Abstract
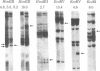
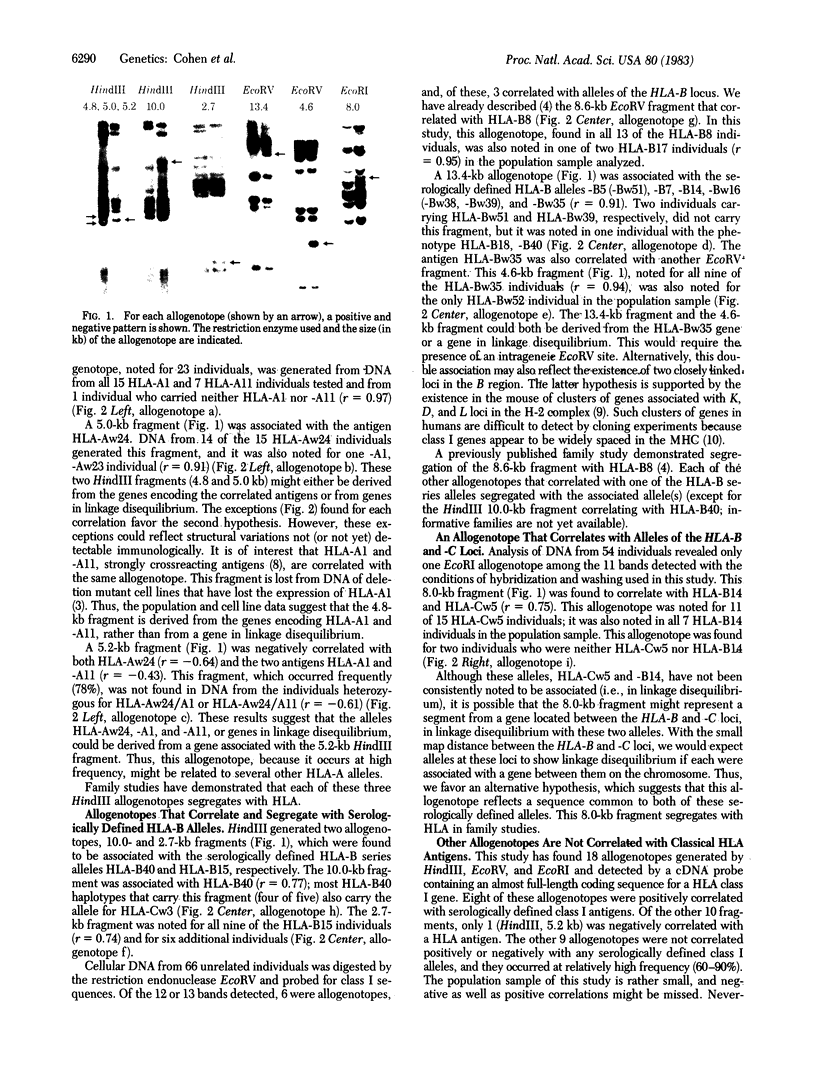
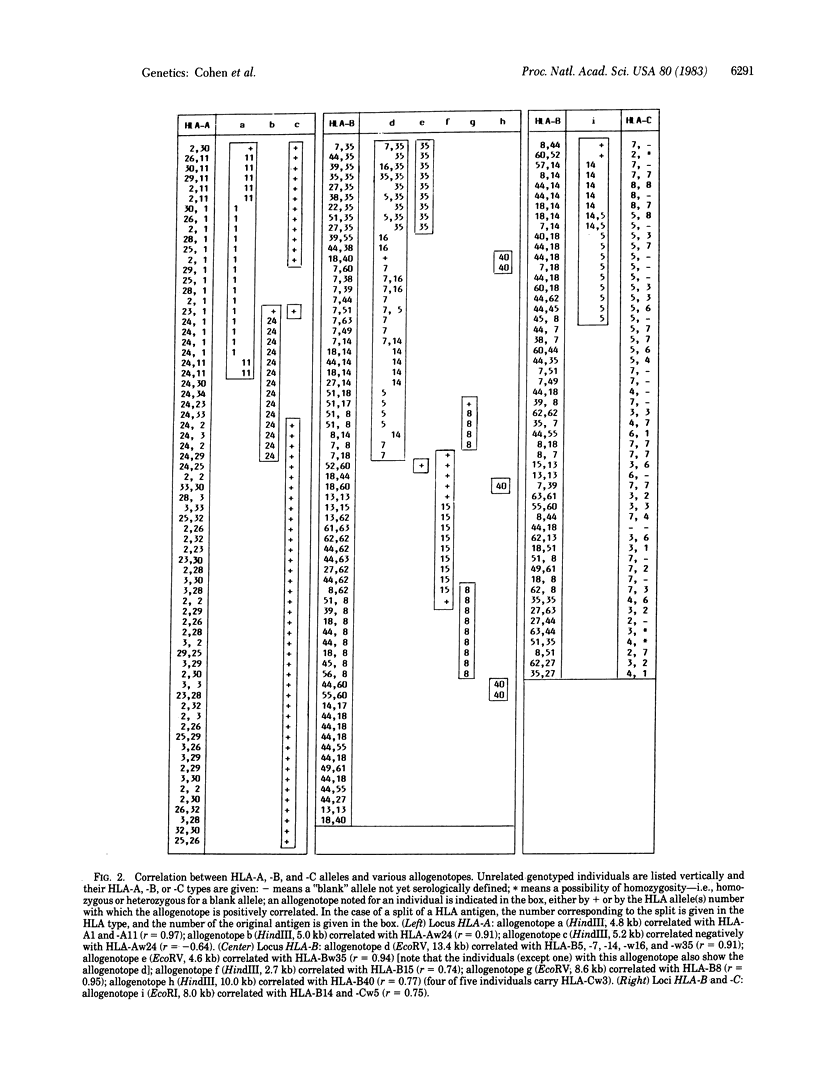
Cellular DNA from HLA-typed individuals was digested with the restriction endonucleases HindIII, EcoRV, and EcoRI. The separated restriction endonuclease fragments were hybridized with a HLA class I cDNA probe by using the Southern transfer technique. Digestion of cellular DNA with HindIII generated 22 restriction endonuclease fragments, 11 of which showed polymorphism for presence or absence in a population sample. With EcoRV, 13 fragments were identified; 6 showed polymorphism. EcoRI generated 11 fragments, of which 1 was polymorphic. Of these 18 polymorphic fragments generated by the three restriction endonucleases, each of 5 was found to be positively associated with one allele of the HLA-A or -B allelic series (HLA-Aw24, -B8, -B15, -Bw35, and -B40). One fragment was positively associated with two HLA-A series alleles (HLA-A1 and -A11). Another fragment was positively associated with five HLA-B series alleles (HLA-B5, -B7, -B14, -Bw16, and -Bw35) and one fragment was positively associated with alleles at two loci (HLA-B14 and -Cw5). The serologically defined allele HLA-Aw24 was associated with two polymorphic fragments, one association showing a positive correlation and the other a negative correlation. Each informative family studied thus far has shown segregation of the restriction fragment with the associated serologically defined allele. The fragments associated with serologically defined alleles occurred in the population sample studied at low or moderate frequencies. The remaining polymorphic fragments occur at high frequency, suggesting that class I genes not serologically detected show less polymorphism than serologically defined class I genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascanio L., Paul P., Marcadet A., Mahouy G., Fradelizi D., Cohen D., Dausset J. Polymorphisme des gènes HLA: I. Mise en évidence d'une étroite corrélation entre des fragments d'ADN déterminés par l'enzyme de restriction BglI et des antigènes HLA de classe I. C R Seances Acad Sci III. 1982 Oct 18;295(6):433–437. [PubMed] [Google Scholar]
- Cann H. M., Ascanio L., Paul P., Marcadet A., Dausset J., Cohen D. Polymorphic restriction endonuclease fragment segregates and correlates with the gene for HLA-B8. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1665–1668. doi: 10.1073/pnas.80.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J. The major histocompatibility complex in man. Science. 1981 Sep 25;213(4515):1469–1474. doi: 10.1126/science.6792704. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Damotte M., Birnbaum D., Trucy J., Jordan B. R. HLA cosmid clones show complete, widely spaced human class I genes with occasional clusters. Gene. 1982 Dec;20(3):485–489. doi: 10.1016/0378-1119(82)90219-0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Rougeon F. Gene conversion and polymorphism: generation of mouse immunoglobulin gamma 2a chain alleles by differential gene conversion by gamma 2b chain gene. Cell. 1983 Feb;32(2):515–523. doi: 10.1016/0092-8674(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Bach F. H., Ploegh H. L., Strominger J. L., Kavathas P., DeMars R. Use of HLA loss mutants to analyse the structure of the human major histocompatibility complex. Nature. 1982 Apr 1;296(5856):454–456. doi: 10.1038/296454a0. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]