Abstract
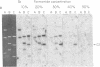
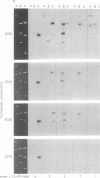
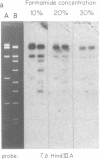
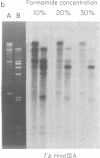
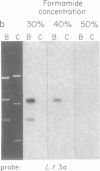
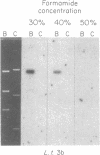
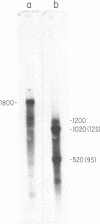
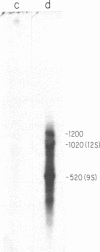
The conserved portions of the maxicircle DNAs of Leishmania tarentolae and Trypanosoma brucei are organized in a basically colinear manner over a 15- to 17-kilobase region that is interrupted by two small less-homologous sequences. The most highly conserved regions are those encoding the 9S and 12S genes. An approximately 12-kilobase region directly upstream of the 12S gene in the L. tarentolae maxicircle showed no sequence homology with the T. brucei maxicircle and also was not transcribed. An approximately 6-kilobase region in the T. brucei maxicircle in the same relative location also showed no sequence homology with the L. tarentolae maxicircle. We propose that evolution of maxicircle DNA occurs mainly within this "divergent region."
Full text
PDF
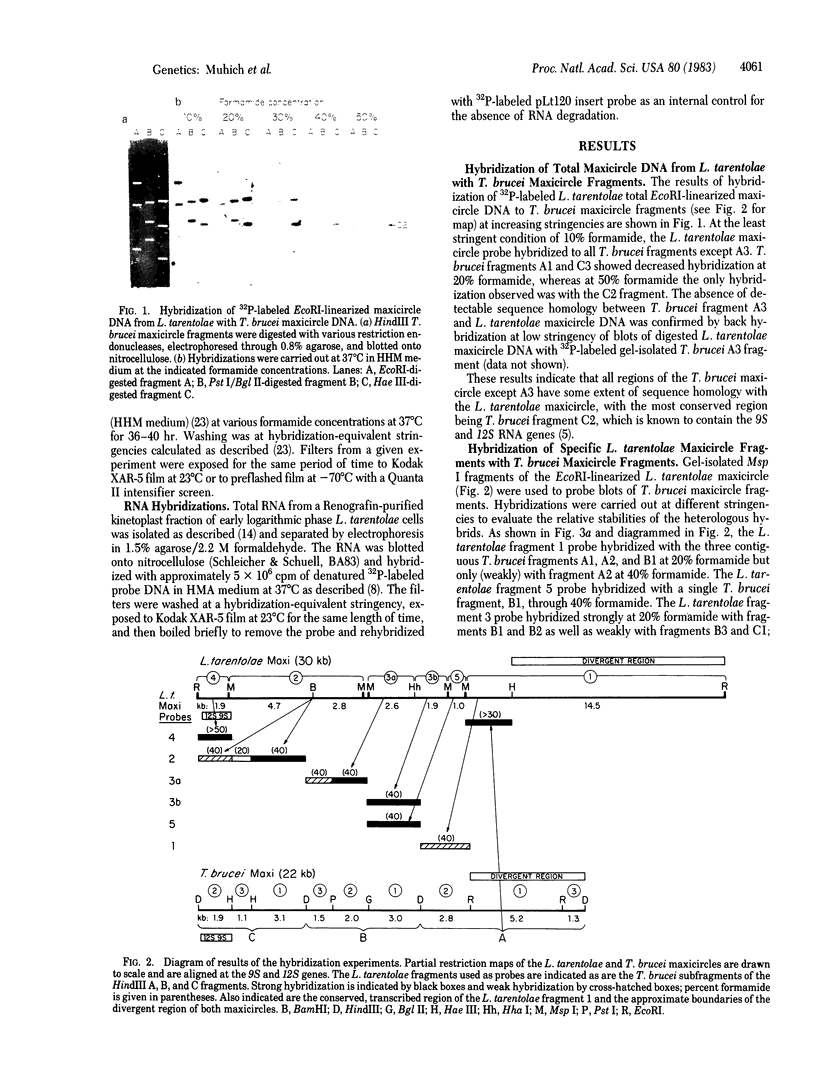



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P., Fase-Fowler F., Gibson W. C. Quantitation of genetic differences between Trypanosoma brucei gambiense, rhodesiense and brucei by restriction enzyme analysis of kinetoplast DNA. Mol Biochem Parasitol. 1981 Jun;3(2):117–131. doi: 10.1016/0166-6851(81)90011-6. [DOI] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F., Hoeijmakers J. H., Frasch A. C. Variations in maxi-circle and mini-circle sequences in kinetoplast DNAs from different Trypanosoma brucei strains. Biochim Biophys Acta. 1980 Dec 11;610(2):197–210. doi: 10.1016/0005-2787(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F. The maxi-circle of Trypanosoma brucei kinetoplast DNA. Biochim Biophys Acta. 1979 Nov 22;565(1):1–12. doi: 10.1016/0005-2787(79)90078-9. [DOI] [PubMed] [Google Scholar]
- Borst P., Weijers P. J., Brakenhoff G. J. Analysis by electron microscopy of the variable segment in the maxi-circle of kinetoplast DNA from Trypanosoma brucei. Biochim Biophys Acta. 1982 Dec 31;699(3):272–280. doi: 10.1016/0167-4781(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Simpson L. Comparison of various ultraviolet sources for fluorescent detection of ethidium bromide-DNA complexes in polyacrylamide gels. Anal Biochem. 1977 Oct;82(2):455–462. doi: 10.1016/0003-2697(77)90183-x. [DOI] [PubMed] [Google Scholar]
- Cheng D., Simpson L. Isolation and characterization of kinetoplast DNA and RNA of Phytomonas davidi. Plasmid. 1978 Jun;1(3):297–315. doi: 10.1016/0147-619x(78)90047-1. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Cunningham I., Gardiner P. R., Taylor A. M., Luckins A. G. Cultivation of infective forms of Trypanosoma congolense from trypanosomes in the proboscis of Glossina morsitans. Parasitology. 1981 Feb;82(1):81–95. doi: 10.1017/s0031182000041883. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Schoutsen B., Borst P. Kinetoplast DNA in the insect trypanosomes Crithidia luciliae and Crithidia fasciculata. I. Sequence evolution and transcription of the maxicircle. Plasmid. 1982 May;7(3):199–209. doi: 10.1016/0147-619x(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Johnson B. J., Hill G. C., Fox T. D., Stuart K. The maxicircle of Trypanosoma brucei kinetoplast DNA hybridizes with a mitochondrial gene encoding cytochrome oxidase subunit II. Mol Biochem Parasitol. 1982 Jun;5(6):381–390. doi: 10.1016/0166-6851(82)90011-1. [DOI] [PubMed] [Google Scholar]
- Leon W., Frasch A. C., Hoeijmakers J. H., Fase-Fowler F., Borst P., Brunel F., Davison J. Maxi-circles and mini-circles in kinetoplast DNA from trypanosoma cruzi. Biochim Biophys Acta. 1980 Apr 30;607(2):221–231. doi: 10.1016/0005-2787(80)90075-1. [DOI] [PubMed] [Google Scholar]
- Masuda H., Simpson L., Rosenblatt H., Simpson A. M. Restriction map, partial cloning and localization of 9S and 12S kinetoplast RNA genes on the maxicircle component of the kinetoplast DNA of Leishmania tarentolae. Gene. 1979 May;6(1):51–73. doi: 10.1016/0378-1119(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Kinetoplast DNA and RNA of Trypanosoma brucei. Mol Biochem Parasitol. 1980 Dec;2(2):93–108. doi: 10.1016/0166-6851(80)90034-1. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L., Livingston L. Transcription of the maxicircle kinetoplast DNA of Leishmania tarentolae. Mol Biochem Parasitol. 1982 Oct;6(4):237–252. doi: 10.1016/0166-6851(82)90057-3. [DOI] [PubMed] [Google Scholar]
- Simpson L., Da Silva A. Isolation and characterization of kinetoplast DNA from Leishmania tarentolae. J Mol Biol. 1971 Mar 28;56(3):443–473. doi: 10.1016/0022-2836(71)90394-9. [DOI] [PubMed] [Google Scholar]
- Simpson L. Isolation of maxicircle component of kinetoplast DNA from hemoflagellate protozoa. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1585–1588. doi: 10.1073/pnas.76.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Simpson A. G. Kinetoplast RNA of Leishmania tarentolae. Cell. 1978 May;14(1):169–178. doi: 10.1016/0092-8674(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Simpson L., Simpson A. M., Kidane G., Livingston L., Spithill T. W. The kinetoplast DNA of the hemoflagellate protozoa. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1053–1063. doi: 10.4269/ajtmh.1980.29.1053. [DOI] [PubMed] [Google Scholar]
- Simpson L., Spithill T. W., Simpson A. M. Identification of maxicircle DNA sequences in Leishmania Tarentolae that are homologous to sequences of specific yeast mitochondrial structural genes. Mol Biochem Parasitol. 1982 Oct;6(4):253–264. doi: 10.1016/0166-6851(82)90058-5. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Steinert M., Van Assel S. Sequence heterogeneity in kinetoplast DNA: reassociation kinetics. Plasmid. 1980 Jan;3(1):7–17. doi: 10.1016/s0147-619x(80)90030-x. [DOI] [PubMed] [Google Scholar]
- Stuart K. D., Gelvin S. B. Localization of kinetoplast DNA maxicircle transcripts in bloodstream and procyclic form Trypanosoma brucei. Mol Cell Biol. 1982 Jul;2(7):845–852. doi: 10.1128/mcb.2.7.845. [DOI] [PMC free article] [PubMed] [Google Scholar]