Abstract
Monoclonal antibodies were prepared against a cell attachment-promoting protein, serum spreading factor, which had been partially purified from human serum by chromatography on glass bead columns. The antibodies selected were those that reacted with polypeptides that had cell attachment-promoting activity after sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Immunochromatography of human plasma on columns containing the monoclonal antibodies followed by affinity chromatography on heparin-Sepharose yielded material that in sodium dodecyl sulfate/polyacrylamide gel electrophoretic analysis gave polypeptides of molecular mass 65 and 75 kilodaltons. Both polypeptides bound each of three monoclonal antibodies and had cell attachment-promoting activity after transfer to nitrocellulose filters. Immunofluorescent staining of tissues with the monoclonal antibodies revealed a fibrillar pattern that was mostly associated with loose connective tissue and overlapped with fibronectin fibrils. Fetal membrane tissue, which showed strong staining with the antibodies in immunofluorescence, also gave 65- and 75-kilodalton polypeptides with cell attachment-promoting activity after chromatography on columns containing the monoclonal antibodies. One source of the tissue protein may be fibroblastic cells, because cultured human fibroblasts also stained with the monoclonal antibodies. The staining was fibrillar and appeared to be associated with the cell surface extracellular matrix. We propose the name "vitronectin" for the various forms of this protein, on the basis of its binding to glass and its adhesive properties.
Full text
PDF

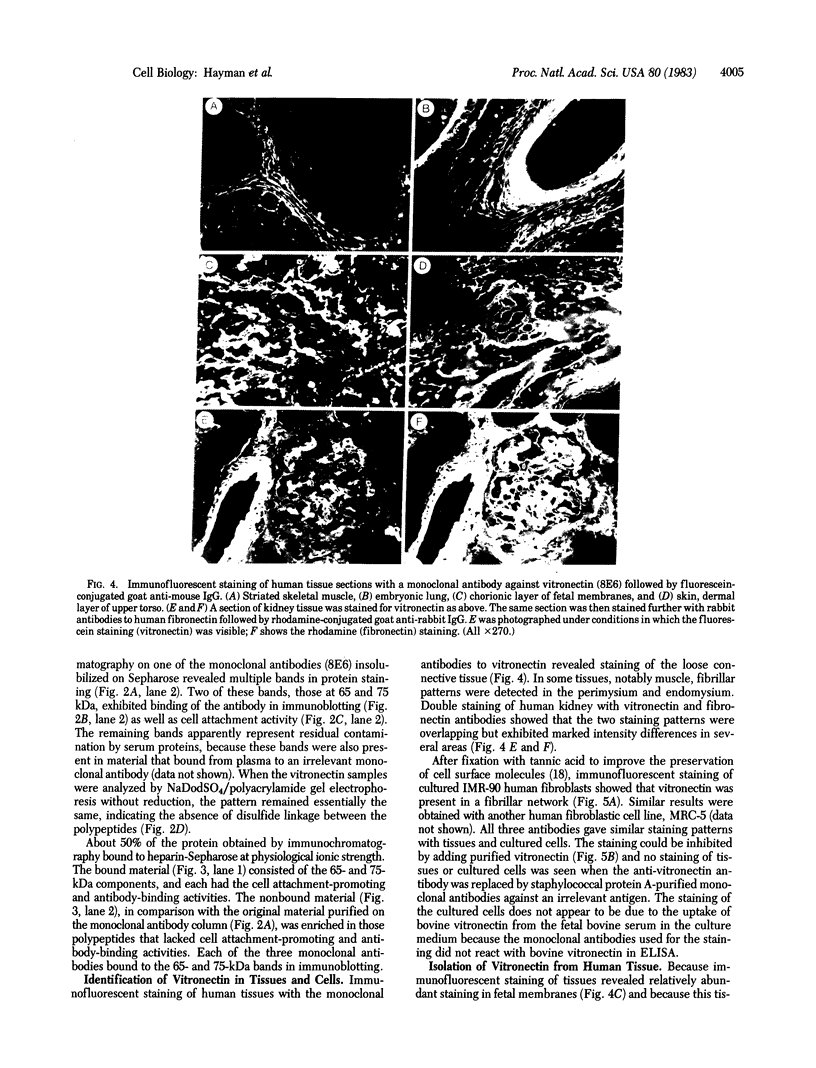


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. W., Silnutzer J., See C., Shaffer M. Characterization of human serum spreading factor with monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1362–1366. doi: 10.1073/pnas.80.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Wolfe R., Serrero G., McClure D., Sato G. Effects of a serum spreading factor on growth and morphology of cells in serum-free medium. J Supramol Struct. 1980;14(1):47–63. doi: 10.1002/jss.400140106. [DOI] [PubMed] [Google Scholar]
- Barnes D., van der Bosch J., Masui H., Miyazaki K., Sato G. The culture of human tumor cells in serum-free medium. Methods Enzymol. 1981;79(Pt B):368–391. doi: 10.1016/s0076-6879(81)79050-5. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., A'Hearn E., Barnes D., Pierschbacher M., Ruoslahti E. Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol. 1982 Oct;95(1):20–23. doi: 10.1083/jcb.95.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., Ruoslahti E. Concomitant loss of cell surface fibronectin and laminin from transformed rat kidney cells. J Cell Biol. 1981 Feb;88(2):352–357. doi: 10.1083/jcb.88.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. Preparation from human serum of an alpha-one protein which induces the immediate growth of unadapted cells in vitro. J Cell Biol. 1967 Feb;32(2):297–308. doi: 10.1083/jcb.32.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Knox P., Griffiths S. The distribution of cell-spreading activities in sera: a quantitative approach. J Cell Sci. 1980 Dec;46:97–112. doi: 10.1242/jcs.46.1.97. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Maupin P., Pollard T. D. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol. 1983 Jan;96(1):51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Murphy D. G., Cristofalo V. J., Toji L. H., Greene A. E., Dwight S. A. Characterization of a new human diploid cell strain, IMR-90. Science. 1977 Apr 1;196(4285):60–63. doi: 10.1126/science.841339. [DOI] [PubMed] [Google Scholar]
- Pekkala-Flagan A., Ruoslahti E. Unfolded transferrin polypeptide chain is immunologically crossreactive with similar derivatives of serum albumin and alpha-fetoprotein. J Immunol. 1982 Mar;128(3):1163–1167. [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 1980 Aug 13;631(2):350–358. doi: 10.1016/0304-4165(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Engvall E., Cothran W. C., Butler W. T. Alignment of biologically active domains in the fibronectin molecule. J Biol Chem. 1981 Jul 25;256(14):7277–7281. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Fukuda M., Hakomori S. Domain structure of hamster plasma fibronectin. Isolation and characterization of four functionally distinct domains and their unequal distribution between two subunit polypeptides. J Biol Chem. 1981 Jun 25;256(12):6452–6462. [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Kimata K., Pratt R. M. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem. 1980 Jul 10;255(13):6055–6063. [PubMed] [Google Scholar]









