Abstract
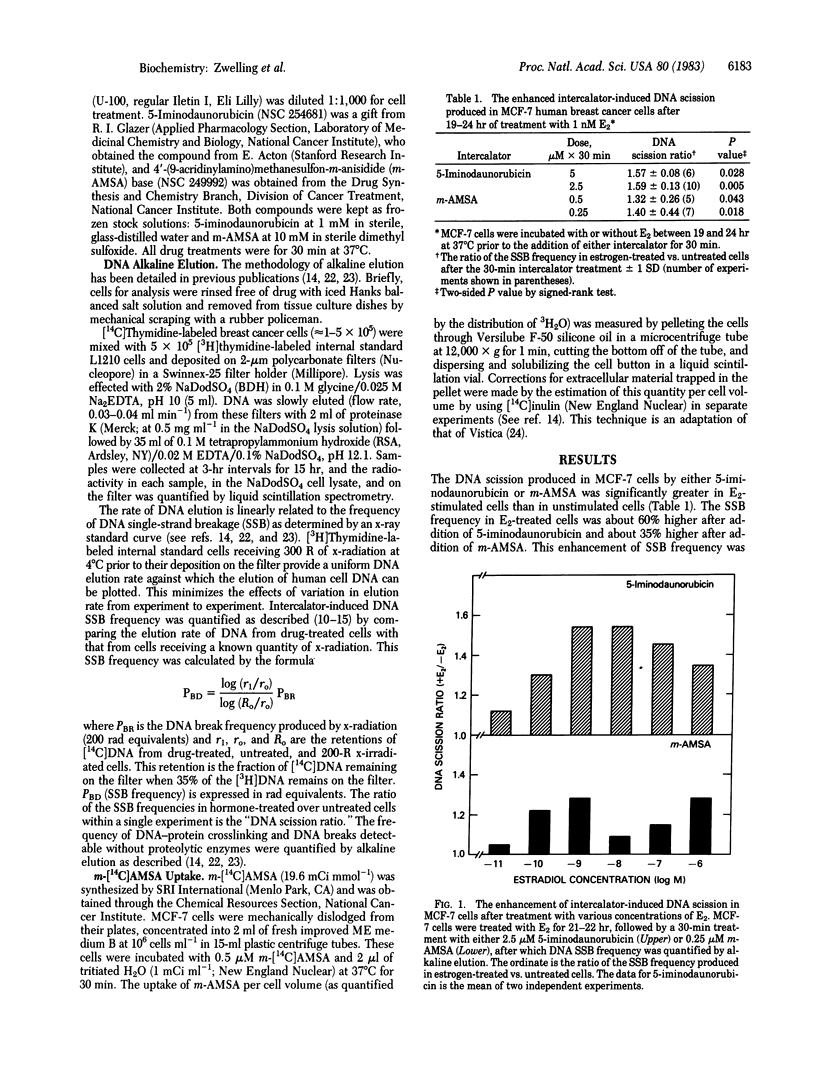
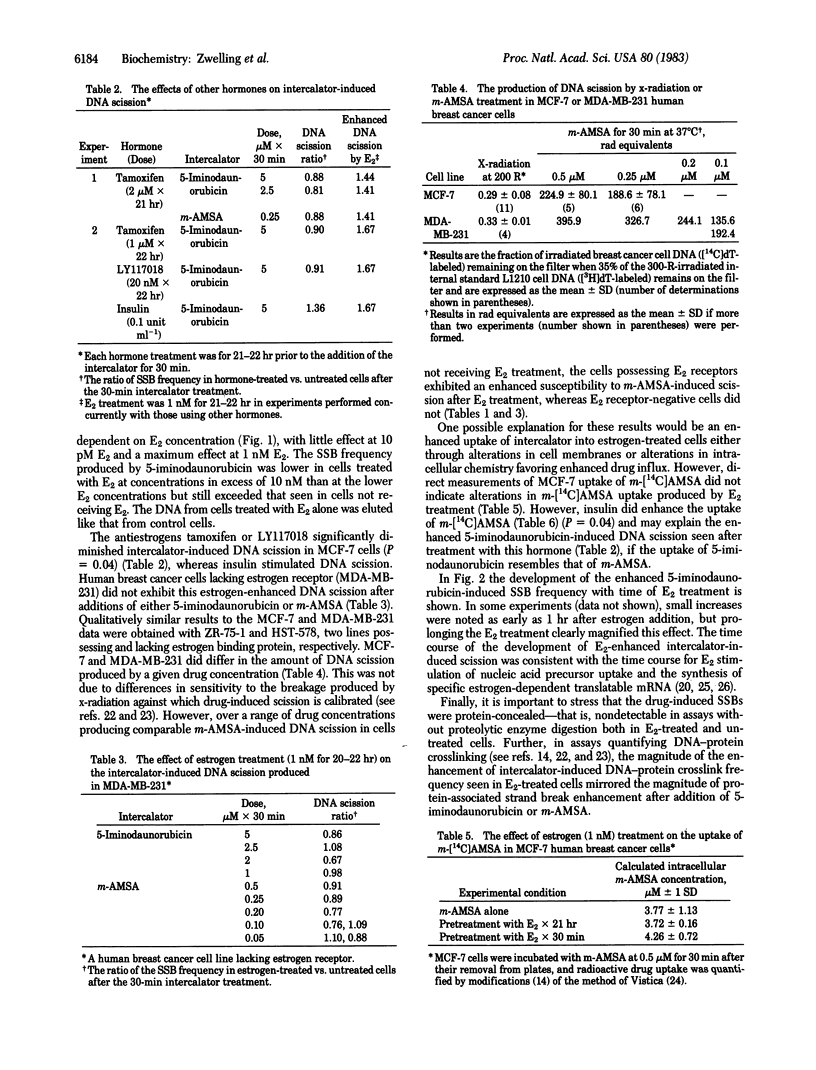
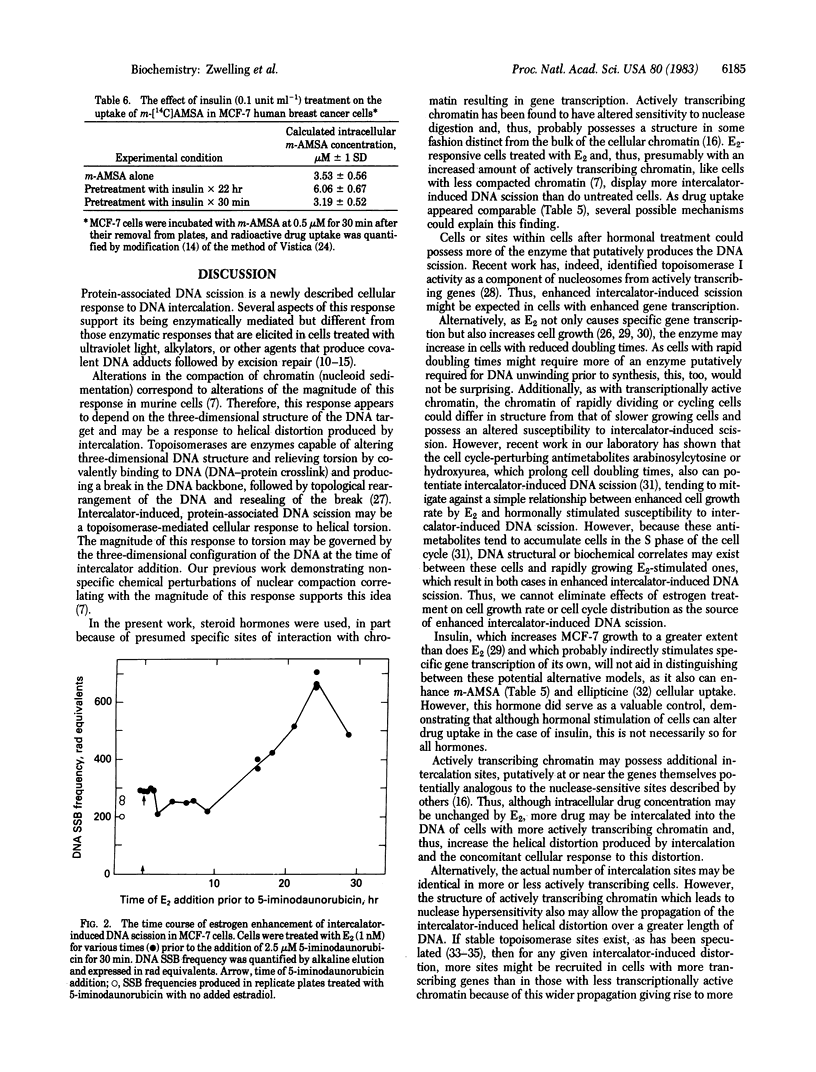
Estrogen-responsive human breast cancer cells (MCF-7) displayed a higher frequency of intercalator-induced protein-associated DNA scission after treatment with 17 beta-estradiol (E2) than did cells that had not received estrogen treatment. This effect was dependent on estrogen concentration (maximum enhancement at approximately equal to 1 nM E2) and time (maximum effect seen approximately equal to 24 hr after E2 addition). Human breast cancer cells lacking estrogen receptors did not display the enhanced response. Antiestrogens produced a slight decrease in intercalator-induced DNA scission, whereas insulin produced an enhanced effect. The DNA breaks produced by the intercalators 5-iminodaunorubicin and 4'-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA) in these cells were undetectable without enzymatic deproteinization of cell lysates prior to quantification by alkaline elution. Intercalator-induced DNA-protein crosslinking also was enhanced in E2-treated MCF-7 cells. Studies with m-[14C]AMSA revealed no estrogen-associated increases in drug uptake. The data suggest that E2 treatment, either by specifically and directly increasing active transcription in chromatin or through secondary effects on DNA that accompany alterations in cell growth or cell cycle distribution, alters the susceptibility of DNA to intercalator-induced protein-associated DNA scission. If this enhanced protein-associated scission is selectively localized to transcriptionally active chromatin, the adsorption of the DNA-bound proteins to membrane filters (DNA-protein crosslinking) may allow identification and isolation of estrogen-regulated gene sequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken S. C., Lippman M. E. Hormonal regulation of net DNA synthesis in MCF-7 human breast cancer cells in tissue culture. Cancer Res. 1982 May;42(5):1727–1735. [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and its coupling to DNA replication in eukaryotic cells. Biochim Biophys Acta. 1978 Dec 11;516(4):489–516. doi: 10.1016/0304-419x(78)90020-3. [DOI] [PubMed] [Google Scholar]
- Cohen M. M., Simpson S. J., Pazos L. Specificity of bleomycin-induced cytotoxic effects on ataxia telangiectasia lymphoid cell lines. Cancer Res. 1981 May;41(5):1817–1823. [PubMed] [Google Scholar]
- Cramer P., Painter R. B. Bleomycin-resistant DNA synthesis in ataxia telangiectasia cells. Nature. 1981 Jun 25;291(5817):671–672. doi: 10.1038/291671a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. P., Adams D. J., McGuire W. L. Specific protein synthesis regulated by estrogen in human breast cancer. J Steroid Biochem. 1981 Dec;15:247–259. doi: 10.1016/0022-4731(81)90281-8. [DOI] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Fisher L. M., Mizuuchi K., O'Dea M. H., Ohmori H., Gellert M. Site-specific interaction of DNA gyrase with DNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4165–4169. doi: 10.1073/pnas.78.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Ewig R. A. Effect of pH on the bleomycin-induced DNA single-strand scission in L1210 cells and the relation to cell survival. Cancer Res. 1976 Oct;36(10):3839–3841. [PubMed] [Google Scholar]
- Morrison A., Cozzarelli N. R. Site-specific cleavage of DNA by E. coli DNA gyrase. Cell. 1979 May;17(1):175–184. doi: 10.1016/0092-8674(79)90305-2. [DOI] [PubMed] [Google Scholar]
- Morrison A., Higgins N. P., Cozzarelli N. R. Interaction between DNA gyrase and its cleavage site on DNA. J Biol Chem. 1980 Mar 10;255(5):2211–2219. [PubMed] [Google Scholar]
- Nickol J., Behe M., Felsenfeld G. Effect of the B--Z transition in poly(dG-m5dC) . poly(dG-m5dC) on nucleosome formation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1771–1775. doi: 10.1073/pnas.79.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne C. K., Bolan G., Monaco M. E., Lippman M. E. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4536–4540. doi: 10.1073/pnas.73.12.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster J. B., Creasey W. A. Enhancement of cellular uptake of ellipticine by insulin preincubation. Eur J Cancer Clin Oncol. 1981 Oct;17(10):1097–1103. doi: 10.1016/0014-2964(81)90294-2. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Schwartz R., Zwelling L. A. The formation and resealing of intercalator-induced DNA strand breaks in isolated L1210 cell nuclei. Biochem Biophys Res Commun. 1982 Jul 30;107(2):576–583. doi: 10.1016/0006-291x(82)91530-3. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D. L., Kohn K. W. Protein-associated DNA breaks in cells treated with adriamycin or ellipticine. Biochim Biophys Acta. 1978 Jun 22;519(1):23–30. doi: 10.1016/0005-2787(78)90059-x. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D., Kohn K. W. Qualitative and quantitative aspects of intercalator-induced DNA strand breaks. Biochim Biophys Acta. 1979 Mar 28;562(1):41–50. doi: 10.1016/0005-2787(79)90124-2. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Rosney C. M., Campbell J. B. Unusual sensitivity of ataxia telangiectasia cells to bleomycin. Cancer Res. 1979 Mar;39(3):1046–1050. [PubMed] [Google Scholar]
- Vistica D. T. Cytotoxicity as an indicator for transport mechanism: evidence that melphalan is transported by two leucine-preferring carrier systems in the L1210 murine leukemia cell. Biochim Biophys Acta. 1979 Jan 19;550(2):309–317. doi: 10.1016/0005-2736(79)90217-7. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. T. Properties of active nucleosomes as revealed by HMG 14 and 17 chromatography. Nucleic Acids Res. 1982 Mar 25;10(6):2017–2042. doi: 10.1093/nar/10.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Kerrigan D., Michaels S. Cytotoxicity and DNA strand breaks by 5-iminodaunorubicin in mouse leukemia L1210 cells: comparison with adriamycin and 4'-(9-acridinylamino)methanesulfon-m-anisidide. Cancer Res. 1982 Jul;42(7):2687–2691. [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Kerrigan D., Pommier Y., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks produced in mouse leukemia L1210 cells by ellipticine and 2-methyl-9-hydroxyellipticinium. Biochem Pharmacol. 1982 Oct 15;31(20):3261–3267. doi: 10.1016/0006-2952(82)90560-3. [DOI] [PubMed] [Google Scholar]


