SYNOPSIS
Correcting ineffective erythropoiesis and iron dysregulation by regulating hepcidin expression
Unbalanced hemoglobin α- and β-chain expression in the thalassemias, results in anemia, extramedullary hematopoiesis and ineffective erythropoiesis leading to secondary iron overload even in the absence of transfusion therapy. Severe, chronic ineffective erythropoiesis also leads to iron overload in other anemias, including the dyserythropoietic and sideroblastic anemias. Hereditary hemochromatosis (HH) is a group of human genetic disorders that share the common pathophysiology of an incrementally inappropriate increase in dietary iron uptake, leading to progressive iron overload over a period of years. In all cases, the iron overload may eventuate in toxic levels of iron in the liver, heart and endocrine tissues, leading to a multiplicity of complications, including organ failure. Despite the diversity of the underlying diseases, in each case, the iron overload is a direct effect of the dysregulation of hepcidin, the hormonal negative regulator of iron absorption by the intestine that is produced in the liver. In this article, we will discuss new approaches to treating iron overload diseases such as these, using hepcidin mimetics or by modulating endogenous hepcidin expression. In particular, we will discuss lipid nanoparticle (LNP) encapsulated siRNA and antisense oligonucleotide (ASO)-mediated inhibition of TMPRSS6, an upstream regulator of hepcidin, and treatment with transferrin or hepcidin mimetics, including the recently described “minihepcidins.” In each case, in animal models of β-thalassemia, not only do the interventions affect iron absorption, but they also act as disease-modifying agents that ameliorate the ineffective erythropoiesis inciting iron metabolism dysreguation in the first place.
Keywords: Iron metabolism, Ineffective erythropoiesis, Hereditary hemochromatosis, β–Thalassemia, Hepcidin/Minihepcidins, Lipid nanoparticle siRNA/antisense oligonucleotide
INTRODUCTION TO IRON METABOLISM
Because iron is highly toxic when present in excess, mammals have evolved elaborate mechanisms for the regulation of iron acquisition, transport, storage and utilization. A typical adult human is endowed with approximately 4g of iron, almost two-thirds of which is distributed in hemoglobin in red blood cells (RBCs). Nearly 25mg of iron is required to support erythropoiesis each day, but most of the iron required for erythropoiesis derives from recycling of iron from effete RBCs by macrophages of the reticuloendothelial system. At a steady state, only 1–2mg of iron is absorbed each day from the diet, and that only to offset iron losses, which are not regulated, and limited to physiological and non-physiological epithelial cell (e.g., skin and intestine) or blood loss. Accordingly, total body iron is regulated entirely at the level of intestinal absorption, which can be modulated according to the body’s needs.
The Hepcidin-Ferroportin Iron Regulatory Axis
Hepcidin is a peptide hormone produced predominately by the liver in response to iron stores.1 As iron levels increase, so does hepcidin,2,3 which, as a negative regulator of iron release from cells, binds to and causes the internalization and degradation of ferroportin (FPN1), the only known iron exporter.4 FPN1 is expressed in abundance on macrophages and duodenal enterocytes, the cells that are directly responsible for iron recycling from senescent RBCs and for iron absorption from the intestine (Figure 1). Thus, hepcidin production simultaneously leads to decreased intestinal iron absorption and sequestration of iron in macrophages, limiting its availability for erythropoiesis. Conversely, decreasing hepcidin expression permits more non-heme iron to be taken up from the diet and released from internal stores. A failure of this “stores regulator” of systemic iron metabolism5 underlies the pathophysiology of most forms of hereditary hemochromatosis (see below).
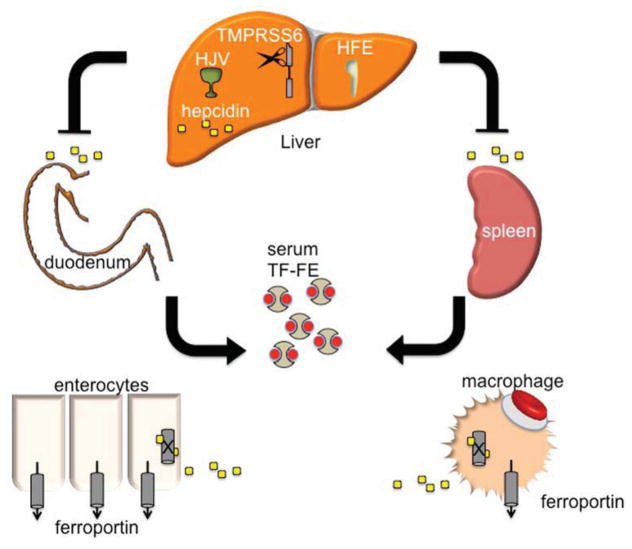
Figure 1. The role of the liver and hepcidin in iron regulation.
Hepcidin, produced in hepatocytes of the liver, is a soluble regulator of iron metabolism. HFE and HJV (hemojuvelin) are necessary for appropriate sensing of TF (transferrin) saturation, and consequently, total body iron burden. TMPRSS6 is a membrane-bound serine-protease thought to regulate HJV function by cleaving the BMP co-receptor HJV at the cell membrane. Upon release from the liver, hepcidin binds to ferroportin on the surface of duodenal enterocytes and macrophages responsible for recycling iron from RBCs, leading to internalization and degradation of the iron transporter FPN1 and diminution of transferrin-bound iron (TF-Fe) in the serum.
In addition to systemic iron deficiency, hepcidin expression is also suppressed by anemia and hypoxia.6 Anemias characterized by ineffective erythropoiesis—bone marrow erythroid hyperplasia with premature, intramedullary death of maturing erythroblasts—appear to uniquely potently suppress hepcidin production even in the presence of systemic iron overload.7 The factor or factors that communicate this signal from the bone marrow to the liver to suppress hepcidin have been termed the “erythroid regulator” of iron metabolism.5 It is the apparent supremacy of the erythroid regulator compared to the stores regulator that underlies the pathogenesis of iron overload in “iron-loading anemias” such as β-thalassemia intermedia, which are characterized by ineffective erythropoiesis. Importantly, in these anemias, as well as in HH, the regulatory dysfunction leading to iron overload is a relative if not absolute deficiency in hepcidin for the degree of iron overload. It is on this theoretical basis that upregulation of hepcidin has been envisioned as a means to treat iron overload in these apparently diverse diseases.
Iron-Responsive Hepcidin Expression by the Hepatocyte
It is now evident that the autosomal recessive forms of hereditary hemochromatosis due to mutations in HFE, HJV, or TFR2 result from a disruption of the hepatocyte’s ability to translate systemic iron stores and availability for erythropoiesis represented by the transferrin saturation (or concentration of diferric transferrin) into a signal that promotes hepcidin gene transcription.8–10 In this way, they are thought to disrupt the “stores regulator” of systemic iron homeostasis. This pathway has been reviewed comprehensively elsewhere.11–13 Only elements that are fundamental to the therapeutic innovations discussed below are highlighted here.
There is strong evidence that the bone morphogenetic protein (BMP)-sons of mothers against decapentaplegic (SMAD) signaling pathway plays a key role in the regulation of hepcidin and systemic iron metabolism (Figure 2). Hemojuvelin (HJV), which is mutated in patients with a severe, juvenile onset from of HH,9 is a BMP co-receptor protein14 that facilitates signaling through the BMP type I receptors (BMPRIs) ALK2 and ALK315,16 in response to BMP6,17,18 which is itself upregulated in the liver by iron. Activated BMP receptors phosphorylate SMADS1, 5, and 8, which in turn phosphorylate SMAD4, which translocates to the nucleus, stimulating transcription by binding to a BMP-response element (BRE) in the hepcidin promoter.
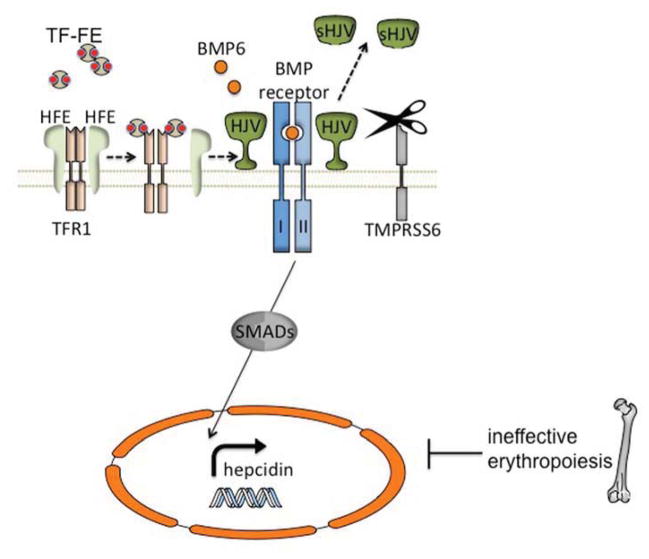
Figure 2. Involvement of the BMP/HJV/SMAD signaling pathway in the regulation of hepatocyte-generated hepcidin.
Hepcidin expression is increased in response to elevated iron and decreased due to ineffective erythropoiesis, such as is found in β-thalassemia intermedia. Mutations in the classic hereditary hemochromatosis gene (HFE) and hemojuvelin (HJV) cause hereditary hemochromatosis. HJV, a bone morphogenetic protein (BMP) co-receptor, plays a central role in hepcidin regulation through a SMAD signaling cascade. BMP6 expression increases with elevated iron conditions and acts as a ligand, binding to HJV and initiating hepcidin expression. Diferric transferrin (TF-Fe) displaces HFE from transferrin receptor-1 (TFR1) likely leading to interaction with the HJV/BMP receptor complex, or a possibly separate signaling pathway. The membrane-bound serine protease TMPRSS6 cleaves HJV from the cell surface, forming a soluble protein (sHJV).
While juvenile hemochromatosis is rare, mutations in HFE account for the vast majority of patients with HH in the western hemisphere.19 Early work demonstrated that HFE interacts with the transferrin receptor (TFRC or TFR1) in a manner that can be competitively inhibited by diferric transferrin binding to TFR1.20,21 A second transferrin receptor, TFR2,22 mutated in a fraction of HH patients,23 also interacts with HFE24,25 in vitro, but it associates with diferric transferrin only very poorly. In this way, there would appear to be a means for hepatocytes to “sense” the amount of iron in the plasma. It is a matter of debate whether TFR2, HFE, or both directly interact with the HJV-BMPR complex,26,27 however, inactivation of either protein diminishes the activation of the downstream SMADS as well as hepcidin transcription in response to iron.
Mutations in any of the HH proteins or certain components of the BMP-SMAD signaling cascade in hepatocytes in model organisms lead to hyporesponsiveness of hepcidin transcription in response to iron. In contrast, mutations in TMPRSS6, a membrane associated protease expressed solely in the liver, cause the opposite phenotype: excessively high hepcidin levels in response to a given iron status, and the clinical phenotype of congenital iron deficiency.28–31 Work in vitro suggests that TMPRSS6 regulates HJV protein levels at the cell membrane by cleaving it to generate a soluble form (sHJV, Figure 2).32,33 Interestingly, it appears that iron, BMP-6 and hypoxia are all able to induce TMPRSS6 transcription in vivo.34,35 In toto, TMPRSS6 activity and expression are coordinated to inhibit hepcidin transcription mediated by the BMP-SMAD pathway, and in doing so may ordinarily help to prevent excessive hepcidin induction and prevent iron deficiency.
PRECLINICAL INVESTIGATION OF HEPCIDIN MIMETIC AND HEPCIDIN-INDUCTION THERAPIES IN MURINE MODELS OF HEREDITARY HEMOCHROMATOSIS
As described above, because of the relative hepcidin deficiency seen in both HH and in the iron loading anemias associated with ineffective erythropoiesis, manipulation of hepcidin levels either through exogenous administration or endogenous stimulation has been envisioned as a potential pharmacological approach to these disorders (Box 1). In many ways, because of the safety, efficacy, and inexpense of phlebotomy for the treatment of HH, many have seen using animal models of HH as proof of principle for these therapies as a prelude to the more complicated secondary hepcidin suppression seen in the iron-loading anemias.
Box 1. Experimental pre-clinical models focused on repressing iron absorption and utilization.
| Genetic ablation of Tmprss6 (Tmprss6−/−)43,57 | Murine β-thalassemia intermedia (Hbbth3/+), murine hereditary hemochromatosis (Hfe−/−) |
| Transferrin (Tf) therapy54 | Murine β-thalassemia intermedia (Hbbth1/th1) |
| Transgenic overexpression of hepcidin (Hamp1)36,55 | Murine β-thalassemia intermedia (Hbbth3/+) and murine hereditary hemochromatosis (Hfe−/−) |
| Dietary iron restriction55 | Murine β-thalassemia intermedia (Hbbth3/+) |
| Pharmacologic repression of Tmprss6 expression (siRNA, ASO) | Murine β-thalassemia intermedia (Hbbth3/+), murine hereditary hemochromatosis (Hfe−/−) |
| Treatment with hepcidin mimetic (mini-hepcidin)48,49 | Murine juvenile hereditary hemochromatosis (Hamp1−/−) |
| BMP6 therapy41 | Murine hereditary hemochromatosis (Hfe−/−) |
Transgenic hepcidin overexpression in HFE HH
Hepcidin therapy was first attempted by Nicolas, et al, in a murine model of HH.36 This group showed that Hfe−/− animals,37 have inappropriately low hepcidin expression that does not change as the animals age and load iron (Figure 3A). Earlier work in this laboratory had generated a transgenic animal that overexpressed the mouse hepcidin gene under control of the liver-specific transthyretin promoter.38 These transgenic animals are extremely pale, have diminished whole body iron stores and a severe hypochromic, microcytic anemia, and, on a C57BL/6 background, die within a few hours of birth. Transgenic mice on a mixed 129Sv; C57BL/6 background are viable, and the anemia eventually subsides as the endogenous hepcidin normalizes approximately nine weeks of life. More severely affected founder animals require treatment with exogenous iron to survive past weaning. As one would expect, overexpression of hepcidin in Hfe−/− animals (Figure 3B) greatly decreased iron loading in whole embryos and also in the livers of one and two month old HH animals.36 In fact, in many animals the correction of the hepcidin deficiency was too great, leading to iron deficiency anemia. In toto, this work was the proof in principle for hepcidin therapy in iron overload diseases, but it equally illustrated the potential complications of an inability to titer the therapy to the physiological state of the animal.
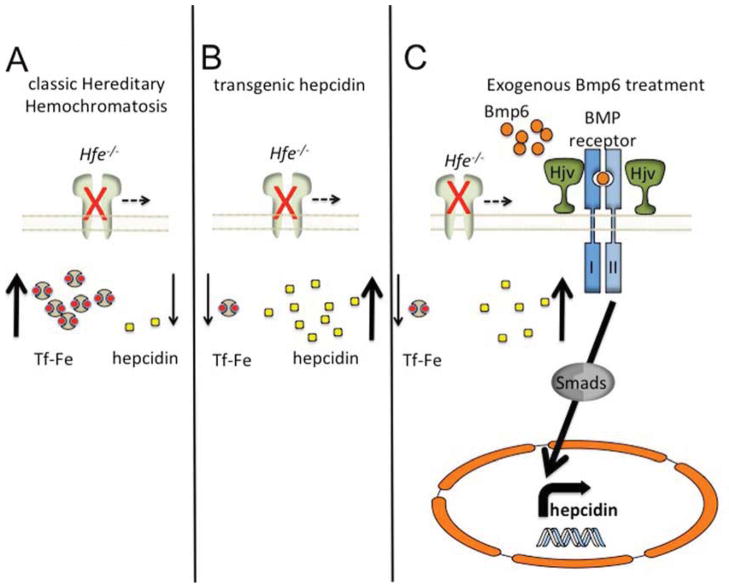
Figure 3. Interventions in a mouse model of hemochromatosis (Hfe−/−).
(A) Genetic ablation of the Hfe protein (Hfe−/−) leads to diminished hepcidin production and elevated iron uptake and distribution. Transgenic overexpression of hepcidin (B) greatly diminishes tissue iron loading and leads to a microcytic, hypochromic anemia. Application of supraphysiological amounts of BMP6 (C) initiates hepcidin production through stimulation of the Bmp/Hjv/Smad signaling pathway, leading to diminished available iron.
Exogenous BMP6 for the treatment of HFE HH
Mice lacking Hfe, although iron overloaded, do not have detectable elevations in hepatic Bmp-Smad pathway signaling.39,40 Furthermore, Bmp6 expression is appropriately upregulated in comparison with the increased tissue iron found in Hfe−/− mice suggesting that Hfe is not necessary for Bmp6 regulation. As a result, Corradini et al,41 hypothesized that treatment of Hfe−/− mice with supraphysiological levels of Bmp6 could compensate for the defect in Smad-mediated hepcidin regulation (Figure 3C). To evaluate this possibility, they administered BMP6 to Hfe−/− mice twice daily for 10 days and found that treatment increased liver hepcidin mRNA expression by almost 2-fold. This resulted in reduced serum iron, transferrin saturation and elevated non-heme iron deposition in the spleen. Improvement of liver, heart and pancreas iron levels was not observed, almost certainly due to the relatively short treatment regimen, which was necessitated by dysplastic calcification at the injection site. This study clearly demonstrated the limitations of systemic BMP6 treatment for iron overload, but equally pointed out the potential for therapeutic efficacy were local, hepatic expression of BMP6 to be achieved.
Genetic and pharmacological inhibition of Tmprss6 in HFE HH
Mice lacking Tmprss6 overexpress hepcidin due to constitutive hyperactivation BMP-SMAD signaling pathway, leading to an iron iron-refractory iron deficiency anemia (IRIDA) phenotype.28,42 A similar IRIDA phenotype occurs in humans with biallelic mutations in TMPRSS6. Current models postulate that TMPRSS6 cleaves HJV from the cell membrane, dampening BMP-SMAD signaling and consequently hepcidin expression, particularly when ambient iron levels are low. As a result, genetic or pharmacologic targeting of TMPRSS6 may be beneficial in disorders of iron regulation dependent upon physiologically “upstream” of HJV (Figure 4A). As a genetic proof of principle, Finberg, et al., showed that deletion of a single Tmprss6 allele greatly diminished iron loading in Hfe−/− animals.43 Complete loss of Tmprss6 ameliorated the iron overload phenotype, but also caused a hypochromic, microcytic iron deficiency anemia on the Hfe−/− background, indicating that a delicate balance must be struck between too much and too little hepcidin.
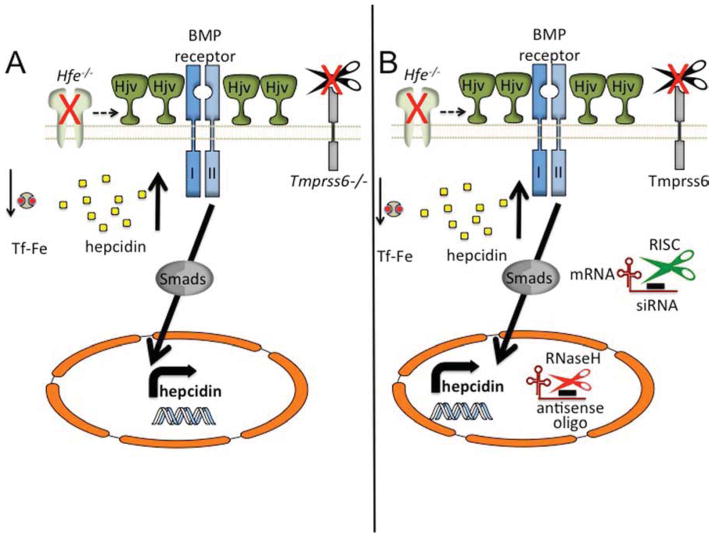
Figure 4. Modulation of hepcidin expression through genetic or pharmacological targeting of Tmprss6.
Loss of all endogenous Tmprss6 protein (A) leads to elevated levels of Hjv, the Bmp co-receptor, on the cell membrane. Significant hepcidin expression causes suppressed iron levels and a hypochromic, microcytic anemia, even in a mouse lacking Hfe. (B) Targeting of Tmprss6 thorough pharmacological means. Targeting of Tmprss6 siRNA to the liver in lipid nanoparticle (LNP)–formulated siRNAs, or by antisense oligonucleotide technology, leads to diminished Tmprss6 mRNA expression through classic RISC-mediated (cytoplasmic) or RnaseH-mediated (nucleus) suppression, respectively. Suppression of Tmprss6 causes elevated levels of Hjv to remain on the cell membrane, triggering heightened hepcidin expression and ameliorating the Hfe−/− phenotype.
Two research groups have sought subsequently to promote hepcidin expression by targeting Tmprss6 mRNA for degradation in vivo (Figure 4B). Schmidt et al. tested an siRNA targeting Tmprss6 mRNA encapsulated in lipid nanoparticles (LNP) composed of an ionizable lipid, disteroylphosphatidyl choline, cholesterol and PEG-DMG.44 Because they are taken up by the chylomicron scavenger receptors, these vesicles can be injected into a peripheral vein, and are avidly taken up by hepatocytes relatively specifically, where they induce Tmprss6 mRNA degradation through a mechanism involving the RNA-induced silencing complex (RISC). Treatment of wild type animals with Tmprss6 siRNA decreased liver expression of Tmprss6 mRNA in a dose dependent manner, leading to prolonged hepcidin induction, and suppression of the transferrin (Tf) saturation and liver non-heme iron levels.45 Silencing Tmprss6 in Hfe−/− mice had similar effects. Furthermore, siRNA treatment also increased total spleen iron, indicating that hepcidin induction also caused splenic macrophages to sequester iron from senescent red blood cells. As might be expected, even in Hfe−/− animals, chronic suppression of Tmprss6 has a cumulative effect on erythropoiesis, leading to a hypochromic, microcytic anemia
Guo, et al., employed a related, antisense oligonucleotide (ASO) approach to suppressing Tmprss6 expression.46,47 Here, antisense oligonucleotides form an RNA:DNA hybrid that is then degraded through a nuclear RNaseH-mediated mechanism. As is true of the siRNA approach, Tmprss6 ASO treatment decreased hepatic expression of Tmprss6 mRNA in a dose dependent manner, leading to elevated hepcidin production, eventuating in a decrease in serum iron and transferrin saturation. Likewise, treatment of Hfe−/− mice also suppressed serum and liver iron parameters and increased spleen iron concentration, indicating, as before, a re-distribution of iron from hepatocytes to recycling macrophages in the spleen. As noted with siRNA treatment, ASO manipulation of hepcidin also leads to a hypochromic, microcytic anemia
Minihepcidins correct hepcidin deficiency in HH
As in other hormone deficiency disorders (e.g., type I diabetes mellitus and hypothroidism) the iron loading in HH could equally be treated by hormone replacement therapy. The relative abundance of hepcidin, which is one of the most highly expressed RNAs in the liver, as well as its short plasma half-life due to proteolysis and renal clearance, however, severely limits the potential for direct hormone replacement. To address the pharmacological shortcomings of the natural peptide, Preza, et al., developed a series of orally bioavailable, long-acting “minihepcidins” for the treatment of iron overload disorders.48 In cell-based assays, they showed that the 7–9 N-terminal amino acids of hepcidin appear to be the minimal sequence able to induce FPN1 degradation. Chemically synthesized N-terminal peptides assembled from D-amino acids in the reverse orientation (so-called retro-inverso peptides) are equally active as the natural L-amino acid N-terminal peptides, but are resistant to proteases. Further modification of the retro-inverso peptides with C-terminal carboxyamide linked polyethylene glycol (PEG) and palmitic acid groups produced biologically active molecules that are both orally bioavailable and have a longer plasma half-life. Indeed, daily intraperitoneal injection of minihepcidins was able to reverse iron loading in mice deficient in hepcidin. Subsequent work by Ramos, et al.,49 employed an optimized minihepcidin, containing only L-amino acids, to treat hepcidin deficient animals that were either dietarily iron-loaded or depleted. Most notably, minihepcidin administration to iron-depleted hepcidin null animal mice prevented liver iron loading, decreased heart iron levels, and caused iron retention in splenic macrophages. However, treatment of already iron-loaded knockout animals caused a significant increase in spleen iron, but only small changes in liver, heart and serum iron measurements. As expected, very high doses of the peptides lead to anemia due to iron restriction, once again highlighting the very narrow therapeutic window for manipulating this pathway.
Small molecule modulation of hepcidin expression
Other recent work has sought to generate small molecules that induce hepcidin. Genistein, a member of the isoflavone family of organic molecules related to estrogens, was found to induce hepcidin transcription in a zebrafish model system.50 This modulation was shown to be both BMP- and Stat3-mediated, but does not require estrogen receptor signaling, in an in vitro cell culture system. Further research will be necessary to determine if this potential treatment modality is viable in a mammalian animal model of iron metabolism.
PRECLINICAL INVESTIGATION OF HEPCIDIN-INDUCTION THERAPIES IN MURINE MODELS OF β-THALASSEMIA INTERMEDIA
Unlike in HH, phlebotomy is not an approach that can be applied to mitigate the iron overload in all but the most mildly anemic patients with ineffective erythropoiesis and secondary iron overload (e.g., some sideroblastic anemia patients). Consequently, if intervention in the hepcidin-ferroportin axis to limit iron absorption is to find a clinical application, it is most likely in the untransfused patient with chronic anemia and iron overload. To this end, several of the strategies described above, as well as several others (Box 1), have been applied to mouse models of non-transfusion dependent β-thalassemia (i.e., β-thalassemia intermedia), which display many of the key characteristics of the human disease. Specifically, two mutant genotypes, Hbbth3/+ and Hbbth1/th1,51,52 have transfusion-independent, moderately severe anemia associated with ineffective erythropoiesis, splenomegaly and secondary iron overload.
Transferrin therapy to modulate iron metabolism in β-thalassemia intermedia
Despite the systemic iron overload, due to the massive erythroid demand and plasma iron turnover, thalassemic erythroblasts are actually functionally iron deficient. Indeed, Ginzburg et al., observed that treatment of Hbbth1/th1 thalassemic animals with iron improves the anemia, but comes at the expense of more marked iron overload.53 Reasoning that Tf therapy could improve iron delivery to the erythron without adding additional iron to the system, this same group of investigators treated thalassemic mice with chronic Tf injections. They observed that Tf treatment increased the hemoglobin and decreased the reticulocytosis, splenomegaly, and plasma erythropoietin as well as decreasing membrane-associated α-globin precipitates and normalizing the RBC half-life.54 Treatment with either apo or holo-Tf significantly increased hepcidin expression in comparison to untreated animals and demonstrated an increase in comparison to WT littermates. Iron staining in treated spleen was decreased, but there was no difference in other tissues.
Despite the rationale leading to its investigation, it is unclear if Tf therapy is effective because it promotes or inhibits iron delivery to the erythron. Some data would suggest that the latter is the case. For example, dietary iron restriction has similar effects on anemia and ineffective erythropoiesis in the Hbbth3/+ mouse model.55 Furthermore, RBC parameters in both these models change in a manner that would suggest more severe iron deficiency: both Tf-treated Hbbth1/th1 and iron deficient Hbbth3/+ mice respond to the treatment by making more RBCs with a smaller MCV and a lower MCH and MCHC, all characteristics of RBC iron deficiency. These observations provided the seminal insight that actual or functional iron deficiency had the potential to modify the disease phenotype in these β-thalassemia models, suggesting that targeting the hepcidin-ferroportin axis to restrict iron availability would effect not only iron loading, but the primary disease itself.
Genetic and pharmacologic induction of hepcidin in β-thalassemia intermedia
Based on the effect of dietary iron deficiency, it was recognized that limiting iron absorption and macrophage iron recycling with hepcidin might equally mitigate the thalassemic and iron overload phenotypes. To test this possibility, Gardenghi et al., transplanted Hbbth3/+ hematopoietic stem cells into mice transgenically expressing hepcidin in a tetracycline-inducible manner.55,56 Here, moderate overexpression of hepcidin in the Hbbth3/+ model reduces iron overload, improves anemia, and decreases splenomegaly. Importantly, in several animals, the anemia actually became worse. These rare individuals, it was found, expressed higher levels of hepcidin than their counterparts that experienced improvement in the anemia, once again illustrating the delicate balance between iron restriction and desirable effects.
Similar to previous work that validated Tmprss6 as a target to moderate murine Hfe−/− HH, Nai, et al., showed that targeted deletion of Tmprss6 not only decreases iron loading, but also uniformly ameliorates the anemia and ineffective erythropoiesis in Hbbth3/+ mice (Figure 4A).57 Importantly, this would suggest that, unlike induction of endogenous hepcidin through other means or potentially administration of hepcidin mimetics, complete inhibition of this target should not have the untoward effect of excessively iron-restricted erythropoiesis.
Using methodologies described above, Schmidt et al.,45 and Guo et al.,46 employed LNP–formulated siRNAs and ASOs, respectively, targeted against Tmprss6 mRNA to enhance hepcidin expression in the Hbbth3/+ thalassemia model. Both groups had qualitatively similar results, demonstrating that suppression of Tmprss6 expression in Hbbth3/+ mice significantly induces liver expressed hepcidin and diminishes tissue and serum iron levels. More importantly, both treatments substantially improved the anemia by altering RBC survival and ineffective erythropoiesis. This improvement in RBC survival was likely a consequence of a decrease in accumulated erythrocyte membrane-associated α-globin precipitates. A reduction in splenomegaly and ineffective erythropoiesis was confirmed by restoration of proper splenic architecture and diminution of serum erythropoietin.
CONCLUSIONS AND FUTURE DIRECTIONS
Although it is quite evident that iron restriction through either dietary limitation or elevating serum hepcidin activity improves anemia in the Hbbth3/+ mouse model of β-thalassemia intermedia the mechanism by which this occurs has not yet been defined. At least in the case of hepcidin and hepcidin mimetics, it is possible that intervening in the hepcidin-ferroportin axis itself may play a direct role in the maturation of erythroblasts. For example, work by Zhang, et al.,58 showed that Fpn1 is highly expressed on the cell membrane in erythroblasts, which may limit may modulate iron availability in early erythroid cells by exporting the metal.59 Nevertheless, as noted earlier, the same effect is observed when thalassemic animals are placed on a low iron diet alone, suggesting that iron-restricted erythropoiesis is the critical factor.55
In humans, it is well documented that induction of γ-globin (and thus hemoglobin F) can mitigate the clinical phenotype of the β-hemoglobinopathies (reviewed in60,61). However, this cannot be the mechanism in rodents as they lack a γ-globin equivalent, switching directly from embryonic to adult β-globins. Similarly, much the same as coinheritance of an α-thalassemia allele mitigates a β-thalassemia genotype,62 it is possible that suppression of available iron decreases the α/β globin synthesis ratio, leading to fewer free α-chains and diminishing damage caused by membrane-associated α-globin. This, however, does not appear to be the case either in this mouse model (PJS and MDF, unpublished). The stability of free α-globin might equally be increased though elevation of AHSP,63 the α-hemoglobin stabilizing protein whose expression is increased in iron deficiency.64 However, while theoretically possible, this is unlikely, as transgenic overexpression of AHSP does not improve the anemia in Hbbth3/+ animals.65 Another, still unconfirmed, hypothesis is that erythroid iron deficiency causes a global decrease in globin protein synthesis, which, due to the absolute decrease in α-chains would lead to less membrane damage and increased intra-and extra-medullary RBC survival. A potential mechanism for this decrease could be through the heme-regulated eIF2α kinase (HRI).66 HRl is an erythroid-specific translational elongation factor 2a (elF-2a) kinase, that, in the absence of heme, is a potent inhibitor of protein synthesis.67–69 Although Hri−/− mice have no baseline phenotype, these animals develop a paradoxical hyperchromic, macrocytic anemia with reduced RBC numbers in the setting of iron deficiency.70 Hri deficiency increases the abundance of α-globin aggregates and in doing so exacerbates the phenotype of the Hbbth1/th1 thalassemia.71 Determining whether this or another mechanism underlies the phenotypic improvement in each of the preclinical models discussed above will most certainly contribute to further refinement of therapeutics designed to enhance these effects.
Application of these experimental therapies has, thus far, been limited to HH and β-thalassemia intermedia mouse models. It is possible, however, that they may eventually find application in transfusion-dependent β-thalassemia major patients, in which the hepcidin levels are at baseline grossly elevated, but may, nonetheless, still be inappropriately low for the systemic iron burden.72 While chronic transfusion therapy is meant to suppress ineffective erythropoiesis and its complications, the episodic nature of transfusions leads to gradually increasing erythropoietic drive pre-transfusion, punctuated by periods of maximal suppression immediately following a transfusion. A recent analysis of β-thalassemia major patients pre- and post-transfusion demonstrated an increase in hepcidin and a decrease in both and erythropoietin and growth differentiation factor-15, the latter thought to be indicative of ineffective erythropoiesis decreased, after transfusion.73 Thus, although the primary source of iron in transfused β-thalassemia is from transfused RBCs, intestinal iron absorption may, nonetheless, be relatively stimulated, particularly as the hemoglobin approaches a nadir. Thus, in this situation, where ongoing erythropoiesis is entirely undesirable, a compromise between erythropoiesis and iron absorption need not be struck, and maximal suppression of the pathway, as might be achieved with a hepcidin mimetic, in particular, is a desirable goal. Furthermore, combination of these therapies with state-of-the art iron chelation strategies may further decrease the burden of iron and iron-related complications in transfusion-dependent and - independent β-thalassemias.
Key Points.
Dysregulation of iron metabolism is a primary or secondary cause of morbidity and mortality in a number of diverse diseases, including Hereditary Hemochromatosis (HH) and β-thalassemia.
Hepcidin is the central hormonal regulator of iron metabolism.
Tmprss6 is a serine protease that regulates hepcidin expression by the hepatocyte through a mechanism that involves several of the hereditary hemochromatosis proteins.
Modulation of hepcidin activity has demonstrated potential as treatment modality to treat iron overload disorders in pre-clinical animal models.
Acknowledgments
Funding sources:
Dr. Schmidt: none
Dr. Fleming: Alnylam Pharmaceuticals, NIH R01 DK087992 and R01 DK100806
Footnotes
Conflict of interest:
Dr. Schmidt: none
Dr. Fleming: Receives research funding from Alnylam Pharmaceuticals and has served as a paid consultant to Bayer, Eli Lilly and Co., Novartis, and FerruMax.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 2.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 3.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 5.Finch C. Regulators of iron balance in humans. Blood. 1994;84(6):1697–1702. [PubMed] [Google Scholar]
- 6.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamsky K, Weizer O, Amariglio N, et al. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol. 2004;124(1):123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105(4):1803–1806. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 9.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36(1):77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 10.Bridle KR, Frazer DM, Wilkins SJ, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt P. Molecular Basis of Hemochromatosis. In: Culotta VC, Scott RS, editors. Metals in Cells. Chichester: John Wiley & Sons, Ltd; 2013. pp. 361–372. [Google Scholar]
- 12.Corradini E, Babitt JL, Lin HY. The RGM/DRAGON family of BMP co-receptors. Cytokine & growth factor reviews. 2009;20(5–6):389–398. doi: 10.1016/j.cytogfr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babitt JL, Lin HY. The molecular pathogenesis of hereditary hemochromatosis. Seminars in liver disease. 2011;31(3):280–292. doi: 10.1055/s-0031-1286059. [DOI] [PubMed] [Google Scholar]
- 14.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 15.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nature chemical biology. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118(15):4224–4230. doi: 10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 19.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 20.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci U S A. 1998;95(4):1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West AP, Jr, Giannetti AM, Herr AB, et al. Mutational analysis of the transferrin receptor reveals overlapping HFE and transferrin binding sites. J Mol Biol. 2001;313(2):385–397. doi: 10.1006/jmbi.2001.5048. [DOI] [PubMed] [Google Scholar]
- 22.Kawabata H, Yang R, Hirama T, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274(30):20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 23.Camaschella C, Roetto A, Cali A, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25(1):14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 24.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281(39):28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9(3):217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt PJ, Fleming MD. Transgenic HFE-dependent induction of hepcidin in mice does not require transferrin receptor-2. American journal of hematology. 2012;87(6):588–595. doi: 10.1002/ajh.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. Journal of hepatology. 2012;57(5):1052–1060. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folgueras AR, de Lara FM, Pendas AM, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112(6):2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 31.Melis MA, Cau M, Congiu R, et al. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93(10):1473–1479. doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- 32.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxson JE, Chen J, Enns CA, Zhang AS. Matriptase-2- and proprotein convertase-cleaved forms of hemojuvelin have different roles in the down-regulation of hepcidin expression. The Journal of biological chemistry. 2010;285(50):39021–39028. doi: 10.1074/jbc.M110.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meynard D, Vaja V, Sun CC, et al. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood. 2011;118(3):747–756. doi: 10.1182/blood-2011-04-348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurer E, Gutschow M, Stirnberg M. Matriptase-2 (TMPRSS6) is directly up-regulated by hypoxia inducible factor-1: identification of a hypoxia-responsive element in the TMPRSS6 promoter region. Biological chemistry. 2012;393(6):535–540. doi: 10.1515/hsz-2011-0221. [DOI] [PubMed] [Google Scholar]
- 36.Nicolas G, Viatte L, Lou DQ, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34(1):97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 37.Levy JE, Montross LK, Cohen DE, Fleming MD, Andrews NC. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94(1):9–11. [PubMed] [Google Scholar]
- 38.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99(7):4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kautz L, Meynard D, Besson-Fournier C, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114(12):2515–2520. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 40.Corradini E, Garuti C, Montosi G, et al. Bone morphogenetic protein signaling is impaired in an Hfe knockout mouse model of hemochromatosis. Gastroenterology. 2009;137(4):1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corradini E, Schmidt PJ, Meynard D, et al. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. 2010;139(5):1721–1729. doi: 10.1053/j.gastro.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finberg KE, Whittlesey RL, Fleming MD, Andrews NC. Down-regulation of Bmp/Smad signaling by Tmprss6 is required for maintenance of systemic iron homeostasis. Blood. 2010;115(18):3817–3826. doi: 10.1182/blood-2009-05-224808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. 2011;117(17):4590–4599. doi: 10.1182/blood-2010-10-315507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semple SC, Akinc A, Chen J, et al. Rational design of cationic lipids for siRNA delivery. Nature biotechnology. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt PJ, Toudjarska I, Sendamarai AK, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(−/−) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood. 2013;121(7):1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo S, Casu C, Gardenghi S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. The Journal of clinical investigation. 2013;123(4):1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annual review of pharmacology and toxicology. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 48.Preza GC, Ruchala P, Pinon R, et al. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. The Journal of clinical investigation. 2011;121(12):4880–4888. doi: 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos E, Ruchala P, Goodnough JB, et al. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. 2012;120(18):3829–3836. doi: 10.1182/blood-2012-07-440743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhen AW, Nguyen NH, Gibert Y, et al. The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology. 2013;58(4):1315–1325. doi: 10.1002/hep.26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skow LC, Burkhart BA, Johnson FM, et al. A mouse model for beta-thalassemia. Cell. 1983;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- 52.Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O. A mouse model for beta 0-thalassemia. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(25):11608–11612. doi: 10.1073/pnas.92.25.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ginzburg YZ, Rybicki AC, Suzuka SM, et al. Exogenous iron increases hemoglobin in beta-thalassemic mice. Experimental hematology. 2009;37(2):172–183. doi: 10.1016/j.exphem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Rybicki AC, Suzuka SM, et al. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nature medicine. 2010;16(2):177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 55.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. The Journal of clinical investigation. 2010;120(12):4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109(9):4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nai A, Pagani A, Mandelli G, et al. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of beta-thalassemia. Blood. 2012;119(21):5021–5029. doi: 10.1182/blood-2012-01-401885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell metabolism. 2009;9(5):461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang DL, Senecal T, Ghosh MC, Ollivierre-Wilson H, Tu T, Rouault TA. Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood. 2011;118(10):2868–2877. doi: 10.1182/blood-2011-01-330241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bauer DE, Orkin SH. Update on fetal hemoglobin gene regulation in hemoglobinopathies. Current opinion in pediatrics. 2011;23(1):1–8. doi: 10.1097/MOP.0b013e3283420fd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sankaran VG. Targeted therapeutic strategies for fetal hemoglobin induction. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2011;2011:459–465. doi: 10.1182/asheducation-2011.1.459. [DOI] [PubMed] [Google Scholar]
- 62.Winichagoon P, Fucharoen S, Weatherall D, Wasi P. Concomitant inheritance of alpha-thalassemia in beta 0- thalassemia/Hb E disease. American journal of hematology. 1985;20(3):217–222. doi: 10.1002/ajh.2830200303. [DOI] [PubMed] [Google Scholar]
- 63.Kihm AJ, Kong Y, Hong W, et al. An abundant erythroid protein that stabilizes free alpha-haemoglobin. Nature. 2002;417(6890):758–763. doi: 10.1038/nature00803. [DOI] [PubMed] [Google Scholar]
- 64.Yu X, Kong Y, Dore LC, et al. An erythroid chaperone that facilitates folding of alpha-globin subunits for hemoglobin synthesis. The Journal of clinical investigation. 2007;117(7):1856–1865. doi: 10.1172/JCI31664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nasimuzzaman M, Khandros E, Wang X, et al. Analysis of alpha hemoglobin stabilizing protein overexpression in murine beta-thalassemia. American journal of hematology. 2010;85(10):820–822. doi: 10.1002/ajh.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen JJ, Throop MS, Gehrke L, et al. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(17):7729–7733. doi: 10.1073/pnas.88.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen JJ, Pal JK, Petryshyn R, et al. Amino acid microsequencing of internal tryptic peptides of heme-regulated eukaryotic initiation factor 2 alpha subunit kinase: homology to protein kinases. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(2):315–319. doi: 10.1073/pnas.88.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen JJ, Crosby JS, London IM. Regulation of heme-regulated eIF-2 alpha kinase and its expression in erythroid cells. Biochimie. 1994;76(8):761–769. doi: 10.1016/0300-9084(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 69.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109(7):2693–2699. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han AP, Yu C, Lu L, et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. Embo J. 2001;20(23):6909–6918. doi: 10.1093/emboj/20.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han AP, Fleming MD, Chen JJ. Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Invest. 2005;115(6):1562–1570. doi: 10.1172/JCI24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 73.Pasricha SR, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with beta-thalassemia major: a longitudinal study. Blood. 2013;122(1):124–133. doi: 10.1182/blood-2012-12-471441. [DOI] [PubMed] [Google Scholar]