Abstract
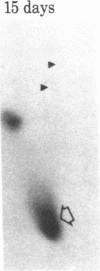
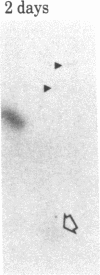
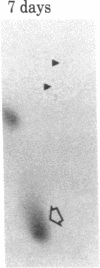
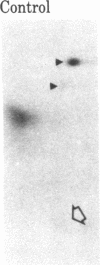
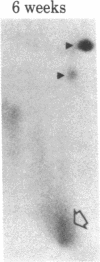
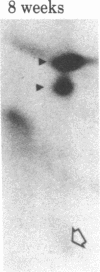
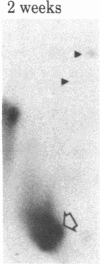
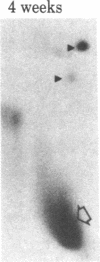
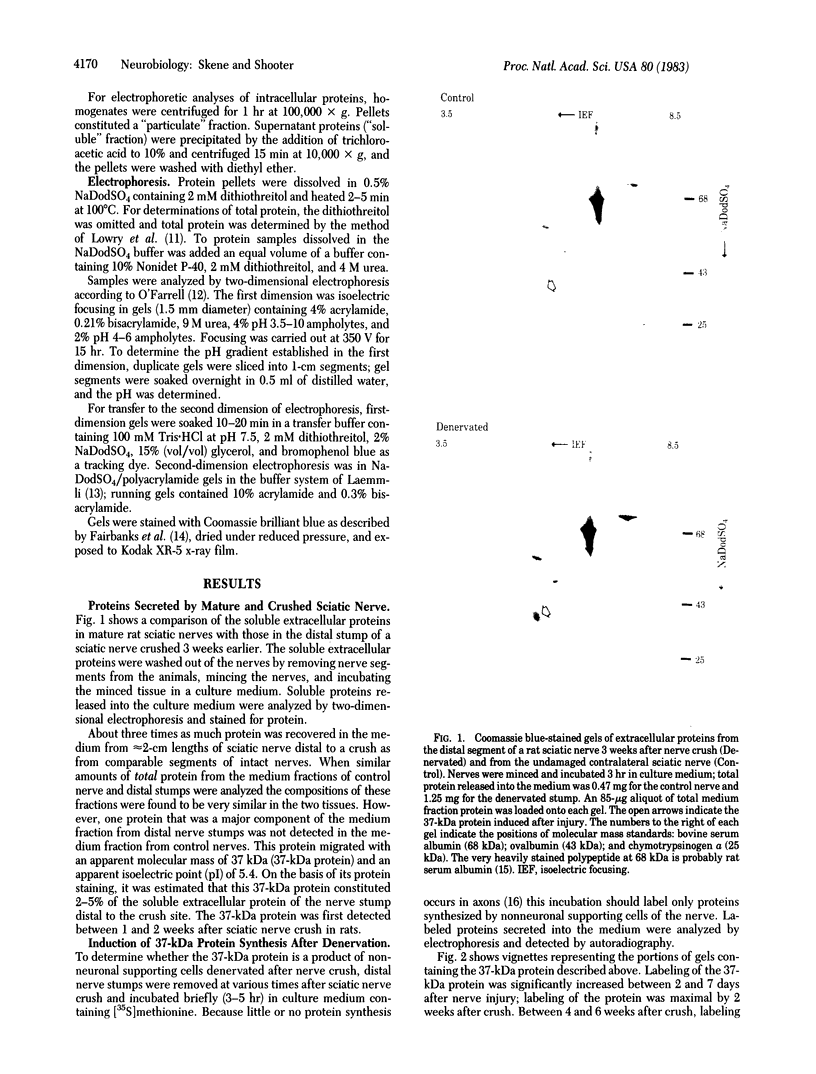
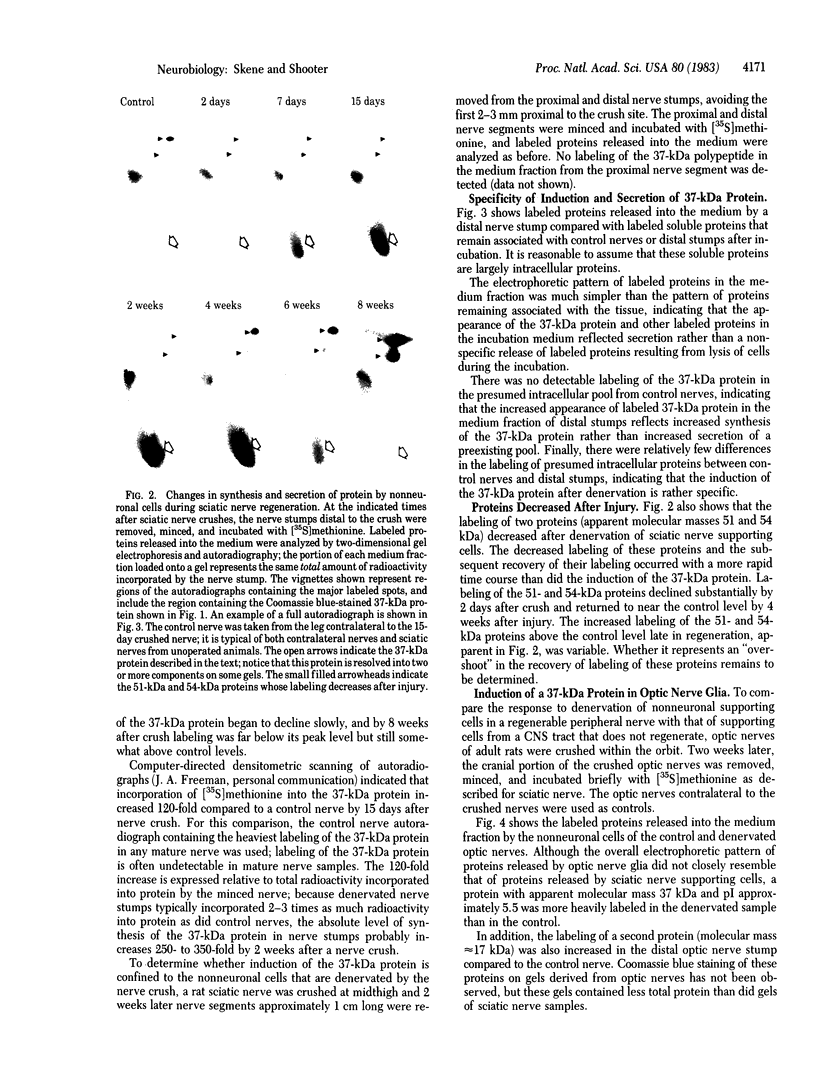
When rat sciatic nerves are crushed, Schwann cells or other supporting cells distal to the injury site begin to synthesize and secrete an acidic 37-kilodalton (kDa) protein. This crush-induced protein accumulates within the nerve sheath and accounts for 2-5% of the total extracellular protein in the distal nerve stump. Synthesis of the 37-kDa protein increased for 2 weeks after nerve crush and declines slowly, beginning 4-6 weeks after the injury. The synthesis of the protein may be regulated by axon-Schwann cell contact. The specific induction of the 37-kDa protein and its accumulation in the extracellular space during nerve regeneration suggest that the protein promotes some aspect of axon growth. Because it is induced slowly after injury, the 37-kDa protein is unlikely to stimulate initial outgrowth of axons; however, it might promote later neuronal responses related to axon growth. The sciatic nerve supporting cells also respond to denervation by reducing the synthesis and release of two proteins of molecular mass 51 and 54 kDa. After crush injury to rat optic nerves, glial cells in the distal optic nerve stump also begin to synthesize and release an acidic 37-kDa protein, although axons of this central nervous system tract do not regenerate. If the 37-kDa protein from peripheral nerves proves to participate in the support of axon regrowth, then the results with rat optic nerve suggest that central nervous system glia initiate at least one part of an appropriate response to nerve injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray G. M., Rasminsky M., Aguayo A. J. Interactions between axons and their sheath cells. Annu Rev Neurosci. 1981;4:127–162. doi: 10.1146/annurev.ne.04.030181.001015. [DOI] [PubMed] [Google Scholar]
- Burrell H. R., Heacock A. M., Water R. D., Agranoff B. W. Increased tubulin messenger RNA in the goldfish retina during optic nerve regeneration. Brain Res. 1979 Jun 8;168(3):628–632. doi: 10.1016/0006-8993(79)90318-4. [DOI] [PubMed] [Google Scholar]
- Calabretta A. M., Munger B. L., Graham W. P. The ultrastructure of degenerating rat sciatic nerves. J Surg Res. 1973 May;14(5):465–471. doi: 10.1016/0022-4804(73)90055-3. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Bunge R. P. Factors influencing the release of proteins by cultured Schwann cells. J Cell Biol. 1981 Dec;91(3 Pt 1):666–672. doi: 10.1083/jcb.91.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Aguayo A. J. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981 Nov 20;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Devor M., Govrin-Lippmann R. Maturation of axonal sprouts after nerve crush. Exp Neurol. 1979 May;64(2):260–270. doi: 10.1016/0014-4886(79)90267-x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forman D. S., Berenberg R. A. Regeneration of motor axons in the rat sciatic nerve studied by labeling with axonally transported radioactive proteins. Brain Res. 1978 Nov 10;156(2):213–225. doi: 10.1016/0006-8993(78)90504-8. [DOI] [PubMed] [Google Scholar]
- Gainer H., Fink D. J. Evidence for slow retrograde transport of serum albumin in rat sciatic nerve. Brain Res. 1982 Feb 11;233(2):404–408. doi: 10.1016/0006-8993(82)91214-8. [DOI] [PubMed] [Google Scholar]
- Ingoglia N. A., Sharma S. C., Pilchman J., Baranowski K., Sturman J. A. Axonal transport and transcellular transfer of nucleosides and polyamines in intact and regenerating optic nerves of goldfish: speculation on the axonal regulation of periaxonal cell metabolism. J Neurosci. 1982 Oct;2(10):1412–1423. doi: 10.1523/JNEUROSCI.02-10-01412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. Retrograde transport of horseradish peroxidase in transected axons. 1. Time relationships between transport and induction of chromatolysis. Brain Res. 1974 Oct 11;79(1):101–109. doi: 10.1016/0006-8993(74)90569-1. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Sjöstrand J. Retrograde transport of protein tracer in the rabbit hypoglossal nerve during regeneration. Brain Res. 1972 Oct 13;45(1):175–181. doi: 10.1016/0006-8993(72)90224-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundborg G., Longo F. M., Varon S. Nerve regeneration model and trophic factors in vivo. Brain Res. 1982 Jan 28;232(1):157–161. doi: 10.1016/0006-8993(82)90618-7. [DOI] [PubMed] [Google Scholar]
- McQuarrie I. G., Grafstein B., Dreyfus C. F., Gershon M. D. Regeneration of adrenergic axons in rat sciatic nerve: effect of a conditioning lesion. Brain Res. 1978 Feb 3;141(1):21–34. doi: 10.1016/0006-8993(78)90614-5. [DOI] [PubMed] [Google Scholar]
- Morton H. J. A survey of commercially available tissue culture media. In Vitro. 1970 Sep-Oct;6(2):89–108. doi: 10.1007/BF02616112. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Politis M. J., Ederle K., Spencer P. S. Tropism in nerve regeneration in vivo. Attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Res. 1982 Dec 16;253(1-2):1–12. doi: 10.1016/0006-8993(82)90667-9. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Ebendal T. Nerve growth activities in rat peripheral nerve. Brain Res. 1982 Aug 19;246(1):57–64. doi: 10.1016/0006-8993(82)90141-x. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., McGuinness U. M., Aguayo A. J. Peripheral nerve autografts to the rat spinal cord: studies with axonal tracing methods. Brain Res. 1982 Apr 8;237(1):147–162. doi: 10.1016/0006-8993(82)90563-7. [DOI] [PubMed] [Google Scholar]
- Rutzky L. P., Pumper R. W. Supplement to a survey of commercially available tissue culture media (1970). In Vitro. 1974 May-Jun;9(6):468–469. [PubMed] [Google Scholar]
- Salzer J. L., Bunge R. P., Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980 Mar;84(3):767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981 Apr;89(1):96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Willard M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. J Cell Biol. 1981 Apr;89(1):86–95. doi: 10.1083/jcb.89.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]