Abstract
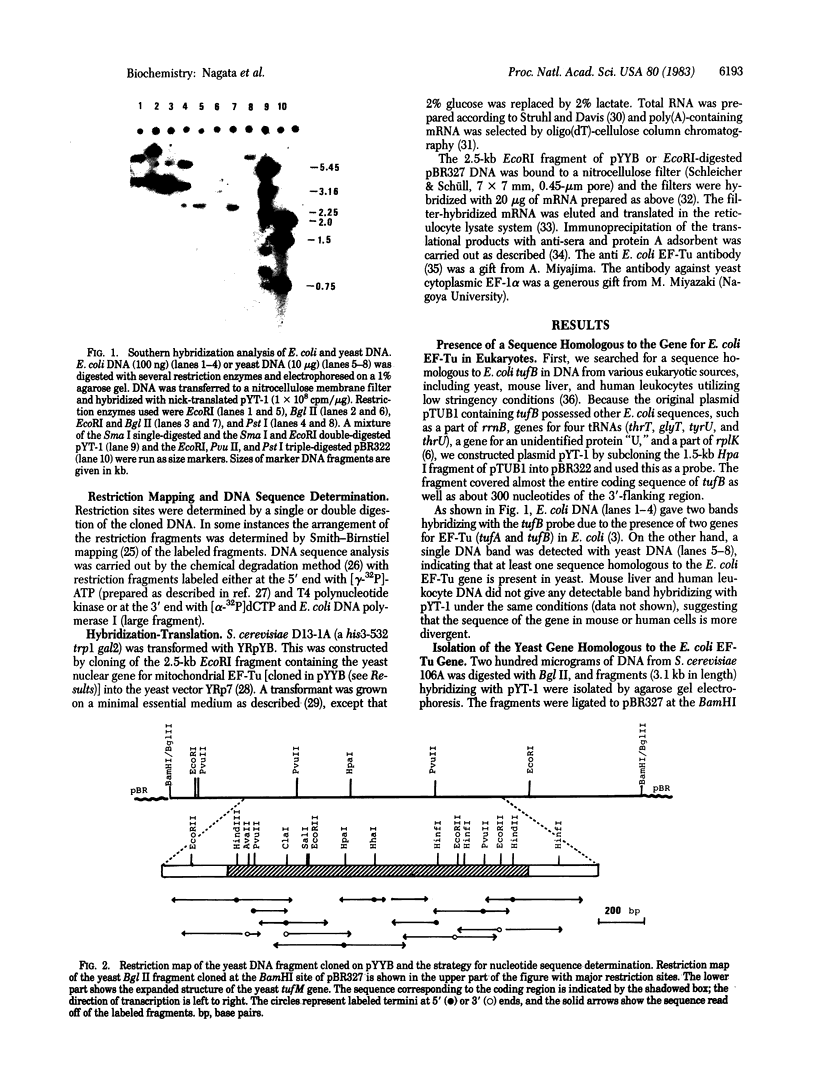
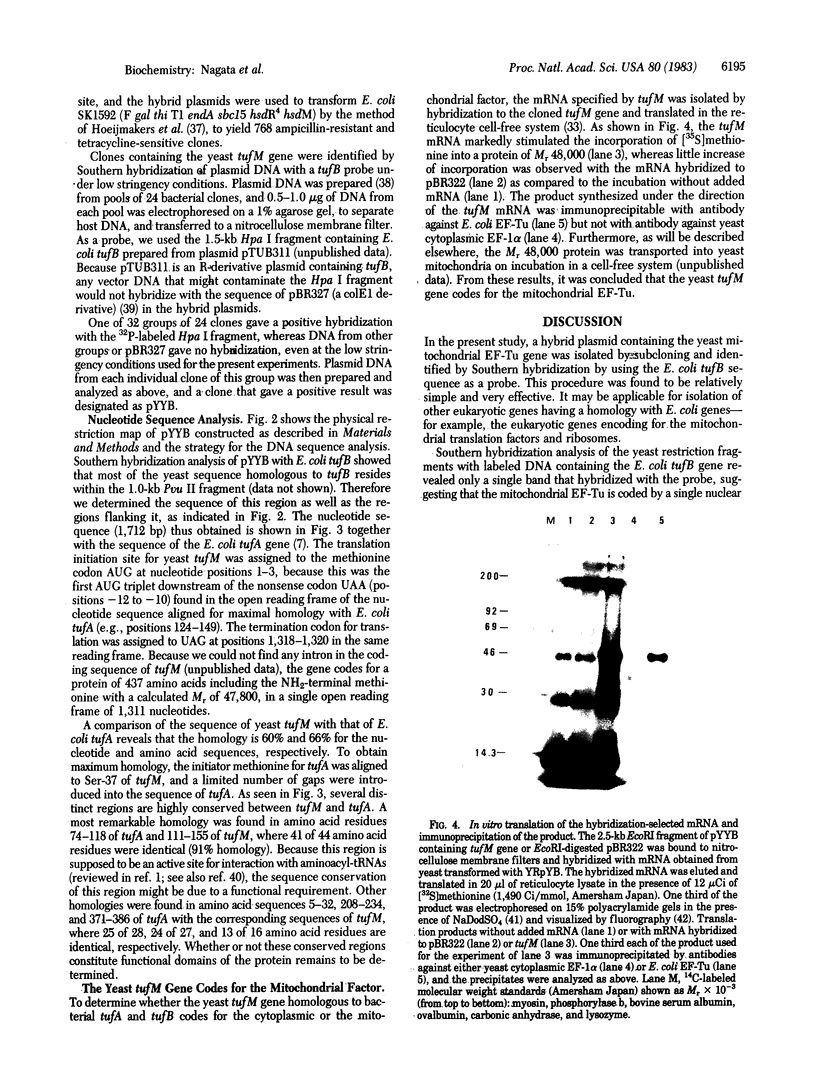
A 3.1-kilobase Bgl II fragment of Saccharomyces cerevisiae carrying the nuclear gene encoding the mitochondrial polypeptide chain elongation factor (EF) Tu has been cloned on pBR327 to yield a chimeric plasmid pYYB. The identification of the gene designated as tufM was based on the cross-hybridization with the Escherichia coli tufB gene, under low stringency conditions. The complete nucleotide sequence of the yeast tufM gene was established together with its 5'- and 3'-flanking regions. The sequence contained 1,311 nucleotides coding for a protein of 437 amino acids with a calculated Mr of 47,980. The nucleotide sequence and the deduced amino acid sequence of tufM were 60% and 66% homologous, respectively, to the corresponding sequences of E. coli tufA, when aligned to obtain the maximal homology. Plasmid YRpYB was then constructed by cloning the 2.5-kilobase EcoRI fragment of pYYB carrying tufM into a yeast cloning vector YRp-7. A mRNA hybridizable with tufM was isolated from the total mRNA of S. cerevisiae D13-1A transformed with YRpYB and translated in the reticulocyte lysate. The mRNA could direct the synthesis of a protein with Mr 48,000, which was immunoprecipitated with an anti-E. coli EF-Tu antibody but not with an antibody against yeast cytoplasmic EF-1 alpha. The results indicate that the tufM gene is a nuclear gene coding for the yeast mitochondrial EF-Tu.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Pluijms W., Roobol K., Möller W. Sequence homology between EF-1 alpha, the alpha-chain of elongation factor 1 from Artemia salina and elongation factor EF-Tu from Escherichia coli. FEBS Lett. 1983 Mar 7;153(1):37–42. doi: 10.1016/0014-5793(83)80115-x. [DOI] [PubMed] [Google Scholar]
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Arai K., Clark B. F., Duffy L., Jones M. D., Kaziro Y., Laursen R. A., L'Italien J., Miller D. L., Nagarkatti S., Nakamura S. Primary structure of elongation factor Tu from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1326–1330. doi: 10.1073/pnas.77.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollini R., Soffientini A. N., Bertani A., Lanzani G. A. Some molecular properties of the elongation factor EF1 from wheat embryos. Biochemistry. 1974 Dec 17;13(26):5421–5425. doi: 10.1021/bi00723a028. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra B., Skogerson L., Chakraburtty K. Protein synthesis in yeast. II. Purification and properties of the elongation factor 1 from Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):10005–10011. [PubMed] [Google Scholar]
- Harpold M. M., Dobner P. R., Evans R. M., Bancroft F. C. Construction and identification by positive hybridization-translation of a bacterial plasmid containing a rat growth hormone structural gene sequence. Nucleic Acids Res. 1978 Jun;5(6):2039–2053. doi: 10.1093/nar/5.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzeman R. A., Hagie F. E., Hayflick J. S., Chen C. Y., Seeburg P. H., Derynck R. The primary structure of the Saccharomyces cerevisiae gene for 3-phosphoglycerate kinase. Nucleic Acids Res. 1982 Dec 11;10(23):7791–7808. doi: 10.1093/nar/10.23.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Kaziro Y. Coordination of levels of elongation factors Tu, Ts, and G, and ribosomal protein SI in Escherichia coli. J Biochem. 1978 Feb;83(2):453–462. doi: 10.1093/oxfordjournals.jbchem.a131932. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Shibuya M., Kaziro Y. Construction and characterization of the two hybrid Co1E1 plasmids carrying Escherichia coli tufB gene. FEBS Lett. 1979 Jun 15;102(2):207–210. doi: 10.1016/0014-5793(79)80001-0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Iwasaki K., Kaziro Y. Purification and properties of polypeptide chain elongation factor-1alpha from pig liver. J Biochem. 1977 Dec;82(6):1633–1646. doi: 10.1093/oxfordjournals.jbchem.a131859. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kaziro Y. Selective photooxidation of histidine residues in polypeptide chain elongation factor Tu from E. coli. J Biochem. 1981 Oct;90(4):1117–1124. doi: 10.1093/oxfordjournals.jbchem.a133563. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Piechulla B., Küntzel H. Mitochondrial polypeptide elongation factor EF-Tu of Saccharomyces cerevisiae. Functional and structural homologies to Escherichia coli EF-Tu. Eur J Biochem. 1983 May 2;132(2):235–240. doi: 10.1111/j.1432-1033.1983.tb07353.x. [DOI] [PubMed] [Google Scholar]
- Richter D., Lipmann F. Separation of mitochondrial and cytoplasmic peptide chain elongation factors from yeast. Biochemistry. 1970 Dec 22;9(26):5065–5070. doi: 10.1021/bi00828a004. [DOI] [PubMed] [Google Scholar]
- Richter D. Production of mitochondrial peptide-chain elongation factors in yeast deficient in mitochondrial deoxyribonucleic acid. Biochemistry. 1971 Nov 23;10(24):4422–4425. doi: 10.1021/bi00800a011. [DOI] [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Shaw G. D., Boll W., Taira H., Mantei N., Lengyel P., Weissmann C. Structure and expression of cloned murine IFN-alpha genes. Nucleic Acids Res. 1983 Feb 11;11(3):555–573. doi: 10.1093/nar/11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Nashimoto H., Kaziro Y. Cloning of an EcoRI fragment carrying E. coli tufA gene. Mol Gen Genet. 1979 Feb 26;170(2):231–234. doi: 10.1007/BF00337801. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Clark R. V., Olson M. O. Functional and structural studies on a tryptic fragment of eucaryotic elongation factor Tu from rabbit reticulocytes. Biochemistry. 1981 Sep 29;20(20):5761–5767. doi: 10.1021/bi00523a019. [DOI] [PubMed] [Google Scholar]
- Slobin L. I. The role of eucaryotic factor Tu in protein synthesis. The measurement of the elongation factor Tu content of rabbit reticulocytes and other mammalian cells by a sensitive radioimmunoassay. Eur J Biochem. 1980 Sep;110(2):555–563. doi: 10.1111/j.1432-1033.1980.tb04898.x. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Transcription of the his3 gene region in Saccharomyces cerevisiae. J Mol Biol. 1981 Nov 5;152(3):535–552. doi: 10.1016/0022-2836(81)90267-9. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]