Abstract
Wnt signalling plays a critical role in both initiating and patterning of the anterior–posterior axis during development. Wnts exert their biological effects, in part, by activating specific target genes through members of the TCF/LEF family of transcription factors. To gain new insight into the role of T-cell factors (or Tcf's) during development, we analysed Tcf4 and Tcf1 compound null embryos. These mutants showed severe caudal truncations, as well as duplications of the neural tube. Unlike other mutations affecting Wnt signalling, paraxial mesoderm formation was not impaired and early caudal markers, such as T, were unaffected. Analysis of endodermal markers uncovered early and specific defects in hindgut expansion, and later an anterior transformation of the gastro-intestinal tract. Our results reveal a novel role for Wnt signalling in early gut morphogenesis and suggest that specific Wnt-driven patterning events are determined by the unique tissue distribution of Tcf/Lef family members.
Keywords: endoderm, gut, Tcf1, Tcf4, Wnt signalling
Introduction
During mouse development, the anterior–posterior (A–P) axis becomes morphologically evident at the onset of gastrulation. At this point, the epiblast cell layer at the posterior end of the embryo forms a structure known as the primitive streak. Cells at the streak delaminate and migrate forward and laterally to form both mesodermal and endodermal layers. As gastrulation proceeds at the posterior end, body structures such as head, trunk, limbs and tail are specified along the A–P axis. This process of patterning and later organogenesis depends on a complex network of instructive signals, ultimately determining whether cells within the developing embryo proliferate, differentiate, or die.
Among the most important regulators of cell fate decisions during both formation and patterning of the A–P axis are members of the Wnt/Wg family of secreted factors. Wnts make up a large family of cystein-rich glycoproteins that activate signalling cascades that induce cytoplasmic responses and/or transcription of target genes (for reviews, see Wodarz and Nusse, 1998; Yamaguchi, 2001; Mlodzik, 2002; He, 2003). The latter depends on cytoplasmic accumulation of β-catenin and its translocation to the nucleus, where it associates with the TCF/LEF family members (comprised of TCF1, LEF, TCF3, TCF4). Formation of this bipartite complex provides a template for the recruitment of additional transcriptional activators that finally turn on expression of specific target genes. Importantly, in the absence of Wnts, TCF/LEF proteins can actively block transcription by recruiting general repressors like Groucho and CtBP (Nusse, 1999; Hurlstone and Clevers, 2002). In this way, TCF/LEF proteins act as molecular switches that control cell fate decisions.
Perhaps the first indication that the Wnt/β-catenin/TCF pathway was required for determining the A–P axis came from microinjection experiments conducted in Xenopus embryos. Ectopic expression of Wnts, for example, induces axis duplications and can rescue UV-irradiated embryos (McMahon and Moon, 1989; Sokol et al, 1991). In addition, gene-targeting experiments in mice have provided a wealth of information confirming an essential role for Wnt signalling in axis formation. Mutations disrupting Wnt signalling, such as Wnt3 and β-catenin knockouts, completely impair formation of the primitive streak and the resulting embryos remain as egg cylinders lacking posterior specification (Liu et al, 1999; Huelsken et al, 2000). On the other hand, mutations in Axin and APC, which lead to increased β-catenin/TCF-mediated transcription, cause axis duplications similar to those observed in Xenopus (Zeng et al, 1997; Ishikawa et al, 2003). Similarly, ablation of Tcf3 results in duplications of the node and notochord, as well as upregulation of axial markers, such as Foxa2, implying that Tcf3 acts primarily as a transcriptional repressor involved in restricting AP axis induction (Merrill et al, 2004).
Deletion of other components of the Wnt cascade has shown that Wnt signals are also required for patterning and/or expansion of the mouse embryonic axis once it has formed. Mutations in Wnt1 lead to deletion of the midbrain and cerebellar component of the hindbrain (McMahon and Bradley, 1990). Dkk−/− embryos display a disruption of tissues forebrain and cephalic neural crest-derived tissues, as well as limb morphogenesis defects (Mukhopadhyay et al, 2001). Wnt3a−/− and Lef−/−/Tcf1−/− embryos show severe caudal truncations of the embryonic axis, resulting from an early loss of mesodermal structures (ie somites) (Takada et al, 1994; Greco et al, 1996; Galceran et al, 1999). The absence of Wnt5a results in a general shortening of the embryonic axis, including limbs (Yamaguchi et al, 1999a). Finally, loss of LRP6, an essential Wnt coreceptor, recapitulates many of the phenotypes described above, with disrupted patterning in the brain, limbs, neural tube and tail (Pinson et al, 2000).
Besides the phenotype of Lef−/−/Tcf1−/− and Tcf3−/− embryos described above, limited information is available about the function of other Tcf/Lef genes during early mouse development. Lef−/− mice die perinatally, lacking teeth, hair follicles and mammary glands (van Genderen et al, 1994). Further analysis has also demonstrated impaired hippocampus development and generation of dentate gyrus granule cells in Lef-deficient mice (Galceran et al, 2000). Tcf1 homozygous null mice are viable but demonstrate an early blockage in thymocyte differentiation, as well as increased susceptibility to form mammary gland and intestinal neoplasms (Verbeek et al, 1995; Roose et al, 1999). Tcf4−/− mice die at birth due to a loss of epithelial stem cells in the small intestine (Korinek et al, 1998a). In order to gain further insight into the function of Tcf/Lef genes during morphogenesis, we generated compound Tcf4−/−/Tcf1−/− mice. These mutants display profound caudal truncations similar to Wnt3a−/− and Lef−/−/Tcf1−/− embryos, although, unlike the latter, Tcf4−/−/Tcf1−/− embryos retain paraxial mesoderm. Rather, the primary patterning defect appears to result from aberrant hindgut expansion, ultimately leading to homeotic shifts of the fetal gastro-intestinal tract.
Results
Tcf4−/−/Tcf1−/− mutants show posterior truncations
To study the consequences of inactivating both Tcf1 and Tcf4 during embryonic development, we crossed Tcf4+/− and Tcf1−/− mice. Although smaller than wild-type counterparts, Tcf4+/−/Tcf1−/− mice appeared normal. Compound homozygous null embryos, however, did not survive past gestation. Viable double mutants were obtained at the expected frequency until E14.5. Examination of Tcf4−/−/Tcf1−/− embryos at this stage showed major abnormalities in the development of caudal structures, while anterior structures were spared. As shown in Figure 1, Tcf4−/−/Tcf1−/− mutants lacked hindlimbs, posterior body and tail. Although the formation of hindlimbs was affected, forelimbs were normal, suggesting that the loss of hindlimbs reflects a general patterning defect, rather than a specific problem in limb development. In more severely truncated embryos, internal organs were exposed, indicating a failure of the abdominal wall to develop properly (Figure 1C and D). Haematoxylin/eosin stainings of sections of the specimen depicted in Figure 1B revealed that most internal structures, including the lungs, heart, pancreas and liver, were clearly distinguished and histologically appeared normal (Figure 1F and data not shown). Even more caudal structures such as kidneys and mesonephric ducts had formed and differentiated. This may imply that Tcf4 and Tcf1 are not required for early morphogenesis of these organs. Closer examination of Tcf4−/−/Tcf1−/− mutants, however, showed a complete absence of the genital tubercle, hindgut and only a remnant of the urogenital sinus, as well as signs of aberrant branching of neural tube (Figure 1D and F and below).
Figure 1.
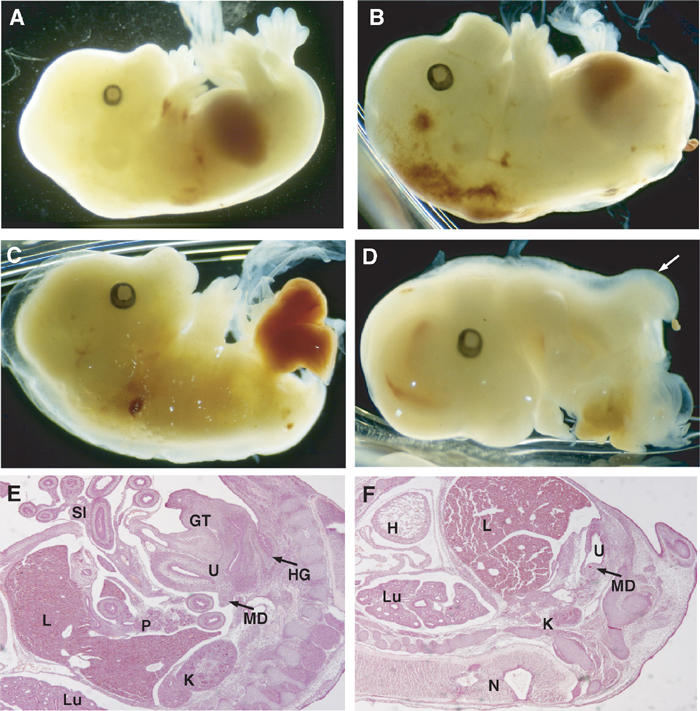
Tcf4−/−/Tcf1−/− embryos show severe caudal truncations. Lateral views of E14.5 embryos. (A) Tcf4+/−/Tcf1−/− and (B–D) Tcf4−/−/Tcf1−/− littermates. Panel B shows vestigial hindlimbs and the absence of a tail in mutant embryos. In more severe cases as depicted in panels C and D, internal organs such as the gut and liver are exposed. In several instances, the neural tube branched out in mutant embryos as shown in (D, F) (see arrow). Panels E and F represent haematoxylin and eosin stainings of sections of embryos depicted in (A, B), respectively. Various structures are identified as follows: (H) heart, (Lu) lungs, (L) liver, (K) kidneys, (U) urogenital sinus, (MD) mesonephric duct, (GT) genital tubercle, (SI) small intestine, (HG) hindgut and (N) neural tube.
Duplications of the neural tube in Tcf4−/−/Tcf1−/− embryos
The posterior truncations observed in Tcf4−/−/Tcf1−/− embryos were reminiscent of Wnt3a−/− mutants. Analysis of these mice, as well as Lef−/−/Tcf1−/− embryos, revealed an early loss of paraxial mesoderm and concomitant formation of ectopic neural tubes (Yoshikawa et al, 1997; Galceran et al, 1999). To characterize the fate of paraxial mesoderm versus neural tissue in Tcf4−/−/Tcf1−/− embryos, we began our analysis by examining the expression of the neural tube marker Wnt-1 in E10.5 embryos. As shown by whole-mount in situ hybridization, Wnt-1 expression along the anterior portion of Tcf4−/−/Tcf1−/− embryos was comparable to that of normal littermates (Figure 2). Towards the posterior end, ectopic Wnt-1 expression was detected, reflecting bifurcations or splitting of the neural tube (Figure 2D, see arrow). Sectioning through the mutant embryos confirmed the occurrence of duplications of the neural tube (Figure 2E).
Figure 2.
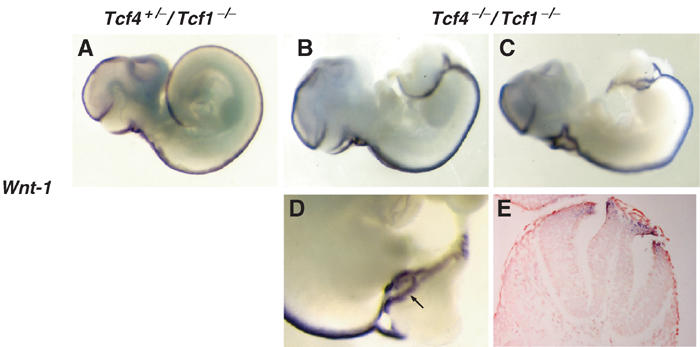
Duplications of the neural tube in Tcf4−/−/Tcf1−/− embryos. Whole-mount in situ hybridizations of E10.5 littermates using a Wnt1 probe as a marker for neural tube. (A) Tcf4+/−/Tcf1−/− embryo; (B) right side, (C) left side and (D) close-up views of a representative Tcf4−/−/Tcf1−/− embryo. Lateral and close-up views reveal that the neural tube appears to split at the caudal end of mutant embryos. In some regions (see the arrow in (D) and the corresponding section in (E)), complete duplications of the neural tube are observed. Note that Wnt1 expression appears normal in the anterior half of Tcf4−/−/Tcf1−/− embryos.
Paraxial mesoderm formation is unaffected in Tcf4−/−/Tcf1−/− embryos
To follow the fate of paraxial mesoderm, we employed three somitic markers, Paraxis, Pax1 and Myf5. Detection of Paraxis transcripts, normally present in sclerotome and dermamyotome compartments, clearly distinguished the presence of somitic tissue along the entire A–P axis of Tcf4−/−/Tcf1−/− embryos (Figure 3B). Note, however, that the somites at the caudal end did appear disorganized. Even in a specimen displaying a more severe truncation, the sclerotome marker Pax1 revealed that, despite being smaller and less well defined, somites were present (Figure 3D). Finally, we tested the myogenic marker Myf5, and found once again that caudal somites had formed (Figure 3F). The maintenance of somitic markers was in stark contrast to the previously described Lef−/−/Tcf1−/− phenotype, in which Pax1 expression was completely absent below the forelimbs (Galceran et al, 1999). To confirm the differences between the two crosses, we generated Lef−/−/Tcf1−/− embryos and verified the expression of Paraxis in these mutants (data not shown). As expected, Paraxis staining was abolished in the caudal portion of these mutants, providing additional proof that, contrary to Tcf4−/−/Tcf1−/− embryos, Lef−/−/Tcf1−/− mutants lack somitic tissue. These results suggest that, despite forming ectopic neural tissue, paraxial mesoderm fate is unaffected in Tcf4−/−/Tcf1−/− embryos.
Figure 3.
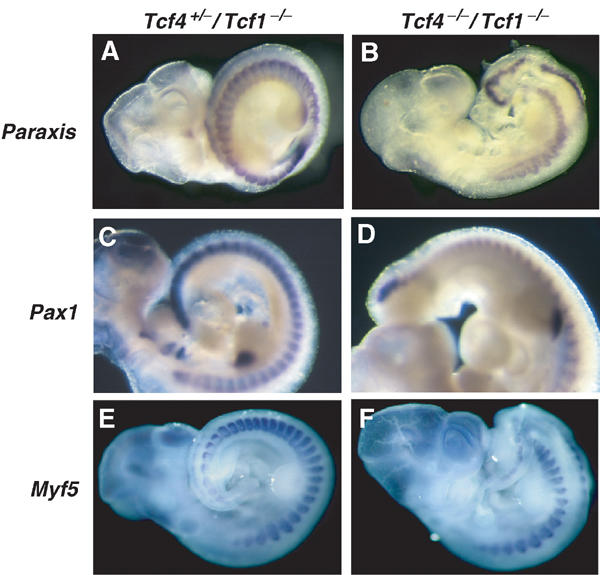
Paraxial mesoderm is intact in Tcf4−/−/Tcf1−/− embryos. Whole-mount in situ hybridizations of E10.5 littermates using markers for paraxial mesoderm. (A, C, E) Tcf4+/−/Tcf1−/− embryos and (B, D, F) Tcf4−/−/Tcf1−/− embryos. Panels A and B show stainings for Paraxis, panels C and D show Pax1 stainings. Panels E and F show expression of Myf5. All three somitic markers confirm the presence of paraxial mesoderm throughout the axis of Tcf4−/−/Tcf1−/− mutants.
Next, we assessed whether expression of early caudal markers was impaired. Several genes expressed in the primitive streak and presomitic mesoderm have been reported to play an essential role during patterning of the caudal end of the mouse embryo. For example, mutations in T (Brachyury) cause a kinked tail phenotype and maintenance of T expression was shown to depend on intact TCF/LEF-binding sites in its promoter (Yamaguchi et al, 1999b; Galceran et al, 2001). However, in situ hybridization on both E8.5 and E9.5 embryos demonstrates that T expression is unaltered in Tcf4−/−/Tcf1−/− embryos (Figure 4A–D). These results contrast the analysis of Lef−/−/Tcf1−/− embryos, where maintenance of T expression was abolished in the tail bud of E9.5 embryos. We also tested Tbx6, another T box-containing family member implicated in posterior patterning (Chapman and Papaioannou, 1998). As shown in Figure 4E and F, Tbx6 transcripts were still detected, although the levels and range of expression were reduced compared to normal littermates. Cdx1 expression was shown to be downregulated as early as E8.5 in Wnt3a-deficient embryos, and in the fetal gut of Tcf4−/− mice (Lickert et al, 2000; Ikeya and Takada, 2001; Prinos et al, 2001). In spite of these observations, Figure 4G and H shows that Cdx1 is not dependant on Tcf1/Tcf4, at least during gastrulation. Finally, we tested Wnt5a because of its essential role in expansion of the tailbud, but as with other markers tested (including Cdx2, Lef, Evx1 and Wnt3a, data not shown) no changes in expression were observed. Altogether, these results provide further evidence that the patterning defects in Tcf4−/−/Tcf1−/− embryos cannot be directly attributed to loss of mesodermal precursors.
Figure 4.
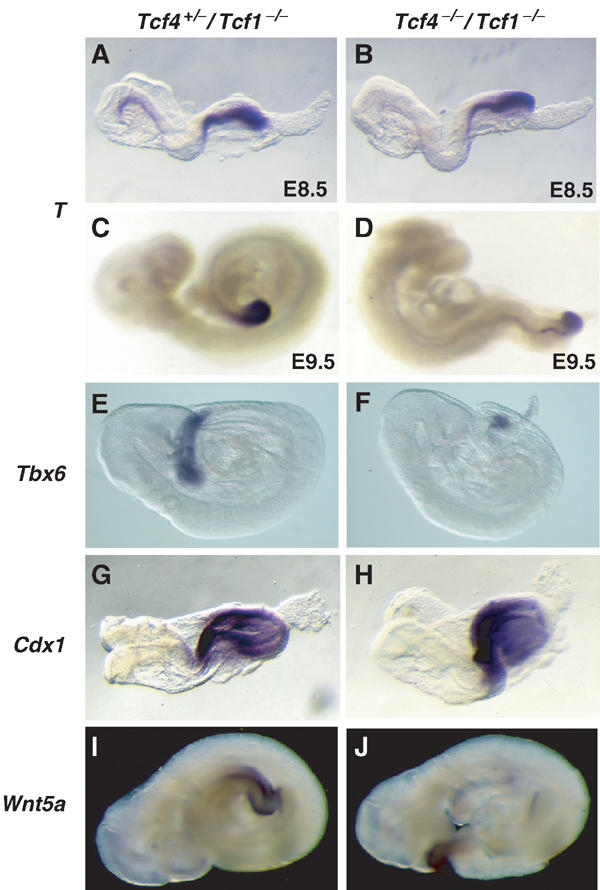
Expression of early posterior markers is unaffected in Tcf4−/−/Tcf1−/− embryos. Whole-mount in situ hybridizations of E8.5–9.5 littermates using various posterior markers (see above). (A, C, E, G, I) Tcf4+/−/Tcf1−/− embryos and (B, D, F, H, J) Tcf4−/−/Tcf1−/− embryos. Panels (A, B) and (C, D) represent E8.5 and E9.5 embryos, respectively, and show that initiation and maintenance of T expression is unchanged in Tcf4−/−/Tcf1−/− embryos.
Expression of Tcf4, Tcf1 and Lef in the primitive gut
To gain further insight into the underlying causes of the Tcf4−/−/Tcf1−/− phenotype, we re-examined the expression of Tcf/Lef family members. Previous analysis has shown overlapping expression of Tcf1 and Lef in the forelimbs and primitive streak region of gastrulating embryos (Oosterwegel et al, 1993; Galceran et al, 1999). Somewhat surprisingly, in the light of the caudal truncations observed in Tcf4−/−/Tcf1−/− embryos, Tcf4 transcripts were mainly detected in rostral structures, such as di- and mesencephalon and pharyngeal arches (Korinek et al, 1998b; Galceran et al, 1999). To address this discrepancy, we performed in situ hybridization on sections of E8.5, and E10.5 wild-type embryos, focusing our attention on posterior structures. Sections through the primitive streak showed that the overall expression pattern of Tcf/Lef genes was only partially overlapping. At E8.5 Tcf4 was specifically detected in the hindgut. Tcf1 was expressed in the primitive ectoderm, presomitic mesoderm and hindgut. Lef was restricted to the presomitic mesoderm (Figure 5A–C). At later stages, we could distinguish Tcf4 expression in the neural tube and hindgut. Comparable sections showed that Tcf1 expression was maintained in all three germ layers, including the neural tube, presomitic mesoderm and hindgut. Importantly, Lef was excluded from the primitive gut and was expressed in mesodermal structures (Figure 5D–F). The tissue specificities of Tcf/Lef genes provided a rationale to explain the disparities between the phenotypes of the compound null mutants. The overlapping expression of Tcf1 and Lef in the presomitic mesoderm, coupled with the exclusion of Tcf4 in this compartment, may explain why Lef−/−/Tcf1−/− embryos, but not Tcf4−/−/Tcf1−/− embryos, lose caudal somites. By analogy, we reasoned that the primary anomaly in Tcf4−/−/Tcf1−/− embryos may be impaired hindgut development. Based on these observations, we decided to analyse endodermal fate in Tcf4−/−/Tcf1−/− embryos.
Figure 5.
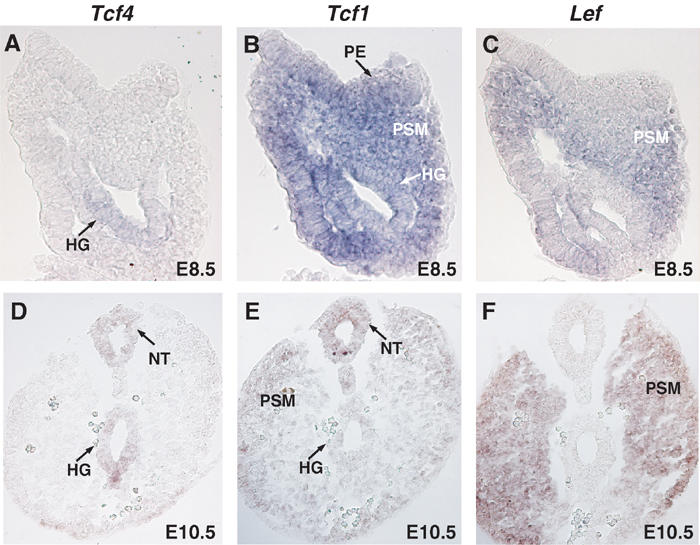
Comparison of Tcf4, Tcf1 and Lef expression. In situ hybridizations on sections of E8.5 and E10.5 wild-type embryos were performed using specific probes recognizing Tcf4 (A, D), Tcf1 (B, E) and Lef (C, F). Top panels A–C depict sections through the primitive streak region of E8.5 embryos, while bottom panels D–E show sections through the tail bud of E10.5 embryos. Various structures are identified as follows: (PE) primitive ectoderm, (NT) neural tube, (PSM) presomitic mesoderm and (HG) hindgut. Tcf4 was specifically expressed in the hindgut at E8.5 and E10.5 in the neural tube and hindgut. Tcf1 was found in all three germ layers at both E8.5 and E10.5. Lef was detected in the presomitic mesoderm in both stages examined and was absent from the primitive gut.
Caudal endoderm is impaired in Tcf4−/−/Tcf1−/− but not Lef−/−/Tcf1−/− embryos
To follow gut development, we analysed the expression of three endodermal markers Sox17, Foxa1 and Shh in Tcf4−/−/Tcf1−/− and Lef−/−/Tcf1−/− embryos. At E8.5, endodermal tube formation is initiated by folding of the endodermal lining at the anterior and posterior ends, creating anterior and caudal intestinal portals (AIP and CIP) (for reviews, see Wells and Melton, 1999; Roberts, 2000). At this stage, Sox17 and Foxa1 are normally expressed along the entire endodermal lining in wild-type embryos (Figure 6A and D). In Tcf4−/−/Tcf1−/− embryos, however, the caudal endoderm showed an absence of both Sox17 and Foxa1 staining (Figure 6B and E, see arrows). Conversely, in Lef−/−/Tcf1−/− embryos, expression of both markers is uninterrupted (Figure 6C and F). At E9.5, the AIP and CIP join to close the endodermal tube, resulting in the formation of a proper foregut, midgut and hindgut. During this stage, Shh expression is detected throughout the newly formed endodermal tube and clearly distinguishes the hindgut from the notochord at the posterior end (Figure 6G–I). In Tcf4−/−/Tcf1−/− embryos, only notochord staining is observed, indicating a complete lack of the hindgut. As a result of the loss of underlying gut, the notochord appears exposed at the tail end of these embryos (Figure 6H, see arrowhead). Shh expression in Lef−/−/Tcf1−/− embryos was maintained in the hindgut (Figure 6I, see arrow), implying once again that the observed effects in Tcf4−/−/Tcf1−/− are specific. To confirm these data, we prepared haematoxylin/eosin sections of wild-type, Tcf4−/−/Tcf1−/−, as well as Lef−/−/Tcf1−/− embryos. Sections through the midgut region (just posterior to the stomach) of Tcf4−/−/Tcf1−/− embryos revealed that the endodermal tube had not closed (Figure 6K). More caudal sections showed ectopic neural tissue and the absence of any hindgut (Figure 6N). Equivalent regions in Lef−/−/Tcf1−/− embryos demonstrated that the gut tube had formed despite the loss of somites and formation of multiple neural tubes (Figure 6L and O). Altogether, these results implied that specification and expansion of the hindgut is aberrant in Tcf4−/−/Tcf1−/− embryos.
Figure 6.
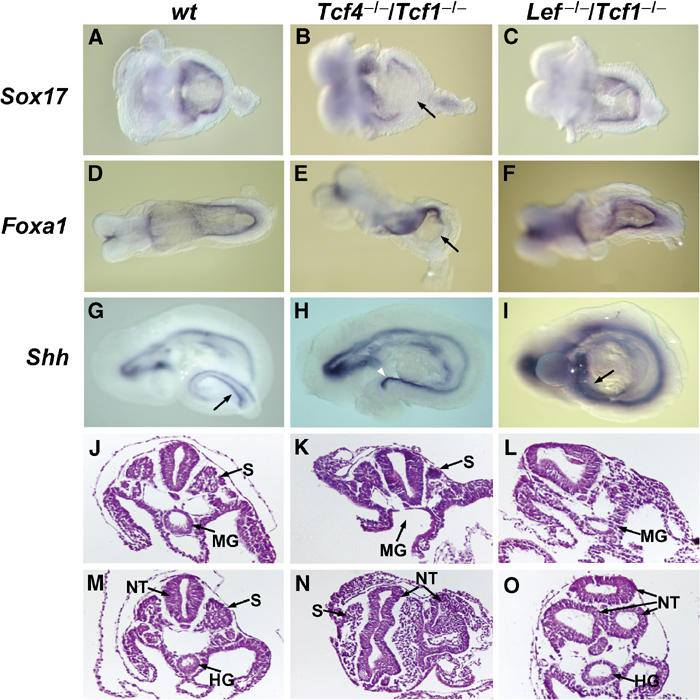
Impaired expansion of caudal endoderm in Tcf4−/−/Tcf1−/−embryos. Whole-mount in situ hybridizations of E8.5–9.5 littermates using endodermal markers and histology of E9.5 normal and mutant embryos. (A, D, G, J, M) Wild-type embryos, (B, E, H, K, N) Tcf4−/−/Tcf1−/−embryos and (C, F, I, L, O) Lef−/−/Tcf1−/− embryos. The arrows in (B) and (E) point to the loss of Sox17 and Foxa1 staining in the caudal endoderm of Tcf4−/−/Tcf1−/−, while staining is normal in Lef−/−/Tcf1−/− embryos. Arrows in (G) and (I) point to Shh expression in the hindgut of wild-type and Lef−/−/Tcf1−/− embryos. The notochord in Tcf4−/−/Tcf1−/−embryos appears exposed due to the absence of an underlying gut tube (arrowhead in (H)). Panels J–L and M–O represent haematoxylin and eosin stainings of sections through the midgut region and hindgut, respectively. Various structures are identified as follows: (S) somites, (NT) neural tube, (MG) midgut and (HG) hindgut. Note that in panel K Tcf4−/−/Tcf1−/− embryos maintain somites but the primitive gut tube has not closed. Similar sections in Lef−/−/Tcf1−/− embryos show a properly formed gut tube and the absence of somites (panel L). The caudal sections (M–O) also reveal the presence of ectopic neural tissue in both Tcf4−/−/Tcf1−/− and Lef−/−/Tcf1−/− embryos.
Anterior transformation in the gastro-intestinal tract of Tcf4−/−/Tcf1−/− embryos
Given the specific defects in the formation of caudal endoderm in Tcf4−/−/Tcf1−/− embryos, we were also interested in examining the later patterning events of the gastro-intestinal tract. As expected, serial transverse sections of E14.5 embryos showed that the intestine of Tcf4−/−/Tcf1−/− mutants was severely truncated and ended blindly (Supplementary Figure 1, bottom panels m–o). A closer inspection of mutant embryos revealed profound patterning defects in the stomach/duodenum region. Normally, the stomach is restricted by the oesophageal junction at the proximal end and by the duodenal junction at the distal end (Supplementary Figure 1, top panels a–e). While the boundary between the oesophagus and stomach was normal in Tcf4−/−/Tcf1−/− embryos, the duodenal opening was not clearly defined. In fact, serial sections suggested that the duodenum was dilated, giving the impression that the stomach had duplicated at the distal end (Supplementary Figure 1, bottom panels). To better visualize this, we dissected out the stomach and intestine from E14.5 embryos in order to verify the expression of the epithelial stomach marker Sox2 (Ishii et al, 1998). In Tcf4+/−/Tcf1−/− littermates, Sox2 expression was restricted to the stomach, with no transcripts detected beyond the duodenal boundary. In Tcf4−/−/Tcf1−/− embryos, Sox2 expression was present throughout the stomach and truncated intestinal tract. These results implied that the Tcf4−/−/Tcf1−/− gastro-intestinal tract was indeed anteriorized. To confirm this, we performed in situ hybridizations on consecutive sections of normal and mutant stomach/duodenum preparations in order to verify the expression of the intestinal marker Cdx2, alongside Sox2. As depicted in Figure 7, Sox2 and Cdx2 are confined to the stomach and duodenum, respectively, in normal littermates (see figure legends for details). In mutant embryos, comparable sections showed that the duodenum only partially expressed Cdx2. Moreover, regions of the duodenum devoid of Cdx2 (see black arrowheads, comparing top and bottom sets of panels i–vi) abundantly expressed Sox2. Thus, the duodenum was apparently transformed into the stomach, effectively leaving Tcf4−/−/Tcf1−/− embryos with little or no intestine.
Figure 7.
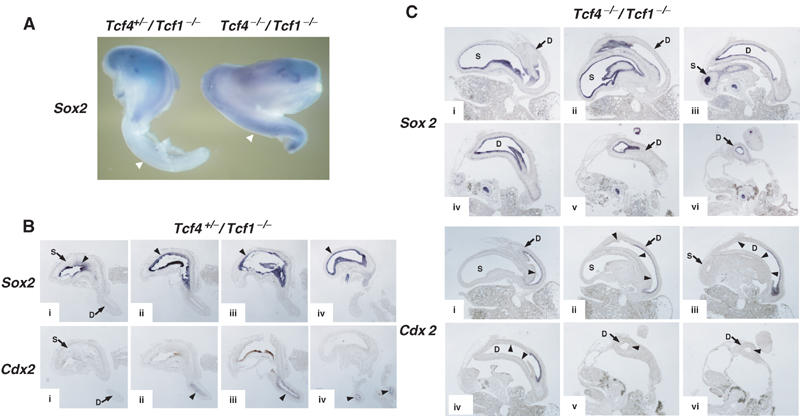
Anteriorization of the gastro-intestinal tract in Tcf4−/−/Tcf1−/− embryos. (A) Whole-mount in situ hybridizations of dissected anterior gastro-intestinal tracts of E14.5 Tcf4+/−/Tcf1−/− (left) and Tcf4−/−/Tcf1−/− (right) embryos using a stomach marker, Sox2. The gastro-intestinal tract of the normal embryo was severed to remove the intestinal tube. The truncated gastro-intestinal tract of Tcf4−/−/Tcf1−/− embryos is shown in its entirety. As shown by the arrowheads, Sox2 expression is confined to the stomach in normal littermates, while ectopic expression in the duodenum is apparent in mutants. (B) In situ hybridizations on consecutive sections (i–iv) of a single E13.5 Tcf4+/−/Tcf1−/− gastro-duodenal preparation. The top and bottom rows of panels were stained for Sox2 (stomach) and Cdx2 (intestine), respectively. The top and bottom sections i–iv represent equivalent regions and should be compared with each other. The arrowheads indicate regions of positive staining for both probes. (C) In situ hybridizations on consecutive sections (i–vi) of a single E13.5 Tcf4−/−/Tcf1−/− gastro-duodenal preparation. The top and bottom sets of panels were stained for Sox2 and Cdx2, respectively. Sections i–vi represent equivalent regions and should be compared with each other. Various structures are identified as follows: (S) stomach, (D) duodenum. The arrowheads in panels stained for Cdx2 represent regions of the duodenum that are devoid of Cdx2 expression but show high expression of Sox2. Note how the duodenum in mutants appears dilated compared to normal littermates (B). In addition, Cdx2 transcripts are confined to a small portion of the duodenum, and the remaining tissue expresses Sox2. Altogether, these data provide evidence for the occurrence of an anterior transformation in the gastro-intestinal tract of Tcf4−/−/Tcf1−/− embryos.
Discussion
In this report, we show that deletion of Tcf1 and Tcf4 leads to an absence of caudal structures in the mouse embryo. These embryos displayed duplications of the neural tube and, unlike other null mutants affecting Wnt signalling, Tcf4−/−/Tcf1−/− embryos retain paraxial mesoderm. By examining the expression of endodermal markers, we found an early defect in the development of the hindgut and later patterning anomalies, consistent with homeotic transformations of the gastro-intestinal tract. The specific defects in caudal endoderm and later axial truncations are reminiscent of recent experiments with chick embryo explants, where surgical removal of the caudal endoderm leads to blunted tail development (de Santa and Roberts, 2002). Likewise, the impaired gut development in Sox17−/− embryos is accompanied by disorganized posterior trunk development, which can partially be rescued in chimaera (Kanai-Azuma et al, 2002; Tam et al, 2003). Together, these findings suggest that posterior endoderm may produce signals required for proper development not only of the hindgut but also adjacent structures. Therefore, in Tcf4−/−/Tcf1−/− embryos, the basis for the severe posterior truncations may be the result of arrested gut development. In the light of this, the disorganized aspect of the paraxial mesoderm in some embryos may be attributed to the hindgut defects. Further analysis will be required to test this hypothesis.
Tcf/Lef genes regulate distinct patterning events
The comparison between Tcf4−/−/Tcf1−/− and Lef−/−/Tcf1−/− embryos has allowed us to uncover distinct roles for Tcf/Lef family members during A–P patterning. Previous work and our own analysis indicate that Lef and Tcf1 are necessary for early specification of paraxial mesodermal Lef−/−/Tcf1−/− embryos phenocopy null mutations in Wnt3a and also the putative target gene Tbx6 (Chapman and Papaioannou, 1998). The primary defect in these mutants appears to result from the formation of ectopic neural tissue at the expense of paraxial mesoderm. One model that has emerged to explain this phenomenon suggests that Wnt signals (namely Wnt3a-LEF/TCF1) instruct epiblast cells to adopt a mesodermal fate. When this signal is disrupted, involuting cells from the primitive streak fail to differentiate into the mesoderm and, possibly by default, adopt a neuronal differentiation programme, ultimately contributing to the formation of ectopic neural tissue where normally somites are formed.
In Tcf4−/−/Tcf1−/− embryos, expression of posterior paraxial mesoderm markers is maintained, suggesting that Tcf4 is not required for specifying this tissue type. Rather, Tcf4 in concert with Tcf1 is necessary for the development of caudal endoderm. By analogy to the above model, our results could also imply that during germ layer formation Tcf4 and Tcf1 regulate the formation and/or expansion of caudal endoderm at the expense of neural tissue. Thus, the neural tube duplications in these embryos may result from unspecified epiblast cells forming ectopic neural tubes, as described in Wnt3a−/− and Lef−/−/Tcf1−/− mutants. Alternatively, the duplications in Tcf4−/−/Tcf1−/− embryos may reflect later intrinsic patterning defects caused by loss of Tcf4 and Tcf1 expression in the neural tube.
Wnt signals, therefore, pattern specific caudal structures through the differential usage or tissue distribution of Tcf4, Tcf1 and Lef. The absence of any defect in either paraxial mesoderm or caudal endoderm development in the single knockouts, however, argues that TCF/LEF factors are functionally redundant at the biochemical level. In other words, LEF/TCF1 in the paraxial mesoderm and TCF4/TCF1 in the caudal endoderm most likely regulate overlapping sets of target genes. However, these results do not exclude the possibility that within other cell types, coexpressed TCF/LEF factors control unique genetic programmes. In favour of this notion, promoter studies have demonstrated that unique domains present within individual TCF/LEF proteins allow for the regulation of specific target genes (Atcha et al, 2003; Hecht and Stemmler, 2003). Moreover, forced expression of mutant version of Tcf3 and Lef in different model systems, such as Xenopus embryos and skin stem cells, results in distinct developmental outcomes (Merrill et al, 2001; Roel et al, 2002). Thus, it appears that the exact contribution of individual TCF/LEF factors in response to Wnt signals depends on the cellular and developmental context.
Both early and late stages of gastro-intestinal development are controlled by Wnt signalling
The specific defects in the fetal gut development of Tcf4−/−/Tcf1−/− embryos are consistent with our previously described single Tcf4 knockout (Korinek et al, 1998a). The phenotype of these mice first becomes evident during villus formation in the small intestine at around E16.5. At this time point, proliferating epithelial cells normally detected in the inter-villus regions are lost and as a result Tcf4−/− mice die perinatally. Given the importance of Tcf4 in maintaining epithelial stem cells in the small intestine, it is intriguing to speculate that Tcf4 may play an equivalent role in the primitive gut. A recent study in the chick provides additional evidence that Tcf/Lef factors are essential for gut development (Theodosiou and Tabin, 2003). However, in this model, expression profile analysis and loss-of-function studies predict a restricted role for Tcf4 and Lef in the development of the gizzard, the duodenum and ceca.
The transformations of the gastro-intestinal tract highlight an unexpected role for Tcf4 and Tcf1 in later patterning of the gut tube. From E9.5 to E14.5, once the endodermal tube has formed, specialized structures such as lungs, stomach, small intestine, colon, etc., develop along the A–P axis. During this patterning phase, both Tcf4 and Tcf1 are expressed in foregut derivatives such as the stomach (data not shown). Tcf4 is strongly expressed in the stomach epithelium. Tcf1 expression appears much less abundant and transiently, with early expression in the stomach mesenchyme and later in the epithelium. As removal of Tcf4 and Tcf1 leads to ectopic stomach tissue, this may reflect a role for both factors in restricting growth of the stomach, while at the same time promoting the growth of more caudal endodermal structures (i.e. the midgut and hindgut).
Besides regulating the expansion and patterning of the gastro-intestinal tract, a number of other functional studies implicate canonical Wnt signalling in other aspects of gut development. For example, the initial formation of definitive endoderm is severely impaired in conditional β-catenin mutant embryos, and as a result these embryos develop ectopic cardiac tissue (Lickert et al, 2002). As a self-renewing tissue, the adult intestine also requires active Wnt signalling to maintain proliferation of epithelial progenitors. This was confirmed recently by two independent studies, which showed that forced expression of the Wnt antagonist Dkk1 blocks proliferation in the intestinal mucosa (Pinto et al, 2003; Kuhnert et al, 2004). On the other hand, several lines of evidence have shown that overstimulation of the Wnt pathway, through activating mutations in components such as β-catenin and APC, promotes tumorigenesis (Bienz and Clevers, 2000; Polakis, 2000). Altogether, therefore, these data imply that Wnt signalling is absolutely required for the formation, growth, patterning and homeostasis of the gut endoderm.
In conclusion, the results presented here establish a novel role for Wnt signalling in early gut morphogenesis and underscore the importance of the Tcf/Lef genes in driving early patterning events during mouse development. Given the abundance of Wnts (19 described genes) and the probability that many have overlapping functions, future analysis of various compound Tcf/Lef null mutants should provide valuable models to dissect the role of canonical Wnt signalling in many other developmental processes.
Materials and methods
Mice
Tcf4-, Tcf1- and Lef-deficient mice were described elsewhere (van Genderen et al, 1994; Verbeek et al, 1995; Korinek et al, 1998a). Genotyping of all embryos was performed by PCR of genomic DNA isolated from extraembryonic tissue. Detailed protocols (including primer sequences) for genotyping will be provided upon request.
Histology and in situ hybridization
Embryos were dissected in PBS, fixed overnight in 4% paraformaldehyde and kept for long-term storage in 100% methanol. For whole-mount in situ hybridization, embryos were rehydrated, digested in proteinase K, post-fixed and hybridized overnight at 70°C with various probes in 5 × SSC (pH 4.5), 50% formamide, 2% blocking powder (Roche), 5 mM EDTA, 50 μg/ml yeast tRNA, 0.1% Tween 20, 0.5% CHAPS and 50 μg/ml heparin. Embryos were washed as follows: 2 × SSC for 4 × 10 min at 70°C, 2 × SSC/0.1% CHAPS for 2 × 30 min at 70°C, 100 μg/ml RNAse A/2 × SSC/0.1% CHAPS for 30 min at 37°C and 100 mM maleic acid/150 mM NaCl for 2 × 30 min at 70°C. Embryos were washed in TBST, and blocked for 2 h in TBST containing 0.5% blocking powder and 1% sheep serum. Next, embryos were incubated in blocking solution overnight at 4°C with preabsorbed alkaline phosphatase-conjugated anti-digoxigenin (1/2000 dilution) (Roche). Embryos were washed several times in TBST and colour reaction was performed with BM Purple AP substrate (Roche). For Wnt1 stainings, embryos were dehydrated, embedded in paraffin, sectioned and counterstained with 1.0% Neutral Red. For in situ hybridization on sections, embryos were embedded in paraffin, sectioned at 10 μM and processed for hybridization as above, with the following modifications. Both CHAPS and Tween 20 were omitted from the hybridization buffer and post-hybridization washes were performed as follows: 2 × SSC for 5 min, 2 × SSC/50% formamide for 3 × 30 min at 65°C. Sections for histology studies were stained with haematoxylin and eosin.
Probes
The following probes were used for in situ hybridization studies: Pax1 (IMAGE clone #1327502), T (Stott et al, 1993), Wnt5a (Gavin et al, 1990), Cdx1 (Meyer and Gruss, 1993), Lef (Oosterwegel et al, 1993), Foxa1 (Kaestner et al, 1994), Sox17 (Kanai et al, 1996), Sox2 (IMAGE clone #5707193), Tcf1 (IMAGE clone #4016305) and Tcf4 (IMAGE clone #4952976).
Supplementary Material
Supplemental material
Acknowledgments
We thank Jacqueline Deschamps and Olivier Destrée as well as all the members of the laboratory for valuable discussions and critically reading the manuscript. This work was supported by the Netherlands Organization for Scientific Research (NWO).
References
- Atcha FA, Munguia JE, Li TW, Hovanes K, Waterman ML (2003) A new beta-catenin-dependent activation domain in T cell factor. J Biol Chem 278: 16169–16175 [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103: 311–320 [DOI] [PubMed] [Google Scholar]
- Chapman DL, Papaioannou VE (1998) Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391: 695–697 [DOI] [PubMed] [Google Scholar]
- de Santa BP, Roberts DJ (2002) Tail gut endoderm and gut/genitourinary/tail development: a new tissue-specific role for Hoxa13. Development 129: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R (1999) Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev 13: 709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Hsu SC, Grosschedl R (2001) Rescue of a Wnt mutation by an activated form of LEF-1: regulation of maintenance but not initiation of Brachyury expression. Proc Natl Acad Sci USA 98: 8668–8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Grosschedl R (2000) Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 127: 469–482 [DOI] [PubMed] [Google Scholar]
- Gavin BJ, McMahon JA, McMahon AP (1990) Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev 4: 2319–2332 [DOI] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA (1996) Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev 10: 313–324 [DOI] [PubMed] [Google Scholar]
- He X (2003) A wnt–wnt situation. Dev Cell 4: 791–797 [DOI] [PubMed] [Google Scholar]
- Hecht A, Stemmler MP (2003) Identification of a promoter-specific transcriptional activation domain at the C terminus of the Wnt effector protein T-cell factor 4. J Biol Chem 278: 3776–3785 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W (2000) Requirement for beta-catenin in anterior–posterior axis formation in mice. J Cell Biol 148: 567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlstone A, Clevers H (2002) T-cell factors: turn-ons and turn-offs. EMBO J 21: 2303–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Takada S (2001) Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev 103: 27–33 [DOI] [PubMed] [Google Scholar]
- Ishii Y, Rex M, Scotting PJ, Yasugi S (1998) Region-specific expression of chicken Sox2 in the developing gut and lung epithelium: regulation by epithelial–mesenchymal interactions. Dev Dyn 213: 464–475 [DOI] [PubMed] [Google Scholar]
- Ishikawa TO, Tamai Y, Li Q, Oshima M, Taketo MM (2003) Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev Biol 253: 230–246 [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Hiemisch H, Luckow B, Schutz G (1994) The HNF-3 gene family of transcription factors in mice: gene structure, cDNA sequence, and mRNA distribution. Genomics 20: 377–385 [DOI] [PubMed] [Google Scholar]
- Kanai Y, Kanai-Azuma M, Noce T, Saido TC, Shiroishi T, Hayashi Y, Yazaki K (1996) Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J Cell Biol 133: 667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y (2002) Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129: 2367–2379 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998a) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Gene 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H (1998b) Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol 18: 1248–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101: 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Domon C, Huls G, Wehrle C, Duluc I, Clevers H, Meyer BI, Freund JN, Kemler R (2000) Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development 127: 3805–3813 [DOI] [PubMed] [Google Scholar]
- Lickert H, Kutsche S, Kanzler B, Tamai Y, Taketo MM, Kemler R (2002) Formation of multiple hearts in mice following deletion of β-catenin in embryonic endoderm. Dev Cell 3: 171–181 [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A (1999) Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22: 361–365 [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A (1990) The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62: 1073–1085 [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT (1989) Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 58: 1075–1084 [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E (2001) Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 15: 1688–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E (2004) Tcf3: a transcriptional regulator of axis induction in the early embryo. Development 131: 263–274 [DOI] [PubMed] [Google Scholar]
- Meyer BI, Gruss P (1993) Mouse Cdx-1 expression during gastrulation. Development 117: 191–203 [DOI] [PubMed] [Google Scholar]
- Mlodzik M (2002) Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet 18: 564–571 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1: 423–434 [DOI] [PubMed] [Google Scholar]
- Nusse R (1999) WNT targets. Repression and activation. Trends Genet 15: 1–3 [DOI] [PubMed] [Google Scholar]
- Oosterwegel M, van de WM, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H (1993) Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development 118: 439–448 [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407: 535–538 [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [PubMed] [Google Scholar]
- Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, Lohnes D (2001) Multiple pathways governing Cdx1 expression during murine development. Dev Biol 239: 257–269 [DOI] [PubMed] [Google Scholar]
- Roberts DJ (2000) Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn 219: 109–120 [DOI] [PubMed] [Google Scholar]
- Roel G, Hamilton FS, Gent Y, Bain AA, Destree O, Hoppler S (2002) Lef-1 and Tcf-3 transcription factors mediate tissue-specific Wnt signaling during Xenopus development. Curr Biol 12: 1941–1945 [DOI] [PubMed] [Google Scholar]
- Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Clevers H (1999) Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285: 1923–1926 [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA (1991) Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67: 741–752 [DOI] [PubMed] [Google Scholar]
- Stott D, Kispert A, Herrmann BG (1993) Rescue of the tail defect of Brachyury mice. Genes Dev 7: 197–203 [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP (1994) Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev 8: 174–189 [DOI] [PubMed] [Google Scholar]
- Theodosiou NA, Tabin CJ (2003) Wnt signaling during development of the gastrointestinal tract. Dev Biol 259: 258–271 [DOI] [PubMed] [Google Scholar]
- Tam PP, Kanai-Azuma M, Kanai Y (2003) Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev 13: 393–400 [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R (1994) Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 8: 2691–2703 [DOI] [PubMed] [Google Scholar]
- Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H (1995) An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 374: 70–74 [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA (1999) Vertebrate endoderm development. Annu Rev Cell Dev Biol 15: 393–410 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14: 59–88 [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP (2001) Heads or tails: Wnts and anterior–posterior patterning. Curr Biol 11: R713–R724 [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999a) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126: 1211–1223 [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP (1999b) T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev 13: 3185–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S (1997) Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol 183: 234–242 [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90: 181–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material


