Abstract
Rapid energy-efficient signaling along vertebrate axons is achieved through intricate subcellular arrangements of voltage-gated ion channels and myelination. One recently appreciated example is the tight colocalization of Kv7 potassium channels and voltage-gated sodium (Nav) channels in the axonal initial segment and nodes of Ranvier. The local biophysical properties of these Kv7 channels and the functional impact of colocalization with Nav channels remain poorly understood. Here, we quantitatively examined Kv7 channels in myelinated axons of rat neocortical pyramidal neurons using high-resolution confocal imaging and patch-clamp recording. Kv7.2 and 7.3 immunoreactivity steeply increased within the distal two-thirds of the axon initial segment and was mirrored by the conductance density estimates, which increased from ∼12 (proximal) to 150 pS μm−2 (distal). The axonal initial segment and nodal M-currents were similar in voltage dependence and kinetics, carried by Kv7.2/7.3 heterotetramers, 4% activated at the resting membrane potential and rapidly activated with single-exponential time constants (∼15 ms at 28 mV). Experiments and computational modeling showed that while somatodendritic Kv7 channels are strongly activated by the backpropagating action potential to attenuate the afterdepolarization and repetitive firing, axonal Kv7 channels are minimally recruited by the forward-propagating action potential. Instead, in nodal domains Kv7.2/7.3 channels were found to increase Nav channel availability and action potential amplitude by stabilizing the resting membrane potential. Thus, Kv7 clustering near axonal Nav channels serves specific and context-dependent roles, both restraining initiation and enhancing conduction of the action potential.
Keywords: axon, excitability, Kv7
Introduction
Axon initial segments (AISs) and nodes of Ranvier are two fundamental specializations in axons of jawed vertebrates for the initiation and conduction of action potentials. These crucial functions are mediated by clustered subcellular expression of specific voltage-gated conductances (Poliak and Peles, 2003; Rasband, 2010; Kole and Stuart, 2012). The M-current (IM) is a non-inactivating voltage-gated potassium (Kv) current characterized by relatively slow kinetics, a low voltage threshold for activation and regulation by muscarinic receptors (Brown and Adams, 1980). In many neurons, IM is carried by heteromultimeric combinations of Kv7.2 and Kv7.3 (also called KCNQ2/3) subunits (Wang et al., 1998). Kv7.2 and Kv7.3 are encoded by paralogous vertebrate genes that share a unique domain for binding to ankyrin G and mediating their coclustering with voltage-gated sodium (Nav) channels to the AIS and nodes of Ranvier (Pan et al., 2006; Cooper, 2011). Loss-of-function mutations in Kv7.2 and Kv7.3 have been found to underlie a spectrum of neurological diseases including neonatal-onset epilepsy, myokymia, and epileptic encephalopathy (Biervert et al., 1998; Jentsch, 2000; Maljevic et al., 2008; Weckhuysen et al., 2012; Kato et al., 2013), highlighting the importance of their expression in these privileged excitable domains. Although inferences of axonal Kv7 channel function have been drawn from somatic and peripheral nerve recordings, the properties and function of Kv7.2/7.3 channels intrinsic to axonal domains of central neurons have remained elusive.
Past research revealed two contrasting roles of axonal Kv7 conductance. In hippocampal CA1 pyramidal neurons, competitive inhibition of Kv7.2/7.3 binding to ankyrin G, or bath application of XE-991, a highly selective Kv7 blocker (Wang et al., 1998), led to spontaneous action potentials and hyperpolarized the voltage threshold for action potential generation, suggesting an attenuating role of the AIS Kv7 channel in excitability (Shah et al., 2008). In contrast, the application of XE-991 reduced both glutamate release and presynaptic fiber volley amplitude at Schaffer collateral synapses, suggesting that the presence of IM in axons may facilitate axonal excitability (Vervaeke et al., 2006). IM-like currents (called IKs currents) have been isolated in recordings from single rat sciatic nerve nodes of Ranvier (Röper and Schwarz, 1989; Safronov et al., 1993; Schwarz et al., 2006) and from the calyx of Held presynaptic terminal (Huang and Trussell, 2008). These studies revealed a large (20–50 mV) hyperpolarizing shift in the voltage dependence of activation, compared with the somatic IM (Safronov et al., 1993; Huang and Trussell, 2011), raising the possibility that perisomatic and more distal axonal Kv7 channels differ greatly.
Here, using whole-cell, cell-attached, and outside-out voltage-clamp recordings of IM combined with high-resolution imaging of Kv7.2/7.3 immunofluorescence, we determined that axonal IM is mediated by Kv7.2/7.3 heterotetramers and exhibits pharmacological and biophysical properties similar to the somatic IM. Using simultaneous recordings from soma and axons combined with conductance-based computational modeling, we found that unlike the perisomatic region, where Kv7 channel activation counteracts the persistent Nav current and restrains repetitive firing, in the node of Ranvier Kv7 channels set the resting membrane conductance and augment the amplitude of the propagating presynaptic action potential.
Materials and Methods
Animals.
All experiments were performed according to guidelines approved by the local animal ethics committees of the Royal Netherlands Academy of Arts and Sciences, Baylor College of Medicine, and the Australian National University. Adult male Wistar rats [postnatal day 28 (P28) to P91; Harlan Laboratories] were deeply anesthetized by 3% isoflurane (v/v) inhalation and subsequently decapitated; brain slices were then prepared as described previously (Kole et al., 2007).
Electrophysiological recordings.
Individual slices were transferred to the stage of a Zeiss Axioskop or Olympus BX51WI microscope. The microscope bath was perfused with normal ACSF consisting of the following (in mm): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 25 glucose, 2 CaCl2, and 1 MgCl2 saturated with 95% O2/5% CO2, pH 7.4. Current-clamp and voltage-clamp recordings from dendrites, soma, and cut axons were all made between 1 and 10 h after slice preparation. Dual whole-cell current-clamp recordings were made using Dagan BVC-700A amplifiers (Dagan). Patch pipettes were pulled from borosilicate glass (outer diameter, 1.5 mm; inner diameter, 0.86 mm) and had an open tip resistance of 5–7 MΩ, filled with the following standard intracellular solution (in mm): 130 K+-gluconate, 10 KCl, 4 Mg2+-ATP, 0.3 Na+-GTP, 10 HEPES, and 10 Na2-phosphocreatine, pH 7.3 with KOH (∼280 mOsm kg−1). Biocytin (5 mg/ml) and Alexa Fluor 488/594 (200 μm; Life Technologies) were routinely added to the intracellular solution. Axonal recording distances were taken as linear estimations from the live bright-field or fluorescence images, or from the axonal path distances of reconstructed neurons filled with biocytin (Kole et al., 2007). Voltage was analog low-pass filtered at 10 kHz (Bessel filter) and digitally sampled at 50 or 100 kHz using an AD/DA converter (ITC-18, HEKA Elektronik GmbH) and using the data acquisition software Axograph X (AxoGraph Scientific). The access resistance during current-clamp experiments ranged between 8 and 20 MΩ for somatic and axonal recordings, up to 30 MΩ for dendritic recordings, and was fully compensated using bridge balance and capacitance neutralization of the amplifiers.
For whole-cell voltage-clamp recordings of IM, the extracellular ACSF was supplemented with 5 mm 4-aminopyridine (4-AP), 1 μm tetrodotoxin (TTX), and 20 μm 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD-7288) to block the fast-activating Kv1, Nav, and hyperpolarization-activated cyclic nucleotide (HCN)-gated currents, respectively. Preliminary experiments showed that the addition of cadmium (Cd2+), often used to block Ca2+ currents at more depolarized potentials, inhibited IM amplitudes and slowed the rise time (see also Robbins et al., 1992), and was therefore omitted from the routine voltage-clamp solution. For cell-attached recordings of K+ currents, we used patch-pipettes (∼10 MΩ) filled with the following (in mm): 125 NaCl, 2.5 KCl, 20 HEPES, 20 glucose, 2 MgCl2, 1 μm TTX, and 5 mm 4-AP, pH 7.3 with NaOH (285 mOsm). CaCl2 was omitted to increase the probability of M-channel opening (Selyanko and Brown, 1999; Selyanko et al., 2001). Voltage-clamp recordings were made with an Axopatch 200B or Multiclamp 700A amplifier (Molecular Devices), and currents were low-pass filtered at 4–10 kHz (eight-pole Bessel filter) with 10–50 kHz sampling frequencies. To subtract the capacitive and leak currents, the membrane patch or axon bleb was held at −92 mV and a P/−6 leak subtraction protocol was applied on-line. In voltage-clamp configuration, the series resistance (Rs) was ∼14 MΩ. Recordings with ∼90% Rs compensation indicated that the current rise times and/or amplitudes were not different from those of uncompensated recordings (p = 0.41 and p = 0.27, respectively; n = 4), which is consistent with the low peak amplitudes of the currents (∼0.5 nA) and their slow kinetics (>10 ms). Rs compensation was therefore not applied. Voltage values for whole-cell recordings were corrected for the calculated liquid junction potential (LJP) of −12 mV of the K+-gluconate intracellular solution. Direct recording of the LJP revealed a nearly similar value of −13.5 ± 0.2 mV (n = 3). All recordings were made at 35 ± 1°C, unless otherwise indicated.
All salt compounds, biocytin, 4-AP, and tetraethylammonium (TEA) were obtained from Sigma-Aldrich. Other drugs, including 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride (XE-991), ZD-7288, [R-(R*,S*)]-6-(5,6,7,8-tetrahydro-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)furo[3, 4-e]-1,3-benzodioxol-8(6H)-one, 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (gabazine), 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μm), 2-amino-6-trifluoromethoxybenzothia-zole hydrochloride (riluzole), and TTX were obtained from Tocris Bioscience. These were dissolved in distilled water, and aliquots were stored at −20°C. Retigabine (a gift from Sigrid Blom, Lundbeck, Valby, Denmark) was dissolved to 100 mm in dimethylsulfoxide and stocks stored at −20°C. Local applications of XE-991 were made by diluting XE-991 in HEPES-buffered ACSF or in normal extracellular ACSF. Puffing solutions were loaded in a patch pipette (∼5 MΩ resistance) connected to a Picospritzer III system (Intracel). By adjusting the pressure (∼1 bar), we aimed for a localized response of ∼50 μm radius as determined from the bright-field image of the slice (Kole et al., 2007).
Analysis.
We determined input resistance by the slope of linear fits to small (<5 mV) voltage responses evoked by positive and negative current injections. To analyze the steady-state voltage dependence of IM, the current amplitudes were converted to conductance values (G) for each step potential (V), normalized to the maximum conductance (Gmax), and fitted with a Boltzmann equation of the form; , in which Vhalf is the half-maximum activation voltage, z the effective valence. The thermodynamic gas constant (R), temperature (T), and Faraday's constant (F) were implemented as a single constant of 26.55 mV for recordings at 35°C.
The sensitivity of the axonal currents to XE-991 and TEA inhibition was analyzed by averaging the raw steady-state amplitudes for voltage steps between 10 and 40 mV, and normalizing the current amplitudes in the presence of the drug to the control amplitudes. Data were averaged over multiple cells and fit with a Hill equation of the following form:
 |
in which yo is the unblocked current amplitude, ymax is the maximally blocked current amplitude, x is the concentration, xhalf is the IC50, and n is the power of the drug block, equivalent to the Hill slope. Fitting was performed in Igor Pro (version 5.05, WaveMetrics).
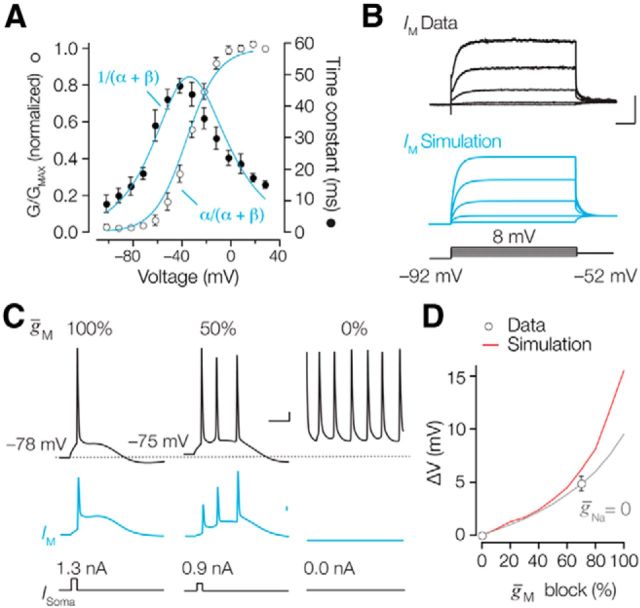
The kinetic description of the axonal M-current followed the classic formalism of Hodgkin and Huxley (1952). Since IM does not inactivate the relationship between the channel conductance and the current, it can be defined as follows: IM = ḡMn(V − EK). In this equation, ḡM is the maximum conductance, n represents an activation gate, EK is the potassium reversal potential, and V is the membrane potential. n follows the differential equation (Zagotta et al., 1994) = α(1 − n) − βn with the forward rate defined by and the backward rate with , where Ca and Cb are the rate constants at 0 mV, and za and zb are the charge movements. Ca, Cb, za, and zb were obtained by fitting the steady-state curve, , and time constants, , simultaneously and weighing with the SEs using the Global Fitting routine in Igor Pro. The fit was optimized with the following parameter values: Ca = 0.036 ms−1, Cb = 0.002 ms−1, za = 0.909 mV, and zb = 1.102 mV (see Fig. 9A). Calculations of conductance densities for outside-out and cell-attached patch-clamp recordings were based on published data of surface area measurements and were 4 μm2 (Schmidt-Hieber and Bischofberger, 2010) and 2 μm2 (Sakmann and Neher, 2009), respectively.
Figure 9.
Simulation of Im in the NEURON model. A, Steady-state activation (open circles) and time constants (closed circles) of axonal Im fit with a Hodgkin-Huxley model (blue). B, Top, average of five experimental whole-axon recordings. Middle, Simulated Kv7 currents activated during a single-electrode voltage-clamp simulation (SEClamp) in NEURON (blue). Bottom, Voltage command protocol for simulation and experiments. Calibration: 100 ms, 100 pA. C, Reducing the Kv7 peak conductance density by 50% (middle) and 100% (right) in the model replicates the experimental XE-991 block (compare Figs. 6 and 7). Blue traces represent the activated Im for the different conditions. Calibration: 10 mV, 10 ms (top); 1 mA/cm2 (middle). D, Summary data showing the effect of reducing ḡM on the simulated resting membrane potential in control conditions (red line) and without sodium conductance (gray line) overlaid with experimentally observed resting potential change (circles). Symbols represent the mean ± SEM.
Compartmental modeling.
Conductance-based multicompartmental simulations used the geometric morphology of an in vitro biocytin-filled thick-tufted L5 pyramidal neuron (cell number 30_08_12_1) three-dimensionally reconstructed with Neurolucida (version 10, MicroBrightField Europe E.K.) and imported into the NEURON simulation environment (version 7.3; Hines and Carnevale, 2001). Since nodes of Ranvier are only barely visible in bright-field microscopy, their approximate positions were also estimated by, for example, the branching of axon collaterals. The dimensions of the nodal compartments, six in total, were assigned nominally and progressively decreased in length (3.0–1.5 μm) with distance from the soma. Throughout all compartments, the membrane resistance (Rm) was set to 25,000 Ω cm2, cytoplasmic resistance (Ri) to 140 Ω cm, and membrane capacitance (Cm) to 1.2 μF cm−2. The resting membrane potential was set to −78 mV. Myelination of internodal sections was represented by increasing internodal Rm fourfold and decreasing Cm sixfold, leading to a conduction velocity in the primary axons of 3.53 m s−1, in accordance with previous estimates (Kole et al., 2007). The axonal cut ending (“bleb”) was connected to the last internode as a single unmyelinated compartment with a diameter of 5 μm.
Kv7 peak conductance (ḡM) was distributed based on the experimental findings (see Fig. 5), and was set to 15 pS μm−2 in all somatodendritic sections and increased linearly from 15 to 150 pS μm−2 from the middle to the end of the AIS. To estimate ḡM in nodal domains, we simulated whole-cell axonal Kv7 currents activated during a single-electrode voltage-clamp simulation, using the point-process manager (SEClamp) of NEURON at the cut-end compartment. The Rs was set to 15.0 MΩ to match experimental conditions and simulations were run with time steps of 200 μs. Capacitive and leak currents were removed by subtracting the transients with current responses when ḡM was set to zero. The axonal whole-cell current amplitudes (see Fig. 9B) will depend both on ḡM as well as the electrode-to-node distance. To test the contribution of both parameters, we modeled the current amplitudes and varied the specific nodal ḡM density (1–300 pS μm−2) and the distance of the cut-end to last node (0.1–140 μm). Assuming that internodes are ∼150 μm in length in the layer 6 region, the electrode-to-node distance will be on average ∼75 μm. At this distance, a nodal peak conductance density of 150 pS μm−2 led to a whole-axon peak amplitude of 475 pA (28 mV step), similar to the experimentally obtained average peak current (478 ± 58 pA, n = 13). This computational estimate is in good agreement with the measured peripheral nodal IK density of 18.8 nS pF−1 (Röper and Schwarz, 1989). In the model, ḡM was therefore set to 150 pS μm−2 in nodes of Ranvier, 15 pS μm−2 in axon collaterals, and 1 pS μm−2 in the internodes.
Figure 5.
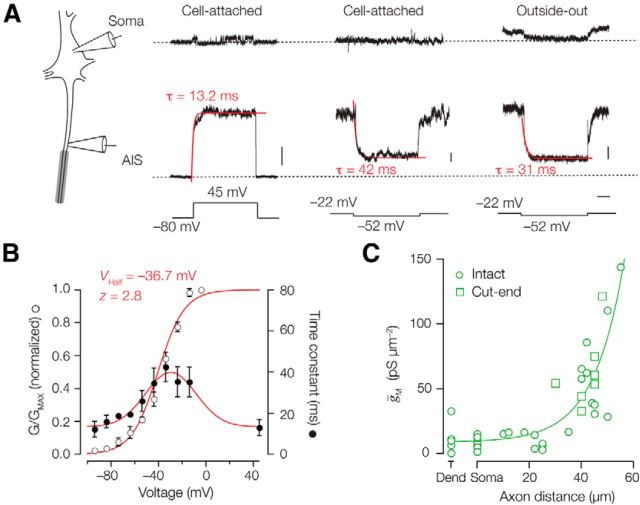
Voltage dependence, kinetics, and current density of IM in the AIS. A, Left, Schematic drawing indicating the different recording positions at the L5 soma and AIS. Right, Putative M-currents obtained in cell-attached and outside-out recordings from the soma (top) and axon initial segment (middle). Voltage-clamp step protocols are indicated at the bottom. Traces represent the average of >5 trials for each single patch. Currents obtained at the AIS were fit with single exponential functions (red). Calibrations: 5, 1, and 5 pA, respectively; 100 ms. B, For three outside-out patch recordings, voltage steps were obtained for a large voltage range between −92 and −2 mV. The 45 mV data were obtained from the cell-attached recordings. Time constants (closed circles) fitted to a Gaussian function (y = 13.4 + 26.3 e [−((x − 29.7)/30.3)2]). Steady-state voltage dependence of activation (open circles) fit with a single-power Boltzmann function with the indicated parameters. Symbols represent the mean ± SEM. C, Subcellular conductance density distribution along the AIS and somatodendritic membrane. Recordings from intact AIS (open circles) and cut-end AIS (open squares) were combined and fit with an exponential function, y = 8.7 + 1.1 e−0.086 x. Dend, Dendrite.
Nav conductance was represented by two separate eight-state allosteric models developed for the soma and the axon (Schmidt-Hieber and Bischofberger, 2010) and distributed in density as described previously (Hallermann et al., 2012). In addition, the AIS sodium channel model was separately implemented by linearly shifting (Vshift) the voltage dependence of inactivation of the axon model to account for the experimentally determined values from direct cell-attached recordings at the AIS (Kole et al., 2008). The Vhalf values of steady-state inactivation were −55 mV (soma), −61 mV (AIS), and −75 mV (axon). For steady-state activation, the Vhalf values were −22 mV (soma), −40 mV (AIS), and −44 mV (axon). With these parameter values, most properties of single action potentials including amplitude, threshold, and rate of rise were well captured (see Fig. 10A,D). K+ conductances were described by the following three Kv channel models: a high-voltage threshold delayed type of non-inactivating model with properties similar to the Kv2 channel (Mainen and Sejnowski, 1996); a recently developed axonal Kv1 channel model (Hallermann et al., 2012), and the new Kv7 model (see above). In addition, Ca2+-dependent K+ channels, and low-voltage and high-voltage Ca2+ channels were assigned to the somatic and dendritic sections with densities as published previously (Mainen and Sejnowski, 1996; Hallermann et al., 2012). The HCN channel model was exponentially increasing in the apical dendrites (Kole et al., 2006) and with a low uniform density in axons (1 pS μm−2). The K+ and Na+ equilibrium potentials were set to −98 and 55 mV, respectively. The final input resistance of the model neuron was 22.4 MΩ. Simulations were performed with a nominal temperature of 35°C and time steps between 5 and 10 μs.
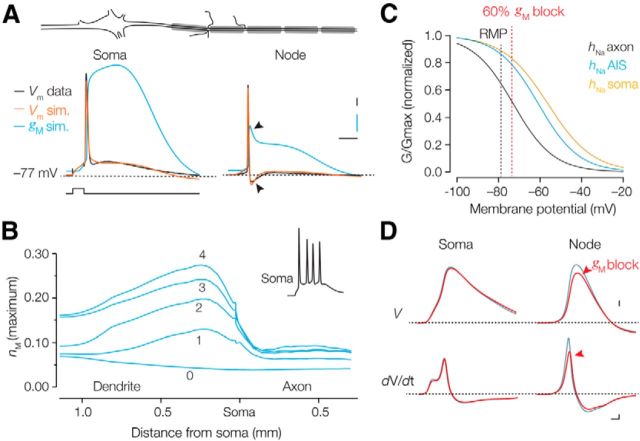
Figure 10.
Voltage and site dependence of Kv7 activation and sodium channel inactivation. A, Top, Schematic of the neuron model. Bottom, Voltage traces of the soma and a node (620 μm distance from the soma). Recorded (black) and simulated (orange) action potential waveforms at soma and node overlaid with the corresponding time course of gM (blue). Arrows at the axonal traces indicate the strong afterhyperpolarization and reduction of gM due to Kv1 activation. Calibration: 5 ms, 10 mV, 1% (blue). B, Space plot of the maximum gM activation along the dendrosomatic–axonal axis during the resting membrane potential (0) and during action potentials (1–4). Inset, Voltage–time plot of the action potentials (170 Hz) in the soma. gM has the highest degree of activation in the proximal dendrite, but the least in the axon. C, Steady-state sodium inactivation curves used for soma (black), AIS (blue), and axon (orange). Dotted vertical lines indicate the resting membrane potential (black) and 70% block of gM (red). D, Simulated somatic and nodal action potentials in control (gray) and during 70% block of gM (red). Note the selective reduction in action potential rate of rise and amplitude at the node. Calibration: 10 mV (top); 100 V/s, 100 μs (bottom).
Immunostaining.
Two alternative strategies for immunostaining were developed, both relying on weak fixation, since even brief 4% paraformaldehyde (PFA) fixation abolished all Kv7 AIS and nodal labeling (Pan et al., 2006). In one protocol (Fig. 1), 300-μm-thick slices were immersion fixed (1% PFA in 0.1 m PBS, pH 7.4, for 30 min), washed several times in 0.1 m PBS, and stored at 4°C for up to 2 weeks before use in immunostaining. Before immunoreactions, antigen retrieval was performed by microwave irradiation in 10 mm citrate, 1 mm EDTA, and 0.05% Tween 20, pH 9.0, in mini-chambers (Shah et al., 2008). Slices were then washed in Tris-buffered saline (TBS), blocked (TBS, 2% Carnation non-fat dry milk, and 0.2% Triton X-100) for 1 h at room temperature, then incubated for 48 h in blocking buffer containing various combinations of the following primary antibodies: rabbit-α-KCNQ2n; guinea pig-α-KCNQ3n; mouse-α-ankyrin G IgG2a; clone 106/36 [from the University of Calfornia, Davis/National Institutes of Health NeuroMab Facility, Davis, CA (UC Davis/Neuromab)]; mouse-α-PanNav IgG1 (Sigma-Aldrich); mouse-α-NeuN IgG1 (Millipore Bioscience Research Reagents); and mouse α-Caspr/paranodin/neurexin IV IgG1 (UC Davis/Neuromab) at 4°C. After extensive washes, the following slices were incubated in secondary antibodies overnight at 4°C: donkey-α-rabbit DyLight 488 (Jackson ImmunoResearch); goat-α-mouse Alexa Fluor 555 IgG2a γ2a (Life Technologies); goat-α-mouse Alexa Fluor 647 IgG1 γ1 (Life Technologies); and donkey-α-guinea pig Cy5 (Jackson ImmunoResearch; Manning et al., 2012). After washing, slices were immersed in TBS containing DAPI (Life Technologies) for 20 min before mounting in Prolong Gold (Life Technologies) in imaging chambers made with plastic spacers (Grace Bio-Labs) and two coverslips (catalog #474030, Zeiss).
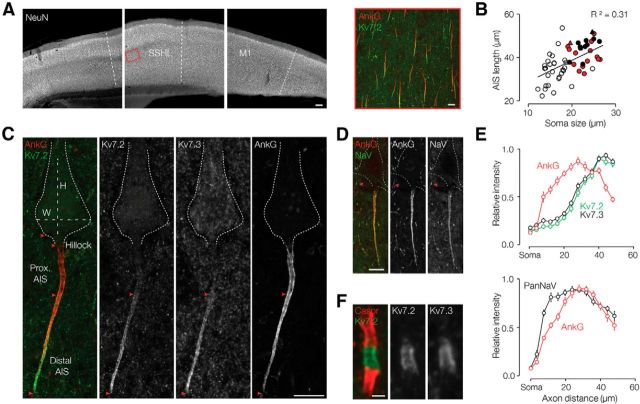
Figure 1.
Kv7 and Nav channels are expressed in nonidentical gradients along neocortical pyramidal neuron AISs and colocalize at nodes of Ranvier. A, Left, At low magnification, NeuN labeling reveals the laminar neocortical structure in a parasagittal brain slice. Somatosensory hindlimb (SSHL) and primary motor (M1) areas are indicated. Scale bar, 100 μm. Right, The L5 somatosensory region (red box, left) is shown at higher magnification, immunolabeled for ankyrin G (AnkG; red) and Kv7.2 (green). Scale bar, 10 μm. B, AIS length is positively correlated with soma size [(height + width)/2]. Least-squares fit shows a positive correlation (R2 = 0.31; fit equation: LAIS = 0.98 (soma size) + 19.082). Red triangle, neuron shown in C; black triangle, neuron shown in D; red circles, set of larger neurons used to plot Kv7.2 and Kv7.3 labeling intensity vs axon distance (shown in E, top); closed circles, larger neurons used to plot PanNav labeling intensity vs axon distance (E, bottom); open circles, smaller neurons that were not included in length measurements. C, Maximal projection image of a large L5 somatosensory neuron, colabeled for Kv7.2, Kv7.3, and ankyrin G. The somatic height (H) and width (W), origin and end of the hillock, proximal AIS, and distal AIS are indicated. Both Kv7 channel subunits are restricted to the distal AIS, wherein intensity increases in a gradient toward the tip. Scale bar, 10 μm. D, Maximal projection image of a large L5 somatosensory neuron, colabeled for ankyrin G and PanNav. The AIS pattern of PanNav (detecting all neuronal Nav channel isoforms) and ankyrin G appear similar. Arrowheads indicate one of many nodes of Ranvier visible in the image (PanNav labeling the unmyelinated node, ankyrin G labeling the node and flanking paranodes). Scale bar, 10 μm. E, Plots of AIS relative labeling intensity vs distance from the axonal origin. Top, Kv7.2 and Kv7.3 labeling profiles are similar: low in the proximal one-third and increasing progressively toward the distal AIS tip (n = 15). Bottom, Ankyrin G and PanNav label the entire AIS, with a broad intensity peak near the midpoint (n = 10). Points show the mean ± SEM of pixel intensity in fractional distance bins along the AIS. F, High-magnification image of a larger node of Ranvier within the L5 somatosensory cortex. Kv7.2 and Kv7.3 colabel the nodal membrane flanked by Caspr at the paranodes. Scale bar, 1 μm.
An alternative protocol was used to detect Kv7.2 and Kv7.3 staining in electrophysiologically characterized cells (Fig. 2). L5 neurons were filled with Alexa Fluor 488 during whole-cell recordings. The 300-μm-thick slices were immersed in precooled methanol for 10 min at −20°C, washed with PBS, and blocked for 2–24 h at room temperature with PBS, 2% non-fat milk, and 0.2%Triton X-100. KCNQ2n and KCNQ3n primary antibodies were added, and the slices were incubated at room temperature overnight. Slices were then washed with PBS and incubated at room temperature with appropriate secondary antibodies conjugated with goat-α-guinea pig Cy3 (Jackson ImmunoResearch) or goat-α-rabbit Cy5 (Jackson ImmunoResearch) for 2 h. Afterward, slices were washed in PBS and mounted in Prolong Gold with DAPI.
Figure 2.
Kv7.2 and Kv7.3 colocalize at AISs and nodes of Ranvier of functionally identified L5 pyramidal neurons. A, Top, z-projected confocal images of the soma and AIS morphology (red, Alexa Fluor 488 fill) and immunofluorescence staining of Kv7.2 (cyan) and Kv7.3 (green) in RS and IB neurons. White arrows indicate the region of the Kv7 immunostaining. Images are background subtracted and pseudo-colored. Scale bars, 10 μm. Bottom, Nodes of Ranvier (NoR) indicated by axonal bifurcation points of identified RS and IB neurons. White arrows indicate positive staining for Kv7.2/7.3 at the NoR. Scale bars, 3 μm. B, Example of a regular firing neuron that was converted into intrinsic burst firing during bath application of XE-991 (red). Calibration: 0.1 s, 20 mV. C, Scatter and bar plots of RS and IB groups show a similar increase in high-frequency action potentials after application of 20 μm XE-991. Scatter plot, Individual experiments. Bars represent mean ± SEM.
Confocal imaging and analysis.
The L5 hindlimb somatosensory cortex region was identified according to atlas coordinates (Paxinos and Watson, 2007). Images were acquired using a Nikon C2 laser scanning confocal microscope, NIS-Elements 4.0 software, and either 2× 0.1 numerical aperture (NA) Planapo or 60× 1.40 NA Planapo VC objectives (Nikon Instruments). For quantification experiments, high-resolution image stacks (z-interval, 0.25 μm; pinhole, 1.0 Airy unit), acquired within the range 10–70 μm below the slice surface, were used for analysis. Only cells whose somata, AISs, and proximal dendrites were entirely contained within the imaged volume were included in subsequent measurements of somatic and AIS size and labeling. Pyramidal cell soma size was estimated as [height (H) + width (W)]/2 (Sloper and Powell, 1979). The mean labeling intensity in the neuropil for ankyrin G, PanNav, Kv7.2, and Kv7.3 was low. Although several methods of setting black levels gave equivalent results, for the calculations shown we measured the mean neuropil intensity (manually adjusted to exclude AISs, somata, and proximal dendrites in each image) for each antibody for each image stack, and subtracted this value from all images. The 3D trajectory and labeling intensity of each proximal axon was measured as follows: using maximal projection and 3D views in NIS-Elements, we drew a 3D polyline along the axon, from within the soma to beyond the AIS. This was converted to a 2D (x and y vs z) plot using the “kymograph” tool, and labeling at each pixel along the trajectory for each fluorophore was read using the “intensity profile” tool and exported to Excel (Microsoft). To average the raw intensity profiles of axons varying in AIS length, we first determined the individual and average lengths of the axon hillocks (i.e., the proximal origin of the axon from the soma or a dendrite, lacking strong Nav and ankyrin G labeling) and AISs. Pixel intensity data from individual axon profiles were binned, and values normalized to the maximum intensity bin for that axon and fluorophore. Normalized intensities of bins representing equivalent fractional distances along the AISs were then averaged (±SEM). To determine the percentage of nodes of Ranvier bearing Kv7.2 and Kv7.3, counts were made in stacks from L5 hindlimb somatosensory cortex region sections colabeled either for ankyrin G or Caspr.
For the Alexa Fluor-filled L5 pyramidal neurons, z-stacks with a step size of 0.25–1 μm were acquired sequentially for each fluorophore using a Leica SP5 confocal microscope, a 63× 1.4 NA Leica Planapo objective, and supplier-provided software (Leica Microsystems). Maximal projection images were adjusted for brightness and contrast in FIJI (ImageJ, version 1.47a). A background value (the mean intensity within a circular region of interest lacking AISs or somata) was subtracted from each channel.
Results
Kv7.2 and Kv7.3 subunits colocalize with Nav channels at neocortical AISs and nodes of Ranvier
To enable correlation of Kv7 channel protein subcellular distribution and function, we en bloc immunostained 300-μm-thick parasagittal brain slices containing primary somatosensory and motor cortex (Fig. 1A, left) using well characterized specific antibodies against Kv7.2, Kv7.3, and Nav channel principal subunits (PanNav), along with markers of neuronal somata (NeuN), initial segments (ankyrin G), and nodes of Ranvier (Caspr/paranodin/neuroexin IV). Single-photon scanning confocal microscopy revealed a strong staining signal to a depth of at least ∼70 μm, allowing the collection of image sets containing complete views of neuronal proximal dendrites, somata, and AISs. Pyramidal cell AISs of layer 5 showed a stereotyped channel distribution: ankyrin G strongly labeled the entire AIS, whereas strong labeling for Kv7.2 was restricted to the distal part of the AIS (Fig. 1A, right).
In L5 of the somatosensory cortex, we measured pyramidal cell soma size (19.3 ± 0.6 μm), hillock length (3.5 ± 0.9 μm), and AIS length (43.68 ± 4.69 μm; all n = 55), and quantified the channel staining intensity profiles along the AIS. In contrast to earlier electron microscope studies based on a smaller number of cells (Sloper and Powell, 1979), we detected a positive correlation between AIS length and soma size (Pearson's, test p = 0.01, Fig. 1B). Although Kv7.2, Kv7.3, ankyrin G, and PanNav staining were concentrated within the AIS, high-resolution imaging showed that the two Kv7 subunits were strictly colocalized within the distal portion of each AIS, whereas Nav staining filled the entire AIS, indicating that the subcellular Nav and Kv7 profiles are distinct. Semiquantitative image analysis showed that staining for Kv7.2/7.3 followed similar profiles, scaled with AIS length, and was confined to the distal fraction of the length of ankyrin G labeling (0.7 ± 0.1, n = 10, for both Kv7.2 and Kv7.3). Kv7.2/7.3 staining peaked at the distal tip and was thus distinct from the subcellular ankyrin G distribution (Fig. 1C–E). The relative Nav intensity, however, peaked broadly near the AIS midpoint and then declined, a pattern that strongly paralleled the ankyrin G distribution (Fig. 1D,E). Nodes of Ranvier were abundant in all layers of the neocortical gray matter and appeared strongly labeled by PanNav (Fig. 1D, arrowhead) or Kv7.2/7.3 flanked by Caspr antibodies (Fig. 1F). In L5, we counted 265 nodes, of which 259 (98%) were labeled by Kv7.2 and 256 (97%) were labeled by Kv7.3, which is indicative of the expression of both subunits in nearly all nodes. Nodes of Ranvier, as estimated from high-magnification images (Fig. 1F), were 0.97 ± 0.02 μm long and 0.66 ± 0.01 μm in diameter (n = 259). In some nodes, especially those well aligned with the x–y plane, the staining for Kv7.2/7.3 (Fig. 1F) and PanNav appeared in parallel lines, suggestive of membrane labeling. These findings show that while Kv7 and Nav are coexpressed in neocortical AISs and nodes, they follow distinct distributions within the AIS with highest absolute and relative Kv7.2/7.3 concentrations at the distal AIS (∼40 μm) coinciding with the site of action potential onset (Palmer and Stuart, 2006; Kole et al., 2007).
Intrinsically bursting and regular firing L5 neurons have similar axonal Kv7 distributions
For our subsequent electrophysiological characterization experiments, we investigated the Kv7 distribution in identified L5 neurons that can be classified by their regular spiking (RS) and intrinsic bursting (IB) phenotypes (Chagnac-Amitai et al., 1990; Kole, 2011). We recorded from large thick-tufted L5 neurons, filled these with Alexa Fluor 488, and performed post hoc Kv7.2 and Kv7.3 staining. z-projected image analysis revealed that neither the soma size (on average, 22.1 ± 0.5 μm; unpaired t test, p = 0.47) nor the AIS length (on average, 45.7 ± 1.1 μm; p = 0.10) was different between the two classes (17 RS and 12 IB neurons). Furthermore, the length of expression in the distal part of the AIS was similar between the two classes for both Kv7.2 (RS, 23.7 ± 1.3 μm, n = 14; IB, 24.9 ± 1.6 μm, n = 9; unpaired t test, p = 0.57; Fig. 2A) and Kv7.3 (RS, 24.6 ± 1.3 μm, n = 12; IB, 25.8 ± 1.7 μm, n = 9; p = 0.59; Fig. 2A). Kv7.2 and 7.3 subunits were also coexpressed at all identified axonal branch points (100%, 11 branch points) in both RS (five of five branch points) and IB neurons (six of six branch points; Fig. 2A).
To determine the physiological contribution of the total Kv7 conductance to the intrinsic excitability in the two classes, we analyzed the change in intrinsic excitability after bath application (10–20 μm) of XE-991, a Kv7 family-selective blocker (Wang et al., 1998). The results indicate that IB and RS neurons similarly increased in input resistance (124 ± 8.6% and 115.7 ± 8.6%, respectively; two-way repeated-measures ANOVA, p = 0.44, n = 6) and depolarized their resting membrane potential (+3.8 ± 0.8 vs +4.3 ± 1.2 mV, IB and RS, respectively; p = 0.60). Furthermore, the percentage increase in the action potential afterdepolarization (ADP) after bath application of 20 μm XE-991 was not different (244.7 ± 48% vs 182.4 ± 17.7%, for IB and RS, respectively; p = 0.28; n = 6), and neither the change in the number of high-frequency action potentials (8.1 ± 1.3 vs 7.2 ± 1.0, IB and RS, respectively; p = 0.54; n = 13 and n = 11; Fig. 2B,C).
Together, these results strongly indicate that Kv7.2 and Kv7.3 subunits are, independent of pyramidal cell subtype, strictly colocalized to the distal region of the AIS and ubiquitously clustered near Nav channels. Although strict colocalization of the two Kv7 subunits at both AISs and nodes is suggestive of the expression of heterotetrameric channels, as noted earlier (Pan et al., 2006), this can only be established by direct M-current recording from the axon.
Pharmacological isolation and identification of IM in neocortical axons
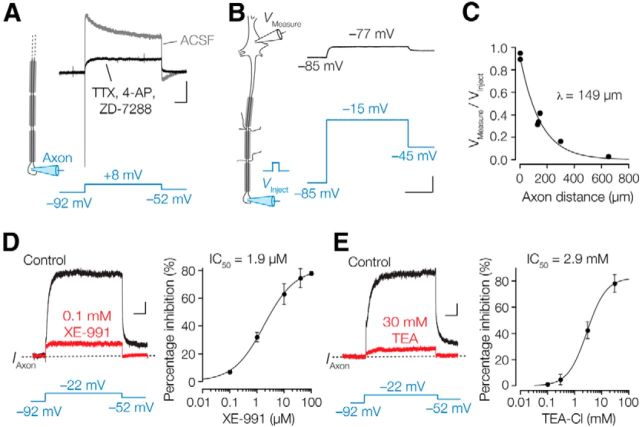
To record axonal IM, we made whole-cell recordings from the unmyelinated cut-ends of visually identified L5 axons (Kole et al., 2007). Using a −92 mV holding potential, a step depolarization in the axon to 8 mV activated a rapid inward Na+ current followed by a slowly inactivating K+ outward current (Fig. 3A). We pharmacologically isolated IM by bath application of TTX, 4-AP, and ZD-7288, blocking Na+ currents, fast-activating Kv1 and Kv3-type K+ currents, and HCN currents, respectively. The remaining outward current was relatively slowly rising (28.8 ± 5.1 ms at 8 mV; n = 5 axons) and non-inactivating, suggestive of IM (Fig. 3A, black trace; Brown and Adams, 1980; Brown and Passmore, 2009). The space-clamp characteristic of this voltage-clamp configuration was assessed by placing a second electrode at the soma while injecting the voltage command steps into the axon (n = 5 soma–axon recordings, 120–650 μm from the soma and n = 2 soma–soma recordings; Fig. 3B,C). Voltage command amplitudes greatly attenuated with increasing distances from the axonal injection site, following a single exponential function with a constant of 149 μm (n = 7; Fig. 3C). These results indicate that axonal recordings beyond 300 μm distance from the soma and injecting, for example, a step potential of 100 mV will depolarize the soma by less than ∼15 mV, minimally activating somatodendritic K+ currents. All axonal whole-cell voltage-clamp recordings were therefore routinely made at a distance of ∼400–800 μm from the soma.
Figure 3.
Isolation and pharmacological characterization of IM in neocortical axons. A, Left, Pipette configuration during single electrode whole-axon voltage-clamp recording. Right, Voltage steps to 8 mV evoked a Na+-mediated fast inward current followed by a large inactivating outward current (gray). In the presence of TTX, 4-AP, and ZD-7288 (black) a slow-activating outward current remained. Calibration: 0.5 nA, 0.1 s. B, Left, Schematic depicting a dual whole-cell recording from soma and axon. Right, Voltage-clamp command steps in the axon (blue traces) were attenuated at the soma (current-clamp, black) to 83%. Calibration: 20 mV, 0.2 s. C, Voltage attenuation plotted vs the distance from the soma and fitted with the exponential function; y = 0.92e−0.0067x. D, E, The Kv7 channel blocker XE-991 (D) and the nonspecific blocker TEA (E) blocked the outward currents. Calibration: 50 pA, 0.1 s. Dose–response curves and corresponding Hill fits to the percentages of current block for XE-991 (n = 18) and TEA (n = 12), respectively. Symbols represent the mean ± SEM.
To pharmacologically characterize the axonal IM, we first bath applied XE-991. Outward currents were dose-dependently blocked to 63.3 ± 7.6% (n = 6) at 10 μm and maximally to 76.7 ± 1.6% (n = 5) at 100 μm (Fig. 3D). The IC50 of XE-991 determined with the Hill equation was 1.9 μm with a Hill slope near unity (∼0.95), which is similar to previous reports (Wang et al., 2000; Schwarz et al., 2006). Since all Kv7 family members are similarly sensitive to XE-991, these results indicated that the current was largely mediated by Kv7 channels, but did not allow identification of the subunits. To achieve this, we exploited the differential sensitivity of homomeric and heteromeric Kv7 channels to the K+ channel blocker TEA (Wang et al., 1998; Hadley et al., 2000, 2003; Schroeder et al., 2000; Shapiro et al., 2000). Homomeric Kv7.2, Kv7.3, Kv7.4, and Kv7.5 channels are blocked with IC50 values of ∼0.17, >200, ∼3.0, and ∼70 mm, respectively. Importantly, Kv7.4 subunits have a restricted expression pattern in brain and have not been found in neocortex (Kharkovets et al., 2000). Heteromeric channels show intermediate sensitivities to TEA block. Kv7.2/7.3 and Kv7.2/7.5 channels have IC50 values near ∼3 and ∼200 mm (Wang et al., 1998; Hadley et al., 2000, 2003; Schroeder et al., 2000; Shapiro et al., 2000). We found that bath application of 3 mm TEA blocked 42.3 ± 6.4% (n = 3) of the 4-AP-insensitive outward currents; 30 mm TEA blocked 78.1 ± 6.8% (n = 5, paired t test p = 0.00058; Fig. 3E). Fitting TEA inhibition results at concentrations of 0.1, 0.3, 3.0, or 30 mm yielded an IC50 of 2.9 mm with a Hill slope coefficient of 1.2. Together, in L5 axons the remaining outward current in the presence of 4-AP resembles the XE991-sensitive IM, predominantly carried by heterotetramers of Kv7.2/7.3 subunits.
Gating properties, voltage dependence, and retigabine sensitivity of axonal Kv7.2/7.3 channels
To examine the precise voltage dependence and kinetics of axonal heterotetrameric Kv7.2/7.3 channels, we used a deactivation protocol from a holding potential of −32 mV (Brown and Adams, 1980; Adams et al., 1982). Between −32 and −62 mV, the inwardly rectifying currents deactivated slowly (∼30 to 40 ms) and inverted beyond voltages of −100 mV (Fig. 4A). Analysis of the intersection of the instantaneous and steady-state currents obtained with the deactivation protocol yielded a reversal potential of on average −92.6 ± 4.3 mV (n = 7), which is consistent with a K+ current. We also explored the voltage and time dependence of IM activation by making steps from a holding potential of −92 mV to a range of test potentials (−102 to 32 mV), followed by a step to −52 mV (Fig. 4B). Outward currents were clearly resolved from −62, and at 28 mV reached peak amplitudes of on average 478.7 ± 58.1 pA, equivalent to a conductance of 4.0 ± 0.5 nS (assuming a −92 mV reversal potential; n = 14; Fig. 4B,C). Bath application of the Kv7.2–7.5 channel opener retigabine (Wickenden et al., 2000) at a concentration of 10 μm led to a nearly twofold increase in peak conductance (7.5 ± 0.22 nS, p = 0.00012, n = 4; Fig. 4A–C).
Figure 4.
Voltage dependence, kinetics, and retigabine sensitivity of nodal M-currents. A, Deactivation currents under control conditions (left) and in the presence of retigabine (right). Traces represent average of five different axons. Calibration: 20 and 100 pA, 0.1 s. B, IM activation in response to six voltage steps (left). Current traces (average of three axons) of IM to the same voltage steps recorded in the presence of 10 μm retigabine (right). Calibration: 0.1 nA; same time scale applies as in A. Single exponential fits (red) of the current rise time are overlaid only for the maximal activated current. C, Current–voltage plots of the peak current amplitude obtained during activation in control, retigabine, and XE-991. D, Normalized conductance–voltage plots fitted with Boltzmann equations with offset for control and in the presence of retigabine. The half-maximum activation and effective valences are indicated. E, Time constants of activation and deactivation obtained from monoexponential fits are shown. IM was significantly slower between −132 and −42 mV in the presence of retigabine. All symbols represent the mean ± SEM.
Tail currents were analyzed for both the activation and deactivation protocols. We found that the midpoints of voltage dependence of activation and effective valances, determined by Boltzmann fits, were not different between the two protocols (p = 0.328 and p = 0.531, respectively) and therefore subsequently pooled the results. The average midpoint for voltage dependence of activation was −33.8 ± 1.8 mV (n = 8) with an effective valence (e−) of 3.1 ± 0.3 (n = 8; Fig. 4D). Retigabine strongly shifted the midpoint of the voltage dependence of activation to −63.1 ± 1.6 mV (unpaired t test, p = 0.00023; n = 6; Fig. 4D), which is in line with the predicted shift of approximately −30 mV for Kv7.2/7.3 heteromers (Tatulian et al., 2001). The time course of activation and deactivation could be well fitted with single exponential functions revealing relatively fast kinetics ranging between 10 and 40 ms at 35°C (Fig. 4E). Bath application of 10 μm retigabine led to a fivefold slowing of the deactivation time constant (at −72 mV; control, 18.9 ± 1.9 ms, n = 10; retigabine 89.6 ± 10.9 ms, n = 4; unpaired t test, p = 0.0013; Fig. 4E) but did not affect activation at depolarized potentials (8 mV; control, 21.7 ± 0.3 ms, n = 10; retigabine, 25.8 ± 1.7 ms, n = 4; p = 0.268; Fig. 4E). The slowing of the IM deactivation of retigabine in L5 pyramidal cell axons is consistent with previous experiments using heterologously expressed Kv7.2/7.3 heteromers (Tatulian et al., 2001). To enable comparison with previous Kv7 studies performed at room temperature, we also recorded IM at a temperature of 25°C. At 32 mV, the activation time constant became significantly slower from an average of 14.8 ± 2.2 ms (35°C; n = 9) to 61.7 ± 6.4 ms (25°C; n = 5; unpaired t test, p = 0.0086), and at −50 mV changed from 42.1 ± 4.8 ms (n = 9) to 114.9 ± 15.1 ms (n = 5; p = 0.0074). Across a range of voltage steps, the temperature coefficient (Q10) was found to be on average ∼2.6, which is in accord with previous work in layer 2/3 neurons (Guan et al., 2011).
In summary, these data show that axonal IM shares the voltage dependence with previously reported somatic IM, but has relatively faster and monoexponential activation and deactivation kinetics.
IM properties in the axon initial segment
Whole-axon recordings most likely recruit ionic current from nodes of Ranvier located at various distances from the recording location, possibly leading to a filtering of the current rise times. Since the AIS contains high densities of Kv7.2/7.3 channels (Figs. 1, 2) and is amenable to direct patch-clamp recording (Kole et al., 2007), we made cell-attached recordings using large depolarizing steps to 45 mV, maximizing the probability to detect small currents. Outward currents evoked in somatic cell-attached patches were <2 pA (n = 8), and in some cases single-channel openings were resolved (Fig. 5A, top traces). Single-channel currents were on average 1.6 ± 0.06 pA (single-channel conductance; 10.7 ± 0.7 pS; n = 4 patches), which is consistent with previously reported single M-channel conductances ranging between 7 and 11 pS (Selyanko et al., 2001; Chen and Johnston, 2004; Miceli et al., 2009). In contrast to the soma, significantly larger and macroscopic currents were obtained at a distance of ≥30 μm from the soma in the AIS (range, 5–38 pA; n = 8; p = 0.0032; Fig. 5A). AIS cell-attached currents strongly resembled IM. First, the time course of activation was on average 13.4 ± 3.5 ms (n = 7). Second, using deactivation steps to −52 mV revealed slower current relaxations (2–8 pA in amplitude) that could be well fitted with a single exponential with a time constant of 43.6 ± 4.6 ms (n = 5). The observed M-current kinetics in the AIS therefore resembled the whole-axon currents (Fig. 4). To rule out any voltage-clamp errors in cell-attached mode (Williams and Wozny, 2011), we also made outside-out patch-clamp recordings from the distal AIS in the presence of 4-AP, ZD-7288, and TTX, and applied deactivation steps. M-currents could successfully be detected in four outside-out recordings and were slowly deactivating at −32 mV (40.7 ± 6.7 ms; n = 4; Fig. 5A,B), which is comparable to the whole-axon kinetics (unpaired t test, p = 0.639). The average half-maximum activation voltage from three separate fits was −38.4 ± 0.1 mV with an effective valence of 2.5 ± 0.1 (n = 3).
The patch recordings allowed us to quantify the density of IM along the somatodendritic and axonal axis by converting the obtained peak current amplitudes to conductance densities, using the experimentally determined reversal potential of −92 mV. On average, the densities in somatic and dendritic patches were determined to be 12.1 ± 0.3 pS μm−2 (n = 22) and 12.2 ± 2.4 pS μm−2 (n = 5), respectively (Fig. 5C), which is equivalent to ∼1–2 Kv7 channels/μm2, assuming a single-channel conductance of 7–11 pS (Selyanko et al., 2001; Chen and Johnston, 2004; Miceli et al., 2009). The collected data demonstrated, however, a steep increase in current amplitudes beyond 30 μm from the soma, reaching a peak conductance density of ∼144 pS μm−2 at the distal end of the AIS at a distance of 55 μm from the soma (Fig. 5C). Interestingly, unlike the AIS Nav currents, which are detectable only when ankyrin G binding is reduced (Kole et al., 2008), the comparison of patch recordings of IM from cut-ends (lacking ankyrin G) and the intact AIS showed no difference in the IM amplitudes (unpaired t test, p = 0.277; n = 8). The distribution of Kv7 along the AIS could be fit with an exponential function (e-fold increase, ∼13 μm; n = 32).
Together, these experiments reveal for the first time the gating properties of M-current native to the axon, which have their highest conductance densities in the distal end of the AIS, ∼10-fold higher compared with the soma.
Local roles of Kv7 channels in dendrites, soma, and axons
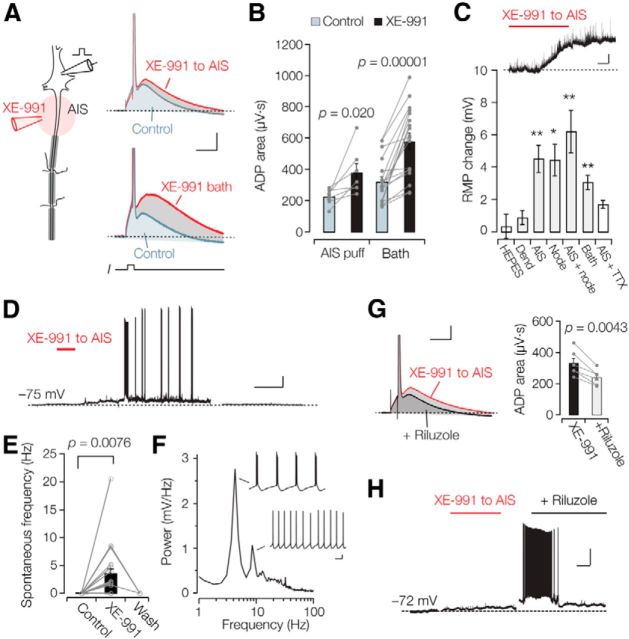
To determine the global impact of a local high density of Kv7 channels in the axon initial segment, we first applied XE-991 focally, via patch pipettes, to visually identified subcellular sites of L5 pyramidal axons using bright-field/fluorescence microscopy combined with somatic whole-cell recording (Fig. 6A). AIS Kv7 channel block led to a significant increase in the somatically recorded ADP (1.56 ± 0.55-fold increase; n = 6; paired t test, p = 0.020; Fig. 6B). In comparison, bath application caused an approximately twofold increase (1.98 ± 0.21-fold increase; n = 18; paired t test, p = 0.000012), suggesting that local AIS expression of Kv7 channels may play a substantial role in counteracting the perisomatic depolarization during the ADP. Furthermore, focal application of XE-991 (10–20 μm) had a clear location-dependent impact on the resting membrane potential (Fig. 6C). Kv7 channel block at the apical dendrite, ∼100 μm from the soma, changed neither the somatically recorded resting membrane potential nor the input resistance (average Vm change, 0.9 ± 0.4 mV; RN change, −0.3 ± 3%; n = 6; paired t test, p = 0.079 and p = 0.89; Fig. 6C). XE-991 applied to the first node or the AIS, however, induced a ∼5 mV depolarization of the resting membrane potential (Vm change, 6.2 ± 1.4 mV, n = 14, paired t test, p = 0.00094; and 4.4 ± 1.0 mV, n = 6, paired t test, p = 0.014). Puffing solution alone (i.e., without XE-991) had no impact on the resting membrane potential when applied to the AIS (0.3 ± 0.75 mV, n = 5, p = 0.14; Fig. 6C). In comparison, bath application of 10–20 μm XE-991 depolarized the resting membrane potential by on average 3.1 ± 0.4 mV (paired t test, p = 0.000035; n = 28; Fig. 6C) and decreased the resting conductance by ∼6 nS (control, 36.8 ± 2.1 nS; XE-991, 30.3 ± 2.9 nS; n = 30; paired t test, p = 0.000013).
Figure 6.
Axonal Kv7 channels maintain resting membrane potential stability. A, Left, Schematic drawing of the drug application (red) and recording pipette arrangement (black). In this example, XE-991 was focally applied to the AIS. Right, Example voltage recordings before (gray) and after XE-991 application (red) locally to the AIS (right top) or in the bath (right bottom) show an augmentation of the ADP. Calibration: 10 ms, 10 mV. B, Line and scatter plots of the individual data reveal an increase in the ADP after local XE-991 application to the AIS (n = 6) or global block in the bath (n = 18). C, Population data of the resting membrane potential change for the different locations and solutions. HEPES, Control solution without XE-991 to the AIS; Dend, XE-991 to the dendrite; AIS, XE-991 to the axon initial segment; Node, XE-991 to the node; AIS + first node, XE-991 to the AIS and node; Bath, XE-991 in the bath; AIS + TTX, XE-991 to the axon initial segment in the presence of 1 μm TTX. **p < 0.001, *p < 0.05. Data represent the mean ± SEM. Inset, Focal AIS application of XE-991 depolarized the resting membrane potential. Calibration: 1 mV, 1 min. D, Example of a recording in which 20 μm XE-991 caused spontaneous firing. A 20 min period before washout was blanked for clarity. Calibration: 4 min, 10 mV. E, Summary data for the 17 experiments in which 10–20 μm was focally applied to the AIS. Washout was tested in three experiments. F, Power spectrum of the spontaneous action potential firing rate during AIS Kv7 channel block. Action potential frequencies ranged between ∼4 and 38 Hz (n = 6). Calibration: 0.1 s, 10 mV. G, After local XE-991 application to the AIS blocking the persistent sodium current by bath application of 5 μm riluzole decreased the ADP (left). Calibration: 10 mV, 10 ms. Population data for the changes of the ADP area during XE-991 application to the AIS and in the presence of riluzole (right). The XE-991 data is the same as in B. H, Recording in which XE-991 puffing to the AIS caused spontaneous firing and application of 5 μm riluzole abolished the spontaneous APs. Gaps in the recording are blanked for clarity. Calibration: 2 min, 20 mV.
The resting membrane depolarization with focal XE-991 application to the AIS, a region only 45 μm in length (<1 nS Kv7 conductance at rest) was surprisingly similar in magnitude compared with bath application of XE-991 (∼4 mV). We hypothesized that the persistent Na+ current in part mediates the depolarizing drive by amplifying the membrane potential depolarization during Kv7 channel block. Indeed, in the presence of TTX the XE-991-induced resting membrane depolarization was significantly reduced (TTX + XE-991, 1.7 ± 0.3 mV; n = 5; unpaired t test vs XE-991 alone, p = 0.0044; Fig. 6C). These data suggest that AIS Kv7 channels prevent subthreshold activation of Nav current.
Interestingly, when applying XE-991 to the AIS the change in resting membrane potential was sufficient to reach action potential threshold and generate spontaneous firing in 61% of the cells (14 of 23 cells; Fig. 6D). Long-lasting periods of unstable membrane potential fluctuations (∼10 mV) persisted for several minutes and were associated with spontaneous action potential firing rates (average rate, 3.6 ± 0.8 Hz; n = 17; Fig. 6E). These large membrane potential fluctuations and periods of spontaneous firing, similar to observations in the CA1 pyramidal neuron (Shah et al., 2008), were also observed in the presence of blockers for inhibitory and excitatory synapses (bicuculline, gabazine, and DNQX; n = 3), in accordance with the idea that these are generated by intrinsic conductances. A power spectrum analysis of the intrinsic spontaneous action potential firing periods showed that the peak of activity occurred at frequencies of ∼4 and 9 Hz, correlating with either high-frequency oscillatory bursts or regular action potential firing (n = 6; Fig. 6F). To test whether block of Kv7 channels by XE-991 unmasks a persistent component of axonal sodium currents (Golomb et al., 2006; Yue and Yaari, 2006), we bath applied 5 μm riluzole, a nonspecific blocker with high sensitivity for the persistent sodium current (Urbani and Belluzzi, 2000). Riluzole did not change the amplitude of single action potentials compared with XE-991 alone (XE-991, 97.4 ± 1.5 mV; XE-991 plus riluzole, 96.3 ± 3.4 mV; n = 6; paired t test, p = 0.70; Fig. 6G). However, the XE-991-induced increase in the ADP was reduced to control values in the presence of riluzole (XE-991, 174.1 ± 25.4%; XE-991 plus riluzole, 114.9 ± 17.7%; n = 6; repeated-measures ANOVA, p = 0.0043; Fig. 6G). In these recordings, one neuron developed sustained action potential firing after XE-991 application, and bath application of 5 μm riluzole reduced the spontaneous action potentials after ∼15 min (Fig. 6H).
Together, these data indicate that AIS Kv7 channels exert hyperpolarizing stabilizing effects upon the resting membrane potential, thereby preventing activation of persistent Na+ current activated at subthreshold potentials and restraining spontaneous firing.
Kv7 channel activation at the resting membrane potential augments propagating axonal action potentials
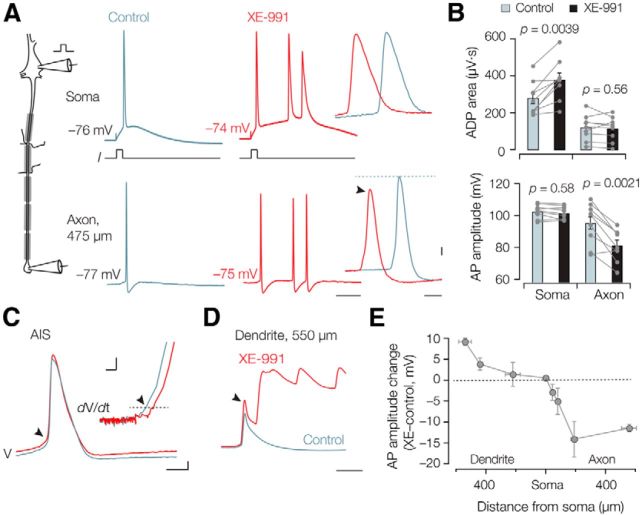
Do Kv7.2/7.3 channels concentrated at nodes of Ranvier also have an attenuating impact on action potential propagation? Direct intracellular recordings from central nodes (∼1 μm in length) are difficult to achieve with patch pipettes (∼1 μm tip diameter). As an alternative, we examined the role of Kv7 in nodal action potentials by using dual pipette recordings from the soma and axon cut-ends (a distance of 130–650 μm from the soma), evoking action potentials at the soma and using bath application of XE-991 (Fig. 7A). The change in the ADP appeared highly location dependent (two-way ANOVA, p = 0.0004; F = 19.82; n = 9). While the ADP significantly increased at the soma (control, 279.0 ± 29.9 μV · s; XE-991, 378.0 ± 36.3 μV · s; Bonferroni correction, t = 5.96, p = 0.0039; n = 9), the voltage–time integral was not affected in distal regions of the axon (control, 119.7 ± 22.7 μV · s; XE-991, 114.0 ± 21.6 μV · s; Bonferroni correction, t = 0.34, p = 0.56; n = 9; Fig. 7B). Interestingly, the action potential amplitude change, measured as the threshold to peak difference, was also location dependent (two-way ANOVA, p = 0.0002, F = 22.81; n = 9) but in an opposite manner. XE-991 led to a significant action potential amplitude reduction in the axon (average reduction, −13.9 mV; Bonferroni correction, t = 7.31, p = 0.0021; n = 9; Fig. 7B) but not in the soma (−1.0 mV reduction; Bonferroni correction, t = 0.59, p = 0.58; n = 9).
Figure 7.
Kv7 channels differentially regulate back-propagating and forward-propagating action potentials. A, Left, Schematic of the simultaneous soma–axon whole-cell recording. Right, Action potentials evoked by brief (3 ms) somatic current injection simultaneously recorded at an axonal recording distance of 475 μm in control condition (gray) and after bath application of XE-991 (10 μm, red). Note the additional high-frequency spikes in the presence of XE-991. Calibration: 10 ms. High magnification of the same traces shows the reduced axonal action potential amplitude (arrow). Calibration: 0.5 ms, 10 mV. B, Bar graphs and individual experiments of the area under the curve reveal an increase in the somatic ADP (n = 9) after XE-991 application but decrease in the distal axons (>150 μm, n = 9). C, Action potentials were evoked at the soma and simultaneously recorded from the AIS. The AIS action potential threshold was significantly increased (n = 5, p = 0.022, arrow). Inset, The phase plot (time derivative vs voltage) highlighting the voltage threshold. Calibration: 0.5 ms, 10 mV (inset: 5 mV, 0.1 kV s−1). D, XE-991 block in the dendrite increased the action potential amplitude (arrow). In this example, the dendrite generated a long-lasting plateau depolarization associated with high-frequency burst firing at the soma. Calibration: 10 ms, same voltage scaling as in C. E, Summary data of all dual whole-cell recordings (n = 25) showing the location dependence of action potential change in XE-991. Action potential amplitudes increase in the distal dendrite, are maintained at the soma, but are substantially reduced in the axon. Symbols represent x and y mean ± SEM.
As a comparison, we also made double whole-cell recordings from soma and axon endings cut at the AIS tip (35–60 μm from the soma; n = 5; Fig. 7C). Bath application of 10 μm XE-991 depolarized the action potential voltage threshold in the AIS by ∼3 mV (control, −54.9 ± 2.6 mV; XE-991, −51.9 ± 2.3 mV; paired t test, p = 0.022; n = 5; Fig. 7C). Furthermore, Kv7 channel block caused only a small reduction in the AIS action potential amplitude (control, 101.8 ± 3.2 mV; XE-991, 96.7 ± 3.9 mV; p = 0.043; n = 5) and the peak of the rate of rise (control, 1.97 ± 0.2 kV s−1; XE-991, 1.6 ± 0.15 kV s−1; p = 0.0017; n = 5). While in these recordings the voltage threshold at the soma did not change (−0.5 ± 0.7 mV; p = 0.432; n = 5), higher concentrations of XE-991 (20 μm), causing a larger fractional block of Kv7 channels (Fig. 3D), significantly depolarized the threshold (+2.6 ± 0.9 mV; paired t test, p = 0.019; n = 12). On the other hand, simultaneous whole-cell recordings from soma and distal dendrites (>400 μm from the soma) revealed that XE-991 increased the backpropagating action potential amplitude by on average 6.5 ± 2.3 mV (p = 0.043, n = 4; Fig. 7D). Plotting the action potential amplitude change in the presence of XE-991 versus all recording locations showed that Kv7 channels differentially affect action potential propagation into dendritic and axonal regions (Fig. 7E). A depolarized voltage threshold and amplitude reduction of fast sodium action potentials is inconsistent with block of an outward current and the experimentally measured IM activation kinetics near action potential threshold (∼40 ms at −55 mV; Figs. 4, 5) are also too slow to affect threshold behavior. Therefore, these data suggest that Kv7 channels open at rest rather regulate the Nav channel availability by setting the resting potential.
Membrane potential regulation of propagating axonal action potentials
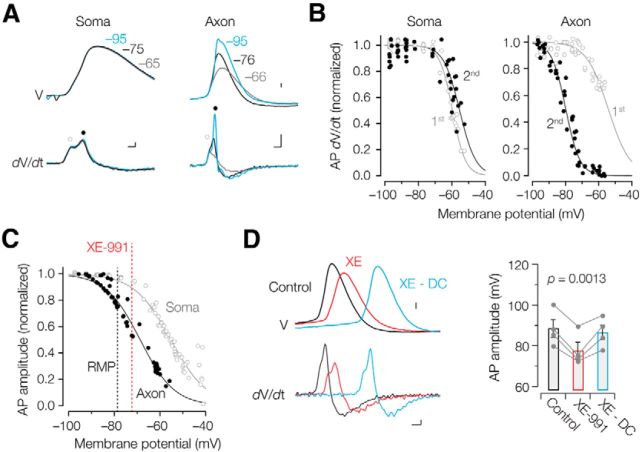
The amplitude of the conducting axonal action potential is known to critically depend on the membrane potential (Waxman et al., 1995; Shu et al., 2006; Kole et al., 2007). Furthermore, recent studies have shown that in central axons the midpoint of steady-state Nav channel inactivation is near −80 mV with a slope of ∼9 mV (Hu et al., 2009; Schmidt-Hieber and Bischofberger, 2010), predicting that even small changes near the resting membrane potential (−77 mV) influence nodal Nav channel availability. To test that prediction, we quantitatively examined action potential amplitudes while varying resting potentials between −110 and −40 mV using local DC injection into the axon (Fig. 8A–C). Action potentials were then activated by somatic current injection, and recorded at the soma and axon, at a distance of between 150 and 250 μm from the soma. Both somatic and axonal action potentials showed two distinct components in the first time derivative. The first component reflects invasion from the antidromically propagating initial segment action potential and the second generated by local Nav channels (Kole and Stuart, 2008). By using Boltzmann fits to the individual component amplitudes at the soma, the first component was found to inactivate at a midpoint voltage of −55.7 ± 0.9 mV (n = 8), significantly more hyperpolarized compared with the second somatodendritic component (−53.7 ± 0.9 mV; paired t test, p = 0.027; n = 8; Fig. 8B). In the axon, the first component showed a midpoint voltage of −52.8 ± 2.3 mV (n = 5), while the second component inactivated at −76.5 ± 2.1 mV (n = 5). These data suggest that steady-state inactivation of Nav channels occurs at much more hyperpolarized potentials in nodal domains. The action potential amplitudes in the axon had a midpoint voltage a few millivolts more depolarized from the resting membrane potential [−71.0 ± 2.4 mV (n = 5, axons) with a slope of 5.5 ± 0.3 mV (n = 5); Fig. 8C]. In contrast, somatic action potentials had a 20 mV more depolarized midpoint of inactivation (−50.2 ± 0.9 mV; n = 10; unpaired t test, p = 5.7 × 10−12) with a shallower slope (6.8 ± 0.4 mV; unpaired t test, p = 0.033). Finally, to determine whether the depolarization of the resting potential causes the axonal action potential amplitude change in XE-991, we corrected the local depolarization in the presence of 10 μm XE-991 (+2.1 ± 0.3 mV; n = 4) by applying negative DC injections into the axon [∼10 pA nullified the resting potential difference with control (−0.1 ± 0.3 mV)]. The membrane potential correction fully restored the action potential amplitudes (one-way repeated-measures ANOVA, p = 0.0013, F = 24.6; Fig. 8D).
Figure 8.
Voltage dependence of action potentials at somatic and axonal locations. A, Top, Voltage dependence of the somatic (left) and axonal action potential amplitude (right) assessed by applying local positive (gray) and negative holding currents (blue). Action potentials evoked from the resting membrane potential (RMP) are indicated in black. Calibration: 10 mV. Bottom, First derivatives of the voltage corresponding to the above-displayed action potentials. Circles indicate the first (open circle) and second peaks (closed circle), respectively. Calibration: 100 μs, 0.1 kV s−1 (left); 100 μs, 0.5 kV s−1 (right). B, Peak amplitudes of the first and second component of the time derivative separately fit with Boltzmann equations. Note the hyperpolarized inactivation at axonal sites compared with the soma. C, Data from two example recordings reveal a ∼20 mV difference in the midpoint of inactivation of action potential amplitudes between axons (black) and soma (gray). Data were fit with Boltzmann equations (lines). The resting membrane potential in control (black) and in 10–20 μm XE-991 (red) are indicated as dotted lines. D, The reduced axonal action potential amplitude after XE-991 application can be recovered by direct negative current injections (top, blue). Calibration: 10 mV. Corresponding differentiated voltage of the action potentials (bottom) and summary data (mean ± SEM) for four similar experiments (right). Calibration: 100 μs, 0.1 kV s−1.
These data show that the propagating axonal action potential is highly sensitive to the resting membrane potential, in part influenced by nodal heterotetrameric Kv7.2/7.3 channels that are open at rest.
Spatial heterogeneity of Kv7 conductances in soma and axons
To quantitatively examine how nodal Kv7.2/7.3 channels interact with Nav channel availability, we generated a Hodgkin–Huxley model of the Kv7 conductance constrained by the experimentally recorded voltage and time dependence of the currents (Figs. 4, 9A; see Materials and Methods). This conductance model, based on a single activation gate, was subsequently implemented into a multicompartmental model of a morphologically reconstructed L5 pyramidal neuron with an axon (see Materials and Methods). Using voltage-clamp protocols similar to the experimental voltage-clamp experiments, the simulated whole-axon currents resembled the experimentally obtained currents, both in amplitude and their time course (Fig. 9B). Several key characteristics of Kv7-mediated excitability were captured in this model. In agreement with our experimental observations, a global reduction of ḡM by 60% (mimicking 10 μm XE-991; Fig. 3D) caused a spatially uniform ∼3 mV depolarization of the resting membrane potential, increased the afterdepolarization, and led to multiple action potentials at high frequency (Fig. 9C,D). The change in resting potential with ḡM reduction was nonlinear and in part dependent on subthreshold Nav channel activation since removing Nav from the model reduced the resting membrane potential depolarization (Fig. 9D).
Next, to understand the differences in the Kv7 regulation of action potentials between compartments we plotted the time course of gM during the action potential in the soma and in a node of Ranvier at a distance of ∼600 μm from the soma (Fig. 10A). At the resting membrane potential (−78 mV), ḡM has a uniform open probability (Po) of 0.04. At the soma, ḡM increased rapidly during the rising phase of the action potential, peaked with a ∼10 ms delay, and slowly returned to the resting conductance shaped by the slow IM deactivation kinetics. In comparison, however, the nodal action potential (250 μs in half-width) recruited gM less efficiently. The maximum Po was 0.065, and subsequently deactivated rapidly due to the large afterhyperpolarization mediated by axonal Kv1 (Fig. 10A). The compartmental differences in gM activation were further explored by examining the accumulation of activation by repetitive action potential generation (170 Hz) during a 30 ms lasting depolarization. Figure 10B shows the resulting maximum gM for each action potential as a function of location along the dendrosomatic–axonal axis. While gM increased progressively in the perisomatic region as a function of action potential number (2.2-fold Po increase), consistent with the rapid repolarization and large afterhyperpolarzation of the nodal action potential, gM showed little increase in the node (1.2-fold). Finally, we assessed in the model the overlap between the IM-mediated resting membrane depolarization and local sodium channel inactivation (hNa). The midpoints of Nav channel inactivation in soma, AIS, and axon were implemented based on available experimental data (Fig. 10C; see Materials and Methods). Reducing gM in the model by 70%, mimicking XE-991 application, uniformly raised the resting membrane potential by ∼5 mV and consequently reduced both the forward-propagating action potential rate of rise and the amplitude (15 mV reduction; Fig. 10D). In contrast to the nodal action potential, the somatic amplitude was largely maintained (3 mV reduction; Fig. 10D), which is consistent with the experimental results (Figs. 7, 8).
In conclusion, the results of the computational model and the experimental work suggest that due to the combined effect of the passive voltage attenuation in central myelinated axons, the active high-pass filtering of nodal action potentials by Kv1 and the compartmentalization of steady-state Nav channel inactivation, the Kv7.2 and Kv7.3 coclustering with Nav channels in the nodal axolemma will primarily influence their availability.
Discussion
We present here the first biophysical characterization and functional analysis of the M-current in a myelinated CNS axon. The axonal M-current, mediated by Kv7.2/7.3 (KCNQ2/KCNQ3) heteromultimers, is ∼4% in the open state at the resting membrane potential, and activates rapidly and monoexponentially with time constants between 15 and 50 ms. In contrast to the well established attenuating effects of IM on excitability in the perisomatic region, coclustering of Kv7.2/7.3 with Nav channels in nodes of Ranvier primarily increases the availability of the transient Nav current, thereby accelerating the action potential upstroke.
In the rat peripheral sciatic nerve, most small- and medium-sized axons (<6 μm) express both Kv7.3 and Kv7.2 subunits in their nodes of Ranvier, while the largest axons (>10 μm) express mostly homomeric Kv7.2 channels (Schwarz et al., 2006). The present results corroborate and extend those observations by showing that central myelinated neocortical axons, ranging between 1 and 3 μm in diameter before entering the white matter, were nearly always immunoreactive for both Kv7.2 and Kv7.3 in the nodes of Ranvier. The limited diversity of Kv channels in axons, compared with the somatodendritic region, allowed isolation of Kv7 by blocking the 4-AP-sensitive fast-activating Kv1 (Kv1.1/1.2) channels, which are densely expressed in L5 axons (Kole et al., 2007; Shu et al., 2007; Hallermann et al., 2012) and clustered in the AIS and juxtaparanodal regions (Poliak and Peles, 2003; Kole and Stuart, 2012). The remaining current has the pharmacological profile of Kv7.2/7.3 heteromers. First, the pharmacological sensitivity to XE-991 implicates the Kv7 family. Second, the hyperpolarizing shift of the voltage dependence, increased current density, and slowed deactivation in the presence of retigabine implicate Kv7.2–7.5. Third, the TEA block results are inconsistent with Kv7.2, Kv7.3, or Kv7.5 homomers (or Kv7.3/7.5 heteromers), and exactly fit those obtained in IM in superior cervical ganglion neurons from adult rats (Hadley et al., 2003), from heterologous coexpression of Kv7.2 and Kv7.3 as separate cDNAs (Wang et al., 1998; Hadley et al., 2000; Shapiro et al., 2000), or as concatamers (Hadley et al., 2003). These results indicate that at least 80–90% of the L5 axonal M-current is mediated by Kv7.2/7.3 heteromultimers, although we cannot exclude a small contribution of alternative combinations and non-Kv7 channels.
Interestingly, we found that activation and deactivation kinetics were faster (∼15 ms at 28 mV, at 35°C) compared with previous reports of the native M-current in superior cervical ganglion neurons or heterologous expressed Kv7.2/7.3 heteromeric channels (Wang et al., 1998, 2000; Selyanko and Brown, 1999; Pan et al., 2001; Selyanko et al., 2001), and had mostly a monoexponential rising phase. Whether this reflects alternative splice variants of KCNQ2 (Pan et al., 2001), post-translational modification, or protein–protein interaction specific to the AIS and nodes remains to be determined. One constraint is that our recordings from distal axonal sites are primarily from cut-ends, which may suffer from damage, voltage-gated channel reorganization, or cytoskeletal rearrangements (Bradke et al., 2012). However, since our voltage-clamp data from the intact AIS were very similar in kinetics and voltage dependence compared with whole-axon recordings, the presumed ion channel modifications due to axotomy may have had a minimal impact on axonal Kv7 channels.
Patch recordings from the distal end of the AIS also enabled us to estimate a peak conductance density of Kv7 as ∼150 pS μm−2. In comparison, the somatic density was >10-fold lower, 12 pS μm−2 (equivalent to ∼1 channel μm−2), which is in reasonable agreement with previous measurements and numerical simulations (2–6 pS μm−2; Chen and Johnston, 2004; Gu et al., 2005; Lawrence et al., 2006; Hu et al., 2007). Interestingly, in sciatic nerve nodes the maximal conductance density of the IKs was estimated at 18.8 nS pF−1 or 188 pS μm−2 (assuming 0.01 pF μm−2; Röper and Schwarz, 1989). The similarity to our peak conductance estimation of IM in the distal end of the AIS adds to the considerable existing evidence of molecular similarity in the composition of AIS and nodes. While both Nav1.6 and Kv7 channels are anchored via ankyrin G to the actin–spectrin filament network (Pan et al., 2006; Hill et al., 2008; Cooper, 2011), the Kv7 peak conductance density is ∼40 fold smaller compared with AIS peak Nav conductance density (∼7000 pS μm−2; Hallermann et al., 2012). How these specific ratios of Nav/Kv7 channels are established and maintained is not well understood. Fluorescence recovery after photobleaching experiments indicates that ankyrin G interacts more weakly with the conserved C-terminal binding domains of Kv7.2/7.3 subunits (Pan et al., 2006) than with neurofascin (Zhang and Bennett, 1998). Relatively weak binding may make Kv7 channels poor competitors with Nav subunits and other ankyrin G ligands. Nonetheless, the distal AIS-predominant Kv7.2/7.3 labeling and conductance profile contrasts markedly with the distribution of ankyrin G. Molecular mechanisms for such subcompartmentalization within the AIS are unknown.
One of the main findings of our study is the identification of a new physiological significance of Nav/Kv7 coclustering. In the dendrites, soma, and AIS, IM accumulates significant outward current during action potential generation and consequently affects spike frequency accommodation and network oscillations (Yue and Yaari, 2004; Lawrence et al., 2006; Safiulina et al., 2008; Brown and Passmore, 2009; Leão et al., 2009). Also in isolated single nodes from peripheral sciatic axons, direct current injection evokes more action potentials when the Kv7 current is pharmacologically blocked, suggesting a role in spike frequency accommodation (Schwarz et al., 2006). Our experimental and computational data show that, due to the cable properties and associated voltage attenuation in the central L5 axons (length constant, ∼0.6 mm; Kole et al., 2007), the propagating axonal action potential, when initiated in the AIS, will minimally activate nodal Kv7 in the subthreshold voltage range. In these axons, the juxtaparanodal activation of Kv1 current rapidly repolarizes the action potential, conferring an active high-pass filtering and leading to a brief half-width duration (<400 μs), causing minimal afterdepolarization. Passive voltage attenuation and local Kv1 activation in central axons therefore leads to a limited recruitment of IM and minimally charges the nodal membrane resistance in the subthreshold range during propagating action potentials.
Rather than attenuating subthreshold depolarization, the high density of nodal Kv7.2/7.3 channels plays a major role in setting the resting membrane potential and indirectly influences the steady-state availability of Nav channels. The basis for the resting membrane potential in central myelinated axons is incompletely known, but in the periphery is determined by a combination of the depolarizing drive from persistent Na+ and HCN channels and the outward currents generated by nodal Kv7 and internodal Na+/K+ pumps (Thomas, 1972; Stys et al., 1993; Waxman et al., 1995). Increasing the action potential peak amplitude by membrane hyperpolarization without changing the half-width may be predicted to enhance Ca2+ current influx at the presynaptic terminal and transmitter release. Whether this occurs in layer 5 axons remains to be determined. Interestingly, in layer 4 pyramidal neurons, hyperpolarization of the presynaptic neuron was found to reduce release failures and increase synaptic currents in the postsynaptic cells (Cowan and Stricker, 2004). An amplifying role of Kv7 channels in transmitter release has been observed in unmyelinated Schaffer collaterals, although these changes were observed only when axons were depolarized by elevation of external K+ (Vervaeke et al., 2006). Kv7 channel-mediated boosting of axonal excitability has also been observed in the peripheral nervous system. Using stimulation of the peripheral myelinated sural nerves, the late superexcitability, determined by the threshold current required 7 ms after a maximal stimulus of the nerve, is reduced in the presence of flupirtine, a Kv7 channel opener, while threshold currents were raised during the subexcitability phase after 30 ms (Sittl et al., 2010). These data are in accord with Kv7 channel opening causing an increase in the ADP in myelinated nerves, which is known to increase in amplitude with steady hyperpolarization of the axonal resting membrane potential (Barrett and Barrett, 1982). Thus, Kv7 channels may serve distinct roles in the myelinated axon by reducing the steady-state inactivation of colocalized nodal Nav channels, thereby elevating short-term axonal excitability.
M-channels are an important target of regulation by neurotransmitters acting through Gq-linked metabotropic receptors, including acetylcholine (Brown and Passmore, 2009). Whether initial segment and/or nodal Kv7 channels are so modulated, thereby altering presynaptic excitability, is not yet known. Our experiments focused on the primary myelinated axon. They do not rule out the possibility that more distally located channels, in axon collaterals and/or presynaptic terminals, formed either by Kv7.2/7.3 or other subunits compositions (e.g., Kv7.2/7.5 or Kv7.5 homomers; Huang and Trussell, 2011), may also regulate local subthreshold voltage and propagating action potentials or be locally regulated by neurotransmitters. Future experiments comparing our findings with results in models of injury and epilepsy will help to illuminate the basis for the spectrum of early-onset epileptic and global development syndromes caused by Kv7.2 loss of function, and will aid the effort to optimize therapeutic use of agents such as retigabine, which increase Kv7 activity.
Footnotes
This work was supported by the European Research Council (ERC) under the European Community's Seventh Framework Program (FP7/2007-2013)/ERC Grant agreement P261114, the Australian National Health and Medical Research Council (Project Grant #525437) to M.H.P.K., and National Institutes of Health (NIH) Grant R01 NS49119 to E.C.C. B.T.T. is the recipient of an Epilepsy Foundation predoctoral fellowship funded by the American Epilepsy Society and the Pediatric Epilepsy Research Foundation. We thank S. de Vries for excellent technical support and S. Hallermann for critical reading of the manuscript. Ankyrin-G and Caspr monoclonal antibodies were developed by the University of California, Davis/NIH NeuroMab Facility (NIH Grant U24 NS050606).
References
- Adams PR, Brown DA, Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol. 1990;296:598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Properties of single voltage-dependent K+ channels in dendrites of CA1 pyramidal neurones of rat hippocampus. J Physiol. 2004;559:187–203. doi: 10.1113/jphysiol.2004.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC. Made for “anchorin”: Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons. Semin Cell Dev Biol. 2011;22:185–192. doi: 10.1016/j.semcdb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan AI, Stricker C. Functional connectivity in layer IV local excitatory circuits of rat somatosensory cortex. J Neurophysiol. 2004;92:2137–2150. doi: 10.1152/jn.01262.2003. [DOI] [PubMed] [Google Scholar]
- Golomb D, Yue C, Yaari Y. Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: combined experimental and modeling study. J Neurophysiol. 2006;96:1912–1926. doi: 10.1152/jn.00205.2006. [DOI] [PubMed] [Google Scholar]
- Guan D, Higgs MH, Horton LR, Spain WJ, Foehring RC. Contributions of Kv7-mediated potassium current to sub- and suprathreshold responses of rat layer II/III neocortical pyramidal neurons. J Neurophysiol. 2011;106:1722–1733. doi: 10.1152/jn.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1–4 potassium channels. Br J Pharmacol. 2000;129:413–415. doi: 10.1038/sj.bjp.0703086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, Brown DA. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J Neurosci. 2003;23:5012–5019. doi: 10.1523/JNEUROSCI.23-12-05012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, de Kock CP, Stuart GJ, Kole MH. State and location dependence of action potential metabolic cost in cortical pyramidal neurons. Nat Neurosci. 2012;15:1007–1014. doi: 10.1038/nn.3132. [DOI] [PubMed] [Google Scholar]
- Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 2008;4:e1000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. NEURON: a tool for neuroscientists. Neuroscientist. 2001;7:123–135. doi: 10.1177/107385840100700207. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27:1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Trussell LO. Control of presynaptic function by a persistent Na(+) current. Neuron. 2008;60:975–979. doi: 10.1016/j.neuron.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Trussell LO. KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci. 2011;14:840–847. doi: 10.1038/nn.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Kato M, Yamagata T, Kubota M, Arai H, Yamashita S, Nakagawa T, Fujii T, Sugai K, Imai K, Uster T, Chitayat D, Weiss S, Kashii H, Kusano R, Matsumoto A, Nakamura K, Oyazato Y, Maeno M, Nishiyama K, Kodera H, et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia. 2013;54:1282–1287. doi: 10.1111/epi.12200. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH. First node of Ranvier facilitates high-frequency burst encoding. Neuron. 2011;71:671–682. doi: 10.1016/j.neuron.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Is action potential threshold lowest in the axon? Nat Neurosci. 2008;11:1253–1255. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kole MH, Hallermann S, Stuart GJ. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci. 2006;26:1677–1687. doi: 10.1523/JNEUROSCI.3664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, Saraga F, Churchill JF, Statland JM, Travis KE, Skinner FK, McBain CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci. 2006;26:12325–12338. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RN, Tan HM, Fisahn A. Kv7/KCNQ channels control action potential phasing of pyramidal neurons during hippocampal gamma oscillations in vitro. J Neurosci. 2009;29:13353–13364. doi: 10.1523/JNEUROSCI.1463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Maljevic S, Wuttke TV, Lerche H. Nervous system KV7 disorders: breakdown of a subthreshold brake. J Physiol. 2008;586:1791–1801. doi: 10.1113/jphysiol.2008.150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CF, Bundros AM, Trimmer JS. Benefits and pitfalls of secondary antibodies: why choosing the right secondary is of primary importance. PLoS One. 2012;7:e38313. doi: 10.1371/journal.pone.0038313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli F, Cilio MR, Taglialatela M, Bezanilla F. Gating currents from neuronal K(V)7.4 channels: general features and correlation with the ionic conductance. Channels (Austin) 2009;3:274–283. [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Selyanko AA, Hadley JK, Brown DA, Dixon JE, McKinnon D. Alternative splicing of KCNQ2 potassium channel transcripts contributes to the functional diversity of M-currents. J Physiol. 2001;531:347–358. doi: 10.1111/j.1469-7793.2001.0347i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 6. Amsterdam: Academic; 2007. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Robbins J, Trouslard J, Marsh SJ, Brown DA. Kinetic and pharmacological properties of the M-current in rodent neuroblastoma x glioma hybrid cells. J Physiol. 1992;451:159–185. doi: 10.1113/jphysiol.1992.sp019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper J, Schwarz JR. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. J Physiol. 1989;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safiulina VF, Zacchi P, Taglialatela M, Yaari Y, Cherubini E. Low expression of Kv7/M channels facilitates intrinsic and network bursting in the developing rat hippocampus. J Physiol. 2008;586:5437–5453. doi: 10.1113/jphysiol.2008.156257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Kampe K, Vogel W. Single voltage-dependent potassium channels in rat peripheral nerve membrane. J Physiol. 1993;460:675–691. doi: 10.1113/jphysiol.1993.sp019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Neher E. Single-channel recording. Ed 2. New York: Springer; 2009. Chapter 21. [Google Scholar]
- Schmidt-Hieber C, Bischofberger J. Fast sodium channel gating supports localized and efficient axonal action potential initiation. J Neurosci. 2010;30:10233–10242. doi: 10.1523/JNEUROSCI.6335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Brown DA. M-channel gating and simulation. Biophys J. 1999;77:701–713. doi: 10.1016/S0006-3495(99)76925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Brown DA. Properties of single M-type KCNQ2/KCNQ3 potassium channels expressed in mammalian cells. J Physiol. 2001;534:15–24. doi: 10.1111/j.1469-7793.2001.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2008;105:7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MS, Roche JP, Kaftan EJ, Cruzblanca H, Mackie K, Hille B. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K(+) channels that underlie the neuronal M-current. J Neurosci. 2000;20:1710–1721. doi: 10.1523/JNEUROSCI.20-05-01710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci U S A. 2007;104:11453–11458. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittl R, Carr RW, Schwarz JR, Grafe P. The Kv7 potassium channel activator flupirtine affects clinical excitability parameters of myelinated axons in isolated rat sural nerve. J Peripher Nerv Syst. 2010;15:63–72. doi: 10.1111/j.1529-8027.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- Sloper JJ, Powell TP. A study of the axon initial segment and proximal axon of neurons in the primate motor and somatic sensory cortices. Philos Trans R Soc Lond, B, Biol Sci. 1979;285:173–197. doi: 10.1098/rstb.1979.0004. [DOI] [PubMed] [Google Scholar]
- Stys PK, Sontheimer H, Ransom BR, Waxman SG. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci U S A. 1993;90:6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972;52:563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Vervaeke K, Gu N, Agdestein C, Hu H, Storm JF. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. J Physiol. 2006;576:235–256. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Wang HS, Brown BS, McKinnon D, Cohen IS. Molecular basis for differential sensitivity of KCNQ and I(Ks) channels to the cognitive enhancer XE991. Mol Pharmacol. 2000;57:1218–1223. [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Stys PK. The axon. New York: Oxford UP; 1995. [Google Scholar]
- Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, Jordanova A, Ceulemans B, Jansen A, Hasaerts D, Roelens F, Lagae L, Yendle S, Stanley T, Heron SE, Mulley JC, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- Williams SR, Wozny C. Errors in the measurement of voltage-activated ion channels in cell-attached patch-clamp recordings. Nat Commun. 2011;2:242. doi: 10.1038/ncomms1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006;95:3480–3495. doi: 10.1152/jn.01333.2005. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bennett V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J Cell Biol. 1998;142:1571–1581. doi: 10.1083/jcb.142.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]