Abstract
Background
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a risk factor for coronary artery disease (CAD) in nontransplant patients. We evaluated the association between Lp-PLA2, cardiac allograft vasculopathy (CAV) assessed by 3D intravascular ultrasound, and incidence of cardiac adverse events in heart transplant recipients.
Materials and Methods
Fasting blood samples were obtained and stored from a cross-section of 112 cardiac transplant recipients attending the Mayo cardiac transplant clinic in 2000 to 2001, mean of 4.7 years after transplant. Lp-PLA2 was measured in plasma aliquots using an enzyme-linked immunoassay. Fifty-six of these patients subsequently underwent two 3D intravascular ultrasound studies in 2004 to 2006 12 months apart. Cardiovascular (CV) events included percutaneous coronary intervention, coronary artery bypass grafting (CABG), reduction in left ventricular ejection fraction (LVEF) ≤45% secondary to CAV and CV death.
Results
High Lp-PLA2 level was associated with increase in plaque volume (r=0.43, P=0.0026) and percent plaque volume (r=0.45, P=0.0004). The association remained significant after adjusting for clinical and lipid variables. During follow-up of 5.1±1.6 years, 24 CV adverse events occurred in 15 of 112 (13%) heart transplant patients. Lp-PLA2 level>236 ng/mL (higher tertile) identified a subgroup of patients having a 2.4-fold increase of relative risk for combined endpoint of CV events (percutaneous coronary intervention, CABG, LVEF<45%, and CV death; 95% CI 1.16–5.19, P=0.012) compared with patients with Lp-PLA2≤236 ng/mL.
Conclusions
Lp-PLA2 is independently associated with progression of CAV and predicts a higher incidence of CV events and CV death in transplant patients. This finding supports the concept that systemic inflammation is an important mediator of CAV. Lp-PLA2 may be a useful marker for risk of CAV and a therapeutic target in posttransplant patients.
Keywords: Cardiac transplantation, Coronary allograft vasculopathy, Lipoprotein-associated phospholipase A2
Cardiac allograft vasculopathy (CAV), a unique form of accelerated atherosclerosis, is a major cause of late morbidity and mortality in heart transplant patients. In the 2006 International Society for Heart and Lung Transplantation registry, after 5 years of posttransplantation, CAV, and late graft failure (likely due to CAV) together account for 30% of deaths (1).
The pathogenesis of CAV seems to be multifactorial, including both immune mediated and nonimmune mechanisms (2). Although the relationship between a number of cardiovascular (CV) risk factors and clinical CAV is well established, it remains unclear whether the presence of risk factors correlates with the extent of plaque burden. Intravascular ultrasound (IVUS) provides a high-resolution technique for quantitative assessment of vessel wall anatomy in transplant patients, and provides a unique opportunity to study the interaction of risk factors with plaque burden and CAV severity (3).
There is a growing body of evidence supporting the major contribution of inflammatory pathways to the etiology of atherosclerosis (4). Inflammatory markers improve the prediction of CV risk achieved through the analysis of traditional risk factors alone (5). Several studies have indicated that inflammatory processes are closely linked with the genesis of CAV (6–9).
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a novel marker of inflammation, produced and secreted by monocytes, macrophages, platelets, and mast cells, (10) which are all involved in atherogenesis. Several studies have found an association of Lp-PLA2 with coronary endothelial dysfunction, (11, 12) coronary events, (13–16) and angiographically documented coronary artery disease (17–20). However, the role of Lp-PLA2 in the development of CAV and its association with other atherogenic substances among transplant recipients has not been delineated.
We prospectively evaluated the association between Lp-PLA2 and other risk factors for CAV, effect of Lp-PLA2 on progression of CAV assessed by 3D IVUS and subsequent incidence of CV adverse events over 5 year follow-up in a cohort of posttransplant patients.
METHODS
After Institutional Review Board (IRB) approval and informed consent, fasting blood samples were obtained and stored from a cross-section of 112 cardiac transplant recipients attending the Mayo cardiac transplant clinic in 2000 to 2001. At the time of enrollment, 4.7±3.1 years after transplantation, all patients were receiving immunosuppressive therapy with cyclosporine/tacrolimus, azathioprine/mycophenolate mofetil, and prednisone if necessary, and were free from acute rejection, clinical signs of infection, or symptoms of acute heart failure.
Frozen plasma aliquots were used for measurement of Lp-PLA2 mass, C-reactive protein (CRP), small dense low-density lipoprotein (LDL), intermediate-density lipoprotein (IDL), and routine lipids.
Lipids (total cholesterol, triglycerides, and HDL cholesterol) were measured using standard automated enzymatic methods on a Roche COBAS MIRA system. LDL cholesterol was calculated as total cholesterol minus HDL cholesterol and 20% of the triglyceride level (all expressed in mg/dL).
Serum CRP concentrations were measured on a Hitachi 912 automated chemistry analyzer using a high-sensitivity polystyrene particle-enhanced immunoturbidimetric assay from DiaSorin (Stillwater, MN). Intraassay coefficients of variation were 8.8%, 1.1%, and 0.4% at 0.028, 0.20, and 1.15 mg/dL, respectively. Interassay coefficients of variation were 8.0%, 2.0%, and 1.0% at 0.05, 0.30, and 1.86 mg/dL, respectively (12).
Particle size of LDL was measured by polyacrylamide gel electrophoresis.
Lp-PLA2 mass was measured as previously described with an enzyme-linked immunoassay (PLAC test, diaDexus, Inc., San Francisco, CA) (11, 17, 21, 22). This assay consists of two high-affinity monoclonal antibodies to Lp-PLA2. The range of detection was 50 to 1,000 ng/mL, and the interassay coefficients of variation were 7.8% at 276 ng/mL, 6.1% at 257 ng/mL, and 13.5% at 105 ng/mL.
Fifty-six patients of the original cohort had undergone two surveillance 3D IVUS 12 months apart during annual angiogram between 2004 and 2006. Of the 112 study patients, 22 patients died before performing IVUS examinations, 19 patients refused, and 15 patients did not undergo angiogram/IVUS study because of comorbidity. As we previously described (3), the study was performed after intracoronary administration of 100 to 200 μg nitroglycerin. Mechanical pullback (0.5 mm/sec) was performed from the mid left anterior descending coronary artery to the ostium of the left main coronary artery using a 20-MHz, 2.9F, solid state IVUS imaging catheter (Eagle Eye™, Volcano Therapeutics, Inc., Rancho Cordova, CA) and a dedicated IVUS scanner (Volcano Therapeutics).
Off-line volumetric analysis of IVUS data was performed (echo Plaque 2™, version 2.5; INDECSystems, Inc., Santa Clara, CA) by two experienced operators, who were unaware of laboratory tests results. Proximal and mid left anterior descending coronary artery regions were defined for the interrogated artery. Starting with the first complete vascular ring distal to the bifurcation with the left circumflex artery lumen, plaque, and vessel volume were analyzed. Each measured volume was normalized to the examined segment length (mm3/mm) to compensate for the difference of the examined vessel segment length in study patients. A plaque index was calculated as (plaque volume/vessel volume)×100%. Simpson’s rule for volumetric measurement was used. The semiautomated contour detection of both the lumen and the media-adventitia interface was performed at intervals of either 16 or 32 frames, depending on the heterogeneity of the image. All other measurements were carried out automatically. Border detection was corrected manually in all frames after automatic border detection. Next, the volume (mm3) of the vessel, lumen, and plaque in the exact examined vessel segment length and frames were obtained and calculated as previously described (23).
Cardiovascular events (percutaneous coronary intervention, coronary artery bypass grafting (CABG), left ventricular ejection fraction (LVEF) ≤45% secondary to CAV, and confirmed CV death) were retrospectively recorded in 112 patients up to September 1, 2006 (duration of follow-up 5.1±1.6 years from blood sample).
Statistical Analysis
Data are displayed as mean±standard deviation or counts and percent, as appropriate. Variables with heavily skewed distribution (CRP, TG/HDL, LIPA, and Lp[a]) are reported as medians with first and third quartiles in parenthesis. Correlation coefficients of Lp-LPA2 with other risk factors for CAV and with plaque burden characteristics were calculated according to Pearson. Univariate and multivariate analyses used least-square regression analysis using IVUS parameters as the dependent variable. The multivariate model included variables with significance level P≤0.05 in univariate analysis and LDL cholesterol. Skewed variables were logarithmically transformed for use in this model providing a better fit model. Kaplan–Meier estimates and log-rank test were used to describe freedom from adverse events and to compare between groups (e.g., LP-PLA2≤236 ng/mL and LP-PLA2>236 ng/mL).
We used ordinal logistic regression for calculation of odds ratio between Lp-PLA2 tertiles. Kaplan–Meier estimates and log-rank test were used to describe freedom from composite endpoint (CV events and CV death) and to compare between the groups (e.g., LP-PLA2≤236 ng/mL and LP-PLA2>236 ng/mL). For all comparisons a value of P<0.05 was considered to be statistically significant.
RESULTS
Demographic, clinical, and laboratory characteristics of the patient’s population are reported in Table 1. At the time of study entry, 87% of the patients were treated with statins and LDL cholesterol level was 99.7±29.6 mg/dL. The time between blood sampling and 3D IVUS was 4.27±0.99 years. Circulatory Lp-PLA2 level (201 ng/mL [174–251], median [IQR]) was positively correlated with recipient age (r=0.28, P=0.004) (Table 2). Patients who undergo transplantation for ischemic CMP had higher Lp-PLA2 levels than those who undergo transplantation for other reasons (249.3l±64.83 ng/mL vs. 206.7±62.11 ng/mL, P=0.002). The difference remained significant after adjustment for age (241±69.74 ng/mL vs. 207±64.26 ng/mL, P=0.044).
TABLE 1.
Patient characteristics
| N=112 | |
|---|---|
| Age (yrs) | 47.58±15.92 |
| Male gender | 90 (82%) |
| Indication for transplant | |
| Ischemic cardiomyopathy | 32 (30%) |
| Dilated cardiomyopathy | 54 (50%) |
| Other | 22 (20%) |
| Time transplant-blood test (yrs) | 5.27±3.35 |
| Donor age (yrs) | 30.06±12.56 |
| Cold ischemic time (min) | 164.57±50.02 |
| BMI | 28.73±6.25 |
| ACE inhibitor | 40 (37%) |
| Statin | 95 (87%) |
| Cyclosporine | 95 (84%) |
| Tacrolimus | 18 (16%) |
| Azathioprine | 75 (68%) |
| Mycophenolate mofetil | 37 (32%) |
| Prednisone | 35 (32%) |
| History of CMV infection | 27 (25%) |
| Hypertension | 45 (41%) |
| Diabetes mellitus | 40 (37%) |
| Rejection episodes (>1B) | 67 (61%) |
| Creatinine (mg/dL) | 2.18±1.16 |
| Glucose (mg/dL) | 100 (92.0–109.75)a |
| Triglycerides (mg/dL) | 182.19±78.48 |
| Total cholesterol (mg/dL) | 175.46±34.31 |
| LDL-cholesterol (mg/dL) | 99.71±29.64 |
| LDL>130 mg/dL, n (%) | 14 (13.5%) |
| LDL particle size (Å) | 271 (266–275)a |
| HDL-cholesterol (mg/dL) | 48.80±15.51 |
| IDL (mg/dL) | 33.70±10.83 |
| Lp-PLA2 (ng/mL) | 201 (174–251)a |
| CRP (mg/L) | 1.40 (0.56–3.45)a |
Median and IQR.
TABLE 2.
Correlation of Lp-PLA2 with clinical and lab parameters
| Variables | Correlation coefficient | P |
|---|---|---|
| Recipient age | 0.28 | 0.004 |
| Recipient gender | 0.13 | 0.17 |
| Time after transplant | 0.12 | 0.22 |
| Ischemic indication for transplantation | 0.30 | 0.002 |
| BMI | 0.06 | 0.54 |
| Creatinine | 0.08 | 0.42 |
| Total cholesterol | 0.33 | 0.0004 |
| LDL cholesterol | 0.40 | 0.0007 |
| IDL cholesterol | 0.28 | 0.003 |
| LDL particle size | −0.27 | 0.004 |
| HDL cholesterol | 0.008 | 0.94 |
| Triglycerides | 0.31 | 0.001 |
| CRP | 0.05 | 0.62 |
Circulatory Lp-PLA2 was not associated with time after transplantation (r=0.12, P=0.22), gender (r=0.13, P=0.17), creatinine (r=0.08, P=0.42), body mass index (r=0.06, P=0.54), or CRP (r=0.05, P=0.61) level. Lp-PLA2 positively correlated with plasma triglycerides (r=0.31, P=0.0012), LDL-cholesterol (r=0.40, P=0.0007), IDL cholesterol (r=0.28, P=0.0034), and total cholesterol level (r=0.33, P=0.0004) and negatively with LDL particle size (r=−0.27, P=0.0044) (Table 2).
Lp-PLA2 Association With Changes in Volumetric Findings Assessed by 3D IVUS
The results of serial 3D IVUS volumetric assessments are presented in Table 3.
TABLE 3.
Volumetric assessment of vascular geometry and progression of allograft vasculopathy during 12 mo follow-up (n=56)
| 1st 3D IVUS | 2nd 3D IVUS | |
|---|---|---|
| SL (mm) | 46.04±6.69 | 42.59±10.55 |
| VV/SL (mm3/mm) | 13.08±3.38 | 13.27±3.91 |
| LV/SL (mm3/mm) | 9.25±2.48 | 8.89±3.04 |
| PV/SL (mm3/mm) | 3.83±1.65 | 4.39±1.88 |
| % PV | 29±9 | 33±11 |
SL, segment length; VV, vessel volume; LV, lumen volume; PV, plaque volume; %PV, percent plaque volume.
A positive correlation existed between circulating Lp-PLA2 value and change in plaque volume (r=0.43, P=0.00026) and percent plaque volume (r=0.45, P=0.0004, Fig. 1A and B). Male gender, donor age, body mass index, triglyceride level, LDL particle size, and time after transplantation were also univariate predictors of either increase in plaque volume or in percent plaque volume (Table 4).
FIGURE 1.
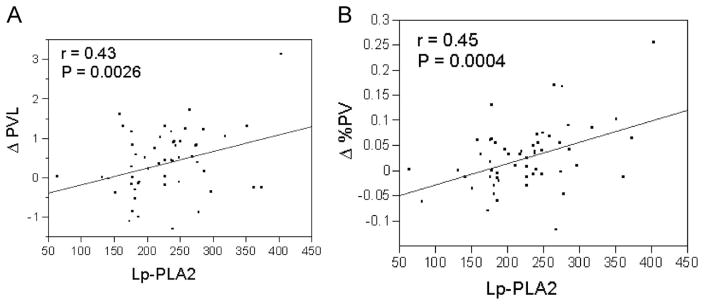
(A) X–Y scatterplot for the correlation between Lp-PLA2 and changes in plaque volume (r=0.43; P=0.0026). (B) X–Y scatterplot for the correlation between Lp-PLA2 and changes in percent plaque volume (r=0.45; P=0.0004).
TABLE 4.
Univariate predictors of changes in PV and %PV, assessed by 3D IVUS
| Univariate predictors of changes in plaque volume (Δ PV/SL)
|
Univariate predictors of changes in percent plaque volume (Δ %PV)
|
|||
|---|---|---|---|---|
| β (95% CI) | Pa | β (95% CI) | Pa | |
| Age | 0.025 (−0.007–0.058) | 0.12 | 0.18 (−0.007–0.368) | 0.06 |
| Male gender | 1.37 (0.204–2.527) | 0.02 | 6.96 (0.083–13.843) | 0.05 |
| Donor age | 0.05 (0.009–0.092) | 0.02 | 0.25 (0.007–0.499) | 0.04 |
| Time after transplant | 0.09 (−0.069–0.257) | 0.25 | 1.2 (0.299–2.130) | 0.01 |
| Indication for transplant-ischemic CMP | 0.64 (−0.433–1.708) | 0.06 | 4.7 (−1.589–10.950) | 0.14 |
| BMI | 0.1 (0.039–0.177) | 0.003 | 0.37 (−0.056–0.796) | 0.09 |
| History of rejection | 0.845 (−0.208–1.897) | 0.11 | 4.407 (−1.925–10.740) | 0.17 |
| Triglycerides | 0.008 (0.003–0.013) | 0.004 | 0.05 (0.020–0.082) | 0.002 |
| HDL cholesterol | −0.04 (−0.069–−0.030) | 0.03 | −0.24 (−0.406–−0.017) | 0.03 |
| LDL cholesterol | −0.001 (−0.018–0.015) | 0.89 | 0.009 (−0.089–0.108) | 0.85 |
| IDL cholesterol | 0.06 (0.017–0.100) | 0.007 | 0.17 (−0.086–0.423) | 0.19 |
| Log-LDL particle size | −20.8 (−36.944–−4.649) | 0.01 | −77.9 (−175.662–19.825) | 0.12 |
| Lp-PLA2 | 0.005 (0.0020–0.0088) | 0.0026 | 0.0004 (0.0002–0.0007) | 0.0004 |
| Log-CRP | 0.02 (−0.393–0.426) | 0.94 | 0.58 (−1.817–−2.971) | 0.63 |
The regression coefficient (β) together with 95% confidence interval (CI) and P-values are based on univariate analysis using IVUS parameters as the dependent variable.
PV/SL, plaque volume/segment length.
Multivariate analysis was performed using the variables with significance level P≤0.05 in univariate analysis and LDL-cholesterol. After adjusting for the other factors in the multivariate analysis, circulatory Lp-PLA2 level and donor age independently associated with increase in plaque volume (P=0.030 and P=0.008, respectively) and percent plaque volume (P=0.016 and P=0.006, respectively).
Clinical Outcome Analyses
Lp-PLA2 Levels and CV Events
During follow-up of 5.1±1.6 years, 24 CV events (percutaneous coronary intervention=5, CABG=3, LVEF<45%=9, and CV death=7) occurred in 15 of 112 (13%) patients. Lp-PLA2 level>236 ng/mL (higher tertile) identified a subgroup of patients having a 2.4-fold increase of relative risk for combined endpoint of CV events (95% CI 1.16–5.19, P=0.012) compared with patients with Lp-PLA2≤236 ng/mL.
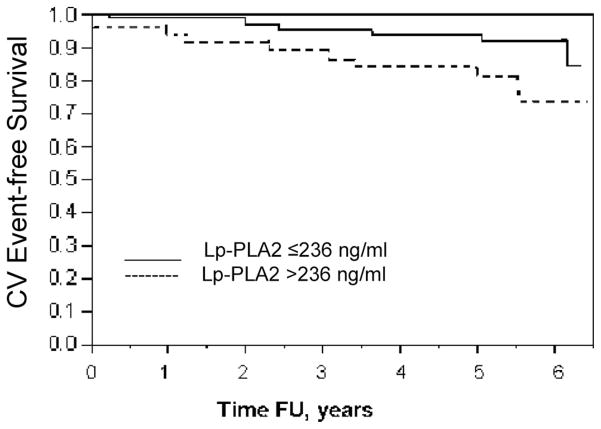
Freedom from CV events over 6 years after blood sample was 72% in the highest tertile of Lp-PLA2>236 ng/mL and was significantly lower compared with 92% in patients Lp-PLA2 level≤236 ng/mL (P=0.02, log-rank test) (Fig. 2).
FIGURE 2.
Cardiovascular event—free survival according to Lp-PLA2 levels. Freedom from CV events 6 years after blood sample was 74% in patients with Lp-PLA2>236 ng/mL (higher tertile) compared with 92% in patients with Lp-PLA2≤236 ng/mL (P=0.02, log-rank test). One patient in the higher tertile had already undergone PCI before the blood sample; results remained significant if that event was excluded from the analysis.
DISCUSSION
This study is the first to show that circulatory Lp-PLA2 levels are significantly and independently associated with subsequent increase in plaque burden assessed by 3D IVUS in the transplanted heart. The higher incidence of CV events in patients with higher Lp-PLA2 during follow-up suggests that Lp-PLA2 may serve as a marker or an active participant in the progression of CAV and predict adverse CV outcome after cardiac transplantation.
Correlation of Lp-PLA2 With Other Risk Factors
Consistent with previous studies in the nontransplant population (16), we found that higher Lp-PLA2 was associated with recipient age. Independent of recipient age, we also found it to be associated with ischemic cause of heart disease as an indication for transplant. This suggests that Lp-PLA2 is not only a marker of atherosclerotic coronary disease, but may also be a marker of increased global CV risk that carries forward after cardiac transplantation. It seems possible that Lp-PLA2 is an active participant in the process of coronary allograft vasculopathy.
In our study, there was an association between Lp-PLA2 and serum lipid concentrations, which is consistent with the results of previous studies (13, 15, 16). There is a direct relationship between Lp-PLA2 and total cholesterol, LDL cholesterol, IDL cholesterol, and triglycerides and an inverse relationship with LDL particle size. The correlation between Lp-PLA2 and non-HDL cholesterol observed in the current and the previous studies in nontransplant populations is expected, because approximately 80% of this enzyme circulates bound to LDL and another 7% with VLDL (21) with preference to atherogenic small dense LDL (24). The small dense LDL particle has enhanced penetration into the vessel wall (25) and enhanced susceptibility to oxidation (26). Only a small proportion of circulating enzyme (<15%) is associated with HDL. Lp-PLA2 hydrolyzes the sn2 ester bond in phospholipids of which the fatty acid moiety has been shortened or altered by oxidation to yield oxidized fatty acid and lysophosphatidylcholine (27). These metabolites have proinflammatory properties, and lead to increased expression of adhesion molecules and vascular inflammation (28). Lysophosphatidylcholine has also been shown to have adverse effects on endothelial function (29, 30). Although Lp-PLA2 is a marker of inflammation, it seems to be independent of CRP. This finding is supported by previous studies (13, 15, 16, 31) and emphasizes the unique and novel characteristics of Lp-PLA2 as a more specific marker of vascular inflammation, coronary endothelial dysfunction, and atherosclerosis.
Lp-PLA2 Association With Plaque Burden Assessed by 3D IVUS
Inflammation has been recognized as a major component in the pathogenesis of atherosclerosis (4) and may occur locally in the vessel wall (32), but signs of inflammation often are found in the systemic circulation. Increased circulating levels of inflammatory biomarkers are found in patients with established coronary artery disease (19, 20) and associated with severity of coronary artery disease (16–18, 20, 21). Recent reports from our group demonstrated that circulating Lp-PLA2 is an independent predictor of coronary endothelial dysfunction, an established stage of early coronary atherosclerosis (11, 12).
Although high CRP level identified patients at high risk for development of CAV, our previous study found no association between CRP levels and angiographic CAV severity (9). In nontransplant population, CRP was a poor predictor of the extent of atherosclerotic disease and demonstrated poor correlations with results of tests that quantify the extent of atherosclerosis (33–35). In our present study, we extended these observations and showed lack of association between CRP levels and the consequent plaque burden in transplant patients. These findings suggest that Lp-PLA2 and CRP may have different pathophysiological mechanisms in the atherosclerotic process (36). CRP is a marker of systemic inflammation, produced mainly in the liver, and may measure characteristics other than atherosclerotic mass, such as the activity of lymphocyte and macrophage populations within plaque or the degree of plaque destabilization (37). Lp-PLA2 may be more directly related to local vascular inflammation and endothelial dysfunction (36). This suggestion is supported by our previous observation of higher Lp-PLA2, but not CRP, production, across the coronary circulation in patients with early atherosclerosis and endothelial dysfunction (12).
Older donor age was independently associated with higher plaque burden in our study. In the 2006 International Society for Heart and Lung Transplantation registry, donor age was an independent risk factor for early CAV. It is unclear, however, whether increasing donor age represents occult pretransplant CAV or a donor age related predisposition to CAV (1).
Lp-PLA2 Association With CV Events
In patients without clinical manifestations of atherosclerosis, levels of inflammatory biomarkers may predict future CV events. At least four large studies have shown an association between Lp-PLA2 and the risk of future CV events in the general population (13–16); however, no previous study has been performed among transplant patients. We have shown that higher Lp-PLA2 levels (>236 ng/mL) are associated with a significantly higher incidence of CV adverse events, increasing relative risk by 2.4 compared with patients having Lp-PLA≤236 ng/mL. Thus, Lp-PLA2 may be a useful marker for risk of CAV and CV events and may be a therapeutic target in posttransplant patients.
Several studies have shown decreases in Lp-PLA2 and LDL-cholesterol levels in response to lipid lowering agents. Atorvastatin therapy can restore, at least partially, the dyslipidemia-induced alterations in Lp-PLA2 distribution by increasing the ratio of HDL-Lp-PLA2 to LDL-Lp-PLA2 levels (38). However, in contrast to CRP, which is reduced by statin therapy in a manner independent of effects on LDL-cholesterol, the effect of pravastatin on Lp-PLA2 levels was significantly attenuated after adjusting for changes in LDL cholesterol level (39). In our study, 87% of patients were treated with statins, and LDL cholesterol level was well controlled. However, patients with higher levels of Lp-PLA2 had increased plaque burden and higher incidence of CV adverse events. This data support the notion that Lp-PLA2 may enhance the atherogenicity of LDL and individuals with low LDL-cholesterol but high Lp-PLA2 may therefore have much greater atherogenicity than would be expected. Animal studies suggest that inhibitors of Lp-PLA2 may confer protection against atherosclerosis (40) and pharmacological interventions aimed at inhibiting the Lp-PLA2 enzyme may provide incremental benefit to intensive lipid-lowering therapy. Phase II studies are currently under way to test such compounds in humans and Lp-PLA2 has the potential to be a therapeutic target in posttransplant patients.
Study Limitations
The cross-sectional design imposes limitation with respect to patient selection for IVUS evaluation. However, serial IVUS was performed systematically and without knowledge of the lipid values, so the observed relationships should not be biased by the study design.
CONCLUSIONS
The present data demonstrate for the first time that circulating Lp-PLA2 levels are significantly and independently associated with subsequent progression of the atherosclerotic burden, and severity of CAV defined by 3D IVUS in the transplanted heart. There was a higher incidence of CV events in patients in the highest tertile of Lp-PLA2 during the subsequent 5 years of follow-up. This finding suggests that Lp-PLA2 may be a useful marker or participant in the progression of CAV and could be a therapeutic target in post-transplant patients.
Acknowledgments
This work was supported by grants R01 HL63911 and K24 HL69840.
References
- 1.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-third official adult heart transplantation report—2006. J Heart Lung Transplant. 2006;25:869. doi: 10.1016/j.healun.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Costanzo MR, Naftel DC, Pritzker MR, et al. Heart transplant coronary artery disease detected by coronary angiography: A multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant. 1998;17:744. [PubMed] [Google Scholar]
- 3.Bae JH, Rihal CS, Edwards BS, et al. Association of angiotensin-converting enzyme inhibitors and serum lipids with plaque regression in cardiac allograft vasculopathy. Transplantation. 2006;82:1108. doi: 10.1097/01.tp.0000230378.61437.a5. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 6.Pethig K, Heublein B, Kutschka I, et al. Systemic inflammatory response in cardiac allograft vasculopathy: High-sensitive C-reactive protein is associated with progressive luminal obstruction. Circulation. 2000;102:III233. doi: 10.1161/01.cir.102.suppl_3.iii-233. [DOI] [PubMed] [Google Scholar]
- 7.Hognestad A, Endresen K, Wergeland R, et al. Plasma C-reactive protein as a marker of cardiac allograft vasculopathy in heart transplant recipients. J Am Coll Cardiol. 2003;42:477. doi: 10.1016/s0735-1097(03)00645-4. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg MS, Chen HJ, Warshofsky MK, et al. Elevated levels of plasma C-reactive protein are associated with decreased graft survival in cardiac transplant recipients. Circulation. 2000;102:2100. doi: 10.1161/01.cir.102.17.2100. [DOI] [PubMed] [Google Scholar]
- 9.Raichlin ER, McConnell JP, Lerman A, et al. Systemic inflammation and metabolic syndrome in cardiac allograft vasculopathy. J Heart Lung Transplant. 2007;26:826. doi: 10.1016/j.healun.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Elstad MR, Stafforini DM, McIntyre TM, et al. Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J Biol Chem. 1989;264:8467. [PubMed] [Google Scholar]
- 11.Yang EH, McConnell JP, Lennon RJ, et al. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2006;26:106. doi: 10.1161/01.ATV.0000191655.87296.ab. [DOI] [PubMed] [Google Scholar]
- 12.Lavi S, McConnell JP, Rihal CS, et al. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: Association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 13.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: The Rotterdam study. Circulation. 2005;111:570. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 14.Koenig W, Twardella D, Brenner H, et al. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 16.Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland coronary prevention study group. N Engl J Med. 2000;343:1148. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 17.Brilakis ES, McConnell JP, Lennon RJ, et al. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J. 2005;26:137. doi: 10.1093/eurheartj/ehi010. [DOI] [PubMed] [Google Scholar]
- 18.Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 as a biomarker for coronary disease and stroke. Nat Clin Pract Cardiovasc Med. 2005;2:529. doi: 10.1038/ncpcardio0321. [DOI] [PubMed] [Google Scholar]
- 19.Winkler K, Winkelmann BR, Scharnagl H, et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: The Ludwigshafen risk and cardiovascular health study. Circulation. 2005;111:980. doi: 10.1161/01.CIR.0000156457.35971.C8. [DOI] [PubMed] [Google Scholar]
- 20.Khuseyinova N, Imhof A, Rothenbacher D, et al. Association between Lp-PLA2 and coronary artery disease: Focus on its relationship with lipoproteins and markers of inflammation and hemostasis. Atherosclerosis. 2005;182:181. doi: 10.1016/j.atherosclerosis.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 21.Caslake MJ, Packard CJ, Suckling KE, et al. Lipoprotein-associated phospholipase A (2), platelet-activating factor acetylhydrolase: A potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150:413. doi: 10.1016/s0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 22.Kardys I, Oei HH, Hofman A, et al. Lipoprotein-associated phospholipase A2 and coronary calcification. The Rotterdam Coronary Calcification Study. Atherosclerosis. 2007;191:377. doi: 10.1016/j.atherosclerosis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Chaves AJ, Sousa AG, Mattos LA, et al. Volumetric analysis of in-stent intimal hyperplasia in diabetic patients treated with or without abciximab: Results of the Diabetes Abciximab steNT Evaluation (DANTE) randomized trial. Circulation. 2004;109:861. doi: 10.1161/01.CIR.0000116752.12261.D4. [DOI] [PubMed] [Google Scholar]
- 24.Tselepis AD, Dentan C, Karabina SA, et al. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler Thromb Vasc Biol. 1995;15:1764. doi: 10.1161/01.atv.15.10.1764. [DOI] [PubMed] [Google Scholar]
- 25.Anber V, Griffin BA, McConnell M, et al. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis. 1996;124:261. doi: 10.1016/0021-9150(96)05842-x. [DOI] [PubMed] [Google Scholar]
- 26.de Graaf J, Hak-Lemmers HL, Hectors MP, et al. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb. 1991;11:298. doi: 10.1161/01.atv.11.2.298. [DOI] [PubMed] [Google Scholar]
- 27.Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol. 2003;14:347. doi: 10.1097/00041433-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 28.MacPhee CH, Moores KE, Boyd HF, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: Use of a novel inhibitor. Biochem J. 1999;338 (Pt 2):479. [PMC free article] [PubMed] [Google Scholar]
- 29.Murugesan G, Sandhya Rani MR, Gerber CE, et al. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J Mol Cell Cardiol. 2003;35:1375. doi: 10.1016/j.yjmcc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Wolfram Kuhlmann CR, Wiebke Ludders D, Schaefer CA, et al. Lysophosphatidylcholine-induced modulation of Ca(2+)-activated K(+)channels contributes to ROS-dependent proliferation of cultured human endothelial cells. J Mol Cell Cardiol. 2004;36:675. doi: 10.1016/j.yjmcc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Koenig W, Khuseyinova N, Lowel H, et al. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: Results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 32.Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: Role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 33.Redberg RF, Rifai N, Gee L, et al. Lack of association of C-reactive protein and coronary calcium by electron beam computed tomography in postmenopausal women: Implications for coronary artery disease screening. J Am Coll Cardiol. 2000;36:39. doi: 10.1016/s0735-1097(00)00680-x. [DOI] [PubMed] [Google Scholar]
- 34.Folsom AR, Pankow JS, Tracy RP, et al. Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol. 2001;88:112. doi: 10.1016/s0002-9149(01)01603-4. [DOI] [PubMed] [Google Scholar]
- 35.Hunt ME, O’Malley PG, Vernalis MN, et al. C-reactive protein is not associated with the presence or extent of calcified subclinical atherosclerosis. Am Heart J. 2001;141:206. doi: 10.1067/mhj.2001.112488. [DOI] [PubMed] [Google Scholar]
- 36.Iribarren C, Gross MD, Darbinian JA, et al. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults: The CARDIA study. Arterioscler Thromb Vasc Biol. 2005;25:216. doi: 10.1161/01.ATV.0000148322.89911.44. [DOI] [PubMed] [Google Scholar]
- 37.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 38.Eisaf M, Tselepis AD. Effect of hypolipidemic drugs on lipoprotein-associated platelet activating factor acetylhydrolase. Implication for atherosclerosis. Biochem Pharmacol. 2003;66:2069. doi: 10.1016/s0006-2952(03)00559-8. [DOI] [PubMed] [Google Scholar]
- 39.Albert MA, Glynn RJ, Wolfert RL, et al. The effect of statin therapy on lipoprotein associated phospholipase A2 levels. Atherosclerosis. 2005;182:193. doi: 10.1016/j.atherosclerosis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Blake GJ, Dada N, Fox JC, et al. A prospective evaluation of lipoprotein-associated phospholipase A (2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol. 2001;38:1302. doi: 10.1016/s0735-1097(01)01554-6. [DOI] [PubMed] [Google Scholar]