Abstract
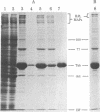
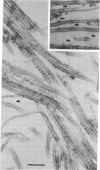
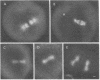
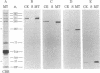
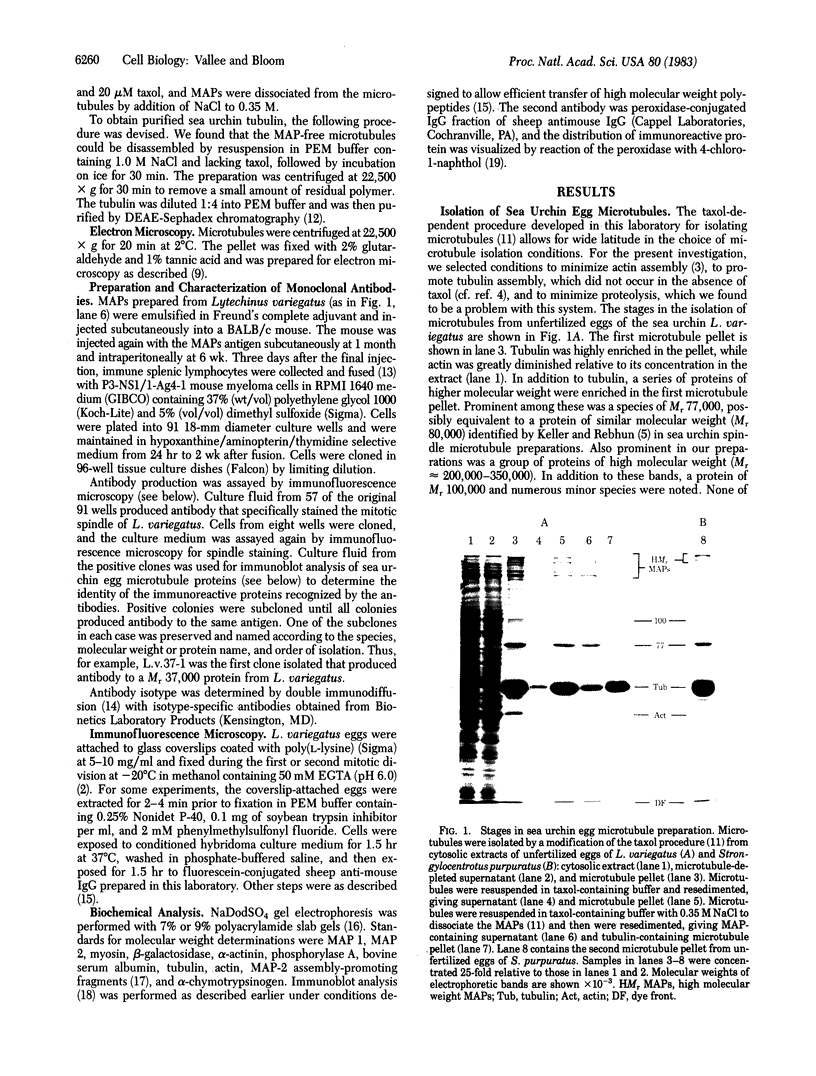
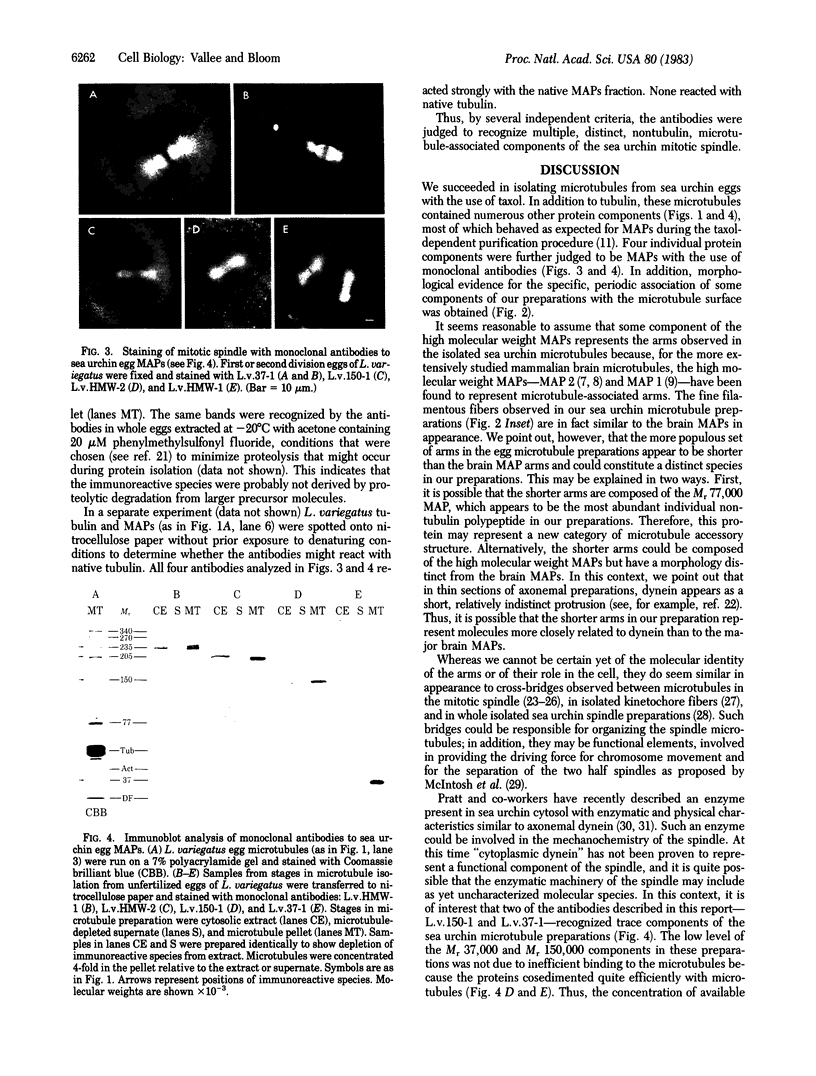
Microtubules were isolated from unfertilized eggs of the sea urchin with the use of the anti-tumor drug taxol. In addition to tubulin, prominent high molecular weight (Mr 205,000-350,000) microtubule-associated proteins (MAPs) were identified as well as MAP species of Mrs 77,000, 100,000, and 120,000. The microtubules were covered with both short periodic arms and longer filamentous arms, both classes of which appeared to crosslink the microtubules into bundles. Monoclonal antibodies were prepared to an unfractionated MAPs preparation. We isolated clonal hybridoma lines producing antibodies to tubulin and to four non-tubulin proteins of Mrs 235,000, 205,000, 150,000, and 37,000. All antibodies strongly and specifically stained the mitotic spindle of dividing sea urchin eggs. All four of the immunoreactive, non-tubulin species behaved as MAPs during microtubule isolation. Thus, we have identified a variety of sea urchin MAPs by biochemical, ultrastructural, and immunochemical means. The immunochemical experiments demonstrated that four of these proteins are microtubule-associated components of the mitotic spindle. We suggest that those proteins that we observed as cross-bridges between the isolated microtublules may be either structural or functional components of the spindle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AFZELIUS B. Electron microscopy of the sperm tail; results obtained with a new fixative. J Biophys Biochem Cytol. 1959 Mar 25;5(2):269–278. doi: 10.1083/jcb.5.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Vallee R. B. Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J Cell Biol. 1983 Jun;96(6):1523–1531. doi: 10.1083/jcb.96.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Cartwright J., Jr Ultrastructural analysis of mitotic spindle elongation in mammalian cells in vitro. Direct microtubule counts. J Cell Biol. 1971 Aug;50(2):416–431. doi: 10.1083/jcb.50.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C. L., Lockwood A. H., Su J. L., Beavo J. A., Steiner A. L. Immunofluorescent localization of cyclic nucleotide-dependent protein kinases on the mitotic apparatus of cultured cells. J Cell Biol. 1980 Nov;87(2 Pt 1):336–345. doi: 10.1083/jcb.87.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Immunofluorescence localization of HeLa cell microtubule-associated proteins on microtubules in vitro and in vivo. J Cell Biol. 1980 Dec;87(3 Pt 1):792–801. doi: 10.1083/jcb.87.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Cleveland D. W., Kirschner M. W. Immunoflourescent staining of cytoplasmic and spindle microtubules in mouse fibroblasts with antibody to tau protein. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2437–2440. doi: 10.1073/pnas.74.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Harris P., Osborn M., Weber K. Distribution of tubulin-containing structures in the egg of the sea urchin Strongylocentrotus purpuratus from fertilization through first cleavage. J Cell Biol. 1980 Mar;84(3):668–679. doi: 10.1083/jcb.84.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Inoué S., Ritter H., Jr Dynamics of mitotic spindle organization and function. Soc Gen Physiol Ser. 1975;30:3–30. [PubMed] [Google Scholar]
- Izant J. G., Weatherbee J. A., McIntosh J. R. A microtubule-associated protein antigen unique to mitotic spindle microtubules in PtK1 cells. J Cell Biol. 1983 Feb;96(2):424–434. doi: 10.1083/jcb.96.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weatherbee J. A., McIntosh J. R. A microtubule-associated protein in the mitotic spindle and the interphase nucleus. Nature. 1982 Jan 21;295(5846):248–250. doi: 10.1038/295248a0. [DOI] [PubMed] [Google Scholar]
- Kane R. E. Preparation and purification of polymerized actin from sea urchin egg extracts. J Cell Biol. 1975 Aug;66(2):305–315. doi: 10.1083/jcb.66.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Rebhun L. I. Strongylocentrotus purpuratus spindle tubulin. I. Characteristics of its polymerization and depolymerization in vitro. J Cell Biol. 1982 Jun;93(3):788–796. doi: 10.1083/jcb.93.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R. In vitro polymerization of marine egg tubulin into microtubules. J Biochem. 1977 Apr;81(4):1115–1125. doi: 10.1093/oxfordjournals.jbchem.a131536. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIntosh J. R. Bridges between microtubules. J Cell Biol. 1974 Apr;61(1):166–187. doi: 10.1083/jcb.61.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B. Identification of microtubule-associated proteins in the meiotic spindle of surf clam oocytes. J Cell Biol. 1980 Feb;84(2):235–245. doi: 10.1083/jcb.84.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M. M., Otter T., Salmon E. D. Dynein-like Mg2+-ATPase in mitotic spindles isolated from sea urchin embryos (Strongylocentrotus droebachiensis). J Cell Biol. 1980 Sep;86(3):738–745. doi: 10.1083/jcb.86.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M. M. The identification of a dynein ATPase in unfertilized sea urchin eggs. Dev Biol. 1980 Feb;74(2):364–378. doi: 10.1016/0012-1606(80)90438-8. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Segall R. R. Calcium-labile mitotic spindles isolated from sea urchin eggs (Lytechinus variegatus). J Cell Biol. 1980 Aug;86(2):355–365. doi: 10.1083/jcb.86.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982 Mar 25;257(6):3284–3290. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem. 1978 Apr 25;253(8):2834–2845. [PubMed] [Google Scholar]
- Vallee R. B., Davis S. E. Low molecular weight microtubule-associated proteins are light chains of microtubule-associated protein 1 (MAP 1). Proc Natl Acad Sci U S A. 1983 Mar;80(5):1342–1346. doi: 10.1073/pnas.80.5.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B. MAP2 (microtubule-associated protein 2). Cell Muscle Motil. 1984;5:289–311. doi: 10.1007/978-1-4684-4592-3_8. [DOI] [PubMed] [Google Scholar]
- Vallee R. Structure and phosphorylation of microtubule-associated protein 2 (MAP 2). Proc Natl Acad Sci U S A. 1980 Jun;77(6):3206–3210. doi: 10.1073/pnas.77.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Calcium-dependent regulator protein: localization in mitotic apparatus of eukaryotic cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1867–1871. doi: 10.1073/pnas.75.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H. J. Arms and bridges on microtubules in the mitotic apparatus. J Cell Biol. 1969 Mar;40(3):854–859. doi: 10.1083/jcb.40.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B., Plummer J., Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol. 1978 Mar;76(3):729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt P. L., Ris H., Borisy G. G. Structure of kinetochore fibers: microtubule continuity and inter-microtubule bridges. Chromosoma. 1981;83(4):523–540. doi: 10.1007/BF00328277. [DOI] [PubMed] [Google Scholar]
- Zieve G., Solomon F. Proteins specifically associated with the microtubules of the mammalian mitotic spindle. Cell. 1982 Feb;28(2):233–242. doi: 10.1016/0092-8674(82)90341-5. [DOI] [PubMed] [Google Scholar]