Abstract
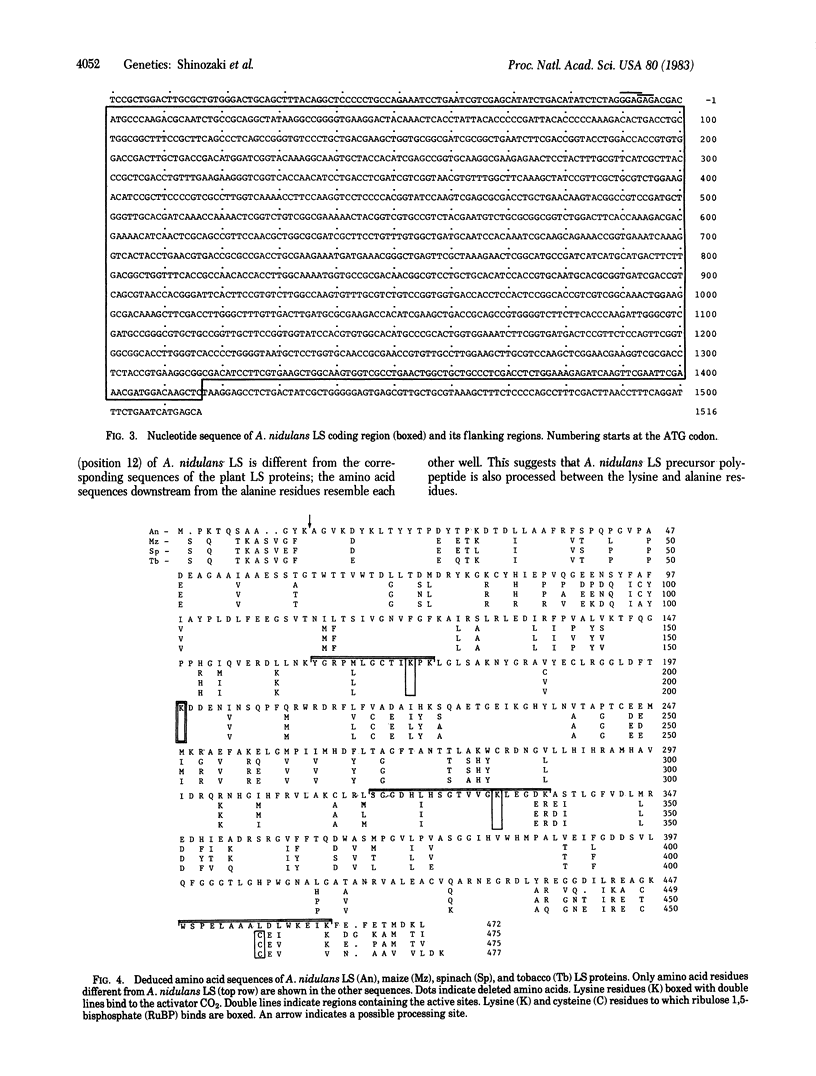
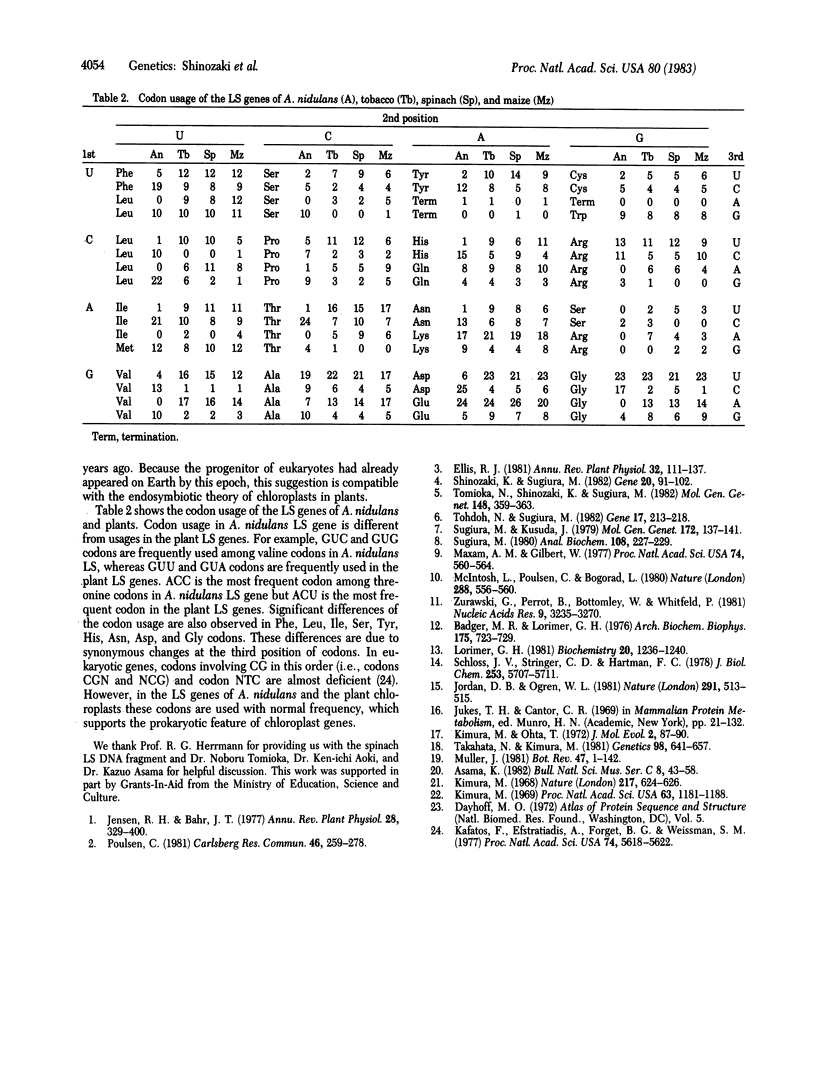
Ribulose-1,5-bisphosphate carboxylase/oxygenase consists of large subunits (LS) and small subunits. In plants, the LS is encoded in chloroplast DNA and the small subunit, in nuclear DNA. In cyanobacteria, both subunits are thought to be encoded in chromosomal DNA because of prokaryotes. The gene for the LS of ribulose-1,5-bisphosphate carboxylase/oxygenase from a cyanobacterium, Anacystis nidulans 6301, has been cloned in pBR322 and subjected to sequence analysis. The coding region contains 1,416 base pairs (472 codons). The deduced amino acid sequence of A. nidulans LS protein shows 80% homology with sequences of maize, spinach, and tobacco LS proteins; the nucleotide sequence of A. nidulans LS gene shows 70% homology with sequences of the plant genes. Between A. nidulans LS and the plant LS proteins there is exact sequence homology around the lysine residue to which the activator CO2 binds and around the two lysine residues to which ribulose 1,5-bisphosphate binds. The amino acid sequence where the LS binds to the small subunit is also highly conserved. From comparison of the LS proteins of A. nidulans and the three plants, the rate of amino acid substitution is estimated to be 0.25-0.5 × 10-9 per year per site, which is far below the median value of various types of proteins (1.2 × 10-9 for hemoglobin α). The LS protein is thus a conserved protein.
Keywords: Anacystis nidulans, amino acid sequence, phylogenetic tree
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Lorimer G. H. Activation of ribulose-1, 5-bisphosphate oxygenase, The role of Mg2+, CO2, and pH. Arch Biochem Biophys. 1976 Aug;175(2):723–729. doi: 10.1016/0003-9861(76)90565-8. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Efstratiadis A., Forget B. G., Weissman S. M. Molecular evolution of human and rabbit beta-globin mRNAs. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5618–5622. doi: 10.1073/pnas.74.12.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Evolutionary rate at the molecular level. Nature. 1968 Feb 17;217(5129):624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Kimura M., Ota T. On the stochastic model for estimation of mutational distance between homologous proteins. J Mol Evol. 1972 Dec 29;2(1):87–90. doi: 10.1007/BF01653945. [DOI] [PubMed] [Google Scholar]
- Kimura M. The rate of molecular evolution considered from the standpoint of population genetics. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1181–1188. doi: 10.1073/pnas.63.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H. Ribulosebisphosphate carboxylase: amino acid sequence of a peptide bearing the activator carbon dioxide. Biochemistry. 1981 Mar 3;20(5):1236–1240. doi: 10.1021/bi00508a028. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. V., Stringer C. D., Hartman F. C. Identification of essential lysyl and cysteinyl residues in spinach ribulosebisphosphate carboxylase/oxygenase modified by the affinity label N-bromoacetylethanolamine phosphate. J Biol Chem. 1978 Aug 25;253(16):5707–5711. [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982 Nov;20(1):91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Sugiura M. Purification of the T4 DNA ligase by Blue Sepharose chromatography. Anal Biochem. 1980 Nov 1;108(2):227–229. doi: 10.1016/0003-2697(80)90573-4. [DOI] [PubMed] [Google Scholar]
- Takahata N., Kimura M. A model of evolutionary base substitutions and its application with special reference to rapid change of pseudogenes. Genetics. 1981 Jul;98(3):641–657. doi: 10.1093/genetics/98.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohdoh N., Sugiura M. The complete nucleotide sequence of 16S ribosomal RNA gene from tobacco chloroplasts. Gene. 1982 Feb;17(2):213–218. doi: 10.1016/0378-1119(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]