, implying that p21Cip1/Waf1 induction may be independent of p53, which agrees with another study that demonstrated induction of p21 Cip1/Waf1 expression by TCDD independent of p53 (37). Furthermore, our results showed that BNF-induced p21Cip1/Waf1 expression is ERα dependent (Figure 4), consistent with previous studies that ERα is a known transcriptional regulator of p21Cip1/Waf1 in MCF-7 cells (31,32). Like other AhR agonists (33), BNF treatment also resulted in ERα protein degradation through activating AhR. ERα knockdown did not impact the phosphorylation of ERK1/2 induced by BNF (Figure 4A), and different ERK1/2-activated transcription factors may regulate promoter activation of p21Cip1/Waf1 (18,50), implicating that ERK1/2 signaling and ERα act together to regulate p21Cip1/Waf1. Although BNF-induced p21Cip1/Waf1 expression is AhR-dependent (Figure 5), and AhR knockdown also blocked BNF-mediated ERK1/2 phosphorylation, implying that BNF-activated AhR increases p21Cip1/Waf1 expression through ERK1/2 phosphorylation, we cannot exclude the possibility that AhR also may directly regulate p21Cip1/Waf1 expression considering TCDD has been shown to regulate p21Cip1/Waf1 expression through a direct transcriptional effect by activated AhR (37). Thus, transcriptional regulation of BNF-induced p21Cip1/Waf1 is very complex. Additional experiments are warranted to investigate how AhR, ERα and ERK-activated transcription factors synergistically regulate BNF-mediated p21Cip1/Waf1 expression.
In summary, we have firstly and clearly demonstrated that BNF exerts an antitumor effect through a completely different mechanism from other AhR agonists and flavonoids, i.e. AhR-mediated PI3K/AKT pathway inhibition, MAPK/ERK signaling activation and ERα signaling work together to prompt a cell cycle arrest. This novel mechanism represents a promising strategy to be exploited in future clinical trials of breast cancer treatment. It is important to further clarify the detailed molecular mechanisms and the exact nature of PI3K/AKT pathway inhibition and MAPK/ERK activation mediated by BNF in our future studies.
Supplementary material
Supplementary Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (ES017774 to S.A.T.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- AhR
aryl hydrocarbon receptor
- BNF
beta-naphthoflavone
- CDK
cyclin-dependent kinases
- DMSO
dimethyl sulfoxide
- ER
estrogen receptor
- MAPK-ERK
mitogen-activated protein kinase-extracellular signal-regulated kinase
- PARP
poly(ADP-ribose) polymerase
- TBST
Tris-buffered saline containing 0.05% Tween 20.
References
- 1. Prochaska H.J., et al. (1988). Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res., 48, 4776–4782 [PubMed] [Google Scholar]
- 2. Yochum L., et al. (1999). Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol., 149, 943–949 [DOI] [PubMed] [Google Scholar]
- 3. Murray T.J., et al. (2006). Growth of a human mammary tumor cell line is blocked by galangin, a naturally occurring bioflavonoid, and is accompanied by down-regulation of cyclins D3, E, and A. Breast Cancer Res., 8, R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lubet R.A., et al. (2011). Effects of 5,6-benzoflavone, indole-3-carbinol (I3C) and diindolylmethane (DIM) on chemically-induced mammary carcinogenesis: is DIM a substitute for I3C? Oncol. Rep., 26, 731–736 [DOI] [PubMed] [Google Scholar]
- 5. Anderson L.M., et al. (1985). Protection against tumorigenesis by 3-methylcholanthrene in mice by beta-naphthoflavone as a function of inducibility of methylcholanthrene metabolism. Cancer Res., 45, 6384–6389 [PubMed] [Google Scholar]
- 6. Izzotti A., et al. (1999). Patterns of DNA adduct formation in liver and mammary epithelial cells of rats treated with 7,12-dimethylbenz(a)anthracene, and selective effects of chemopreventive agents. Cancer Res., 59, 4285–4290 [PubMed] [Google Scholar]
- 7. Denison M.S., et al. (2011). Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci., 124, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van den Berg M., et al. (2006). The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci., 93, 223–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J.S., et al. (2011). AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol., 13, 144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C., et al. (2011). Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces PPAR-alpha pathway activity in mice. Environ. Health. Perspect., 119, 1739–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang S., et al. (2009). The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr. Relat. Cancer, 16, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callero M. A., et al. (2011). The role of aryl hydrocarbon receptor and crosstalk with estrogen receptor in response of breast cancer cells to the novel antitumor agents benzothiazoles and aminoflavone. Int. J. Breast Cancer, 2011, 923250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Safe S., et al. (2000). Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J. Mammary Gland Biol. Neoplasia, 5, 295–306 [DOI] [PubMed] [Google Scholar]
- 14. Thomas C., et al. (2011). The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer, 11, 597–608 [DOI] [PubMed] [Google Scholar]
- 15. Barhoover M. A., et al. (2010). Aryl hydrocarbon receptor regulates cell cycle progression in human breast cancer cells via a functional interaction with cyclin-dependent kinase 4. Mol. Pharmacol., 77, 195–201 [DOI] [PubMed] [Google Scholar]
- 16. Abdelrahim M., et al. (2006). 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha. Cancer Res., 66, 2459–2467 [DOI] [PubMed] [Google Scholar]
- 17. Ariazi E.A., et al. (2010). The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res., 70, 1184–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Siervi A., et al. (2004). Transcriptional activation of p21(waf1/cip1) by alkylphospholipids: role of the mitogen-activated protein kinase pathway in the transactivation of the human p21(waf1/cip1) promoter by Sp1. Cancer Res., 64, 743–750 [DOI] [PubMed] [Google Scholar]
- 19. Zhang K., et al. (2009). Overexpression of RRM2 decreases thrombspondin-1 and increases VEGF production in human cancer cells in vitro and in vivo: implication of RRM2 in angiogenesis. Mol. Cancer, 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zu X., et al. (2007). Reduced 293T cell susceptibility to acrolein due to aldose reductase-like-1 protein expression. Toxicol. Sci., 97, 562–568 [DOI] [PubMed] [Google Scholar]
- 21. Dohr O., et al. (1995). Different response of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-sensitive genes in human breast cancer MCF-7 and MDA-MB 231 cells. Arch. Biochem. Biophys., 321, 405–412 [DOI] [PubMed] [Google Scholar]
- 22. el-Deiry W.S., et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 23. Thaler S., et al. (2009). RASSF1A mediates p21Cip1/Waf1-dependent cell cycle arrest and senescence through modulation of the Raf-MEK-ERK pathway and inhibition of Akt. Cancer Res., 69, 1748–1757 [DOI] [PubMed] [Google Scholar]
- 24. Chang B.D., et al. (2000). Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc. Natl Acad. Sci. USA, 97, 4291–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dimri G.P., et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U S A, 92, 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agarwal R., et al. (2010). PI3K pathway-directed therapeutic strategies in cancer. Curr. Opin. Investig. Drugs, 11, 615–628 [PubMed] [Google Scholar]
- 27. Diehl J.A., et al. (1998). Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev., 12, 3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller T.W., et al. (2011). Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res., 13, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calipel A., et al. (2003). Mutation of B-Raf in human choroidal melanoma cells mediates cell proliferation and transformation through the MEK/ERK pathway. J. Biol. Chem., 278, 42409–42418 [DOI] [PubMed] [Google Scholar]
- 30. Facchinetti M.M., et al. (2004). UCN-01-induced cell cycle arrest requires the transcriptional induction of p21(waf1/cip1) by activation of mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase pathway. Cancer Res., 64, 3629–3637 [DOI] [PubMed] [Google Scholar]
- 31. Mandal S., et al. (2010). Estrogen regulated expression of the p21 Waf1/Cip1 gene in estrogen receptor positive human breast cancer cells. J. Cell. Physiol., 224, 28–32 [DOI] [PubMed] [Google Scholar]
- 32. Maynadier M., et al. (2008). Unliganded estrogen receptor alpha inhibits breast cancer cell growth through interaction with a cyclin-dependent kinase inhibitor (p21(WAF1)). FASEB J., 22, 671–681 [DOI] [PubMed] [Google Scholar]
- 33. Wormke M., et al. (2003). The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell Biol., 23, 1843–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalmes M., et al. (2011). Impact of aryl hydrocarbon receptor (AhR) knockdown on cell cycle progression in human HaCaT keratinocytes. Biol. Chem., 392, 643–651 [DOI] [PubMed] [Google Scholar]
- 35. Orlando D.A., et al. (2008). Global control of cell-cycle transcription by coupled CDK and network oscillators. Nature, 453, 944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang G., et al. (2005). Multiple mechanisms are involved in Ah receptor-mediated cell cycle arrest. Mol. Pharmacol., 67, 88–96 [DOI] [PubMed] [Google Scholar]
- 37. Barnes-Ellerbe S., et al. (2004). 2,3,7,8-Tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol. Pharmacol., 66, 502–511 [DOI] [PubMed] [Google Scholar]
- 38. Ahmed S., et al. (2012). FOXA1 is essential for aryl hydrocarbon receptor-dependent regulation of cyclin G2. Mol. Cancer Res., 10, 636–648 [DOI] [PubMed] [Google Scholar]
- 39. Marlowe J.L., et al. (2004). The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J. Biol. Chem., 279, 29013–29022 [DOI] [PubMed] [Google Scholar]
- 40. Guo M., et al. (2000). Suppression of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated aryl hydrocarbon receptor transformation and CYP1A1 induction by the phosphatidylinositol 3-kinase inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1- benzopyran-4-one (LY294002). Biochem. Pharmacol., 60, 635–642 [DOI] [PubMed] [Google Scholar]
- 41. Tan Z., et al. (2002). Activation of mitogen-activated protein kinases (MAPKs) by aromatic hydrocarbons: role in the regulation of aryl hydrocarbon receptor (AHR) function. Biochem. Pharmacol., 64, 771–780 [DOI] [PubMed] [Google Scholar]
- 42. Reiners J.J., et al. (1998). PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol. Pharmacol., 53, 438–445 [DOI] [PubMed] [Google Scholar]
- 43. Wilson C.L., et al. (1997). Aryl hydrocarbon (Ah) nonresponsiveness in estrogen receptor-negative MDA-MB-231 cells is associated with expression of a variant arnt protein. Arch. Biochem. Biophys., 346, 65–73 [DOI] [PubMed] [Google Scholar]
- 44. Zimmermann S., et al. (1999). Phosphorylation and regulation of Raf by Akt (protein kinase B). Science, 286, 1741–1744 [DOI] [PubMed] [Google Scholar]
- 45. Serra V., et al. (2011). PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene, 30, 2547–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Androutsopoulos V.P., et al. (2009). Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC cancer, 9, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Androutsopoulos V., et al. (2008). Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. Breast Cancer Res., 10, R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bradshaw T.D., et al. (2004). The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate. Curr. Med. Chem., 11, 1009–1021 [DOI] [PubMed] [Google Scholar]
- 49. Chua M.S., et al. (2000). Role of Cyp1A1 in modulation of antitumor properties of the novel agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203, NSC 674495) in human breast cancer cells. Cancer Res., 60, 5196–5203 [PubMed] [Google Scholar]
- 50. Park J.S., et al. (2000). A role for both Ets and C/EBP transcription factors and mRNA stabilization in the MAPK-dependent increase in p21 (Cip-1/WAF1/mda6) protein levels in primary hepatocytes. Mol. Biol. Cell., 11, 2915–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.