Abstract
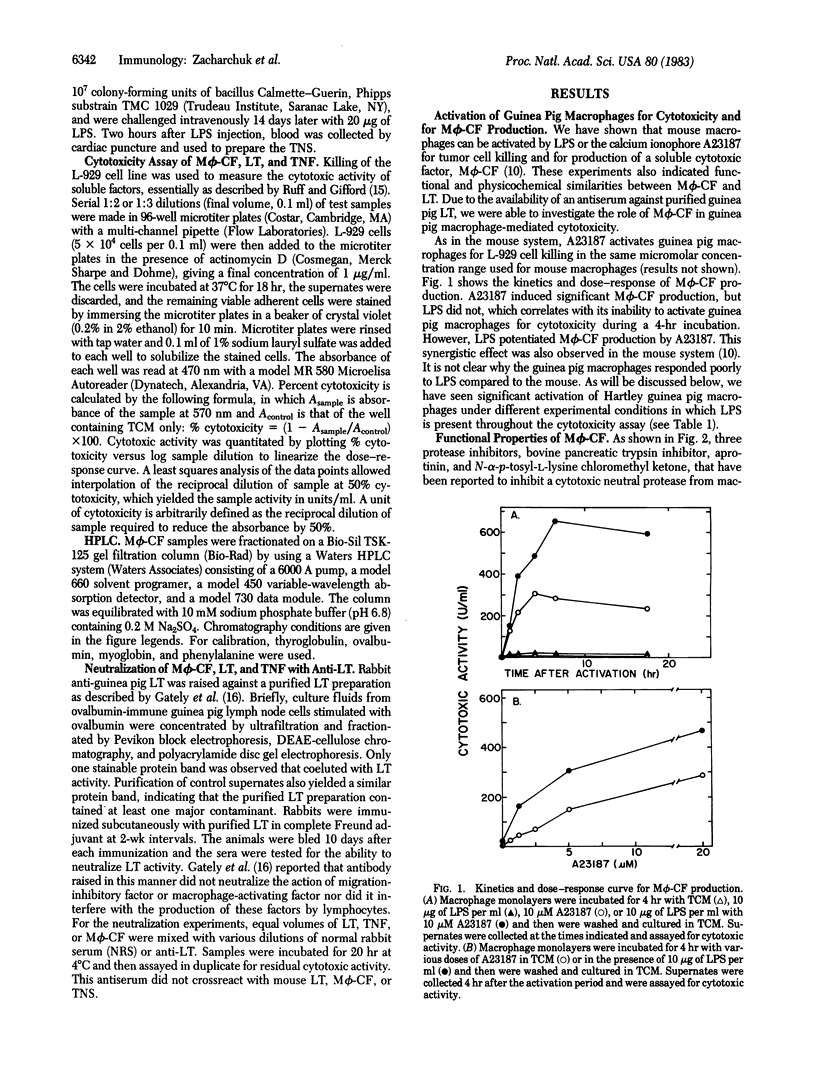
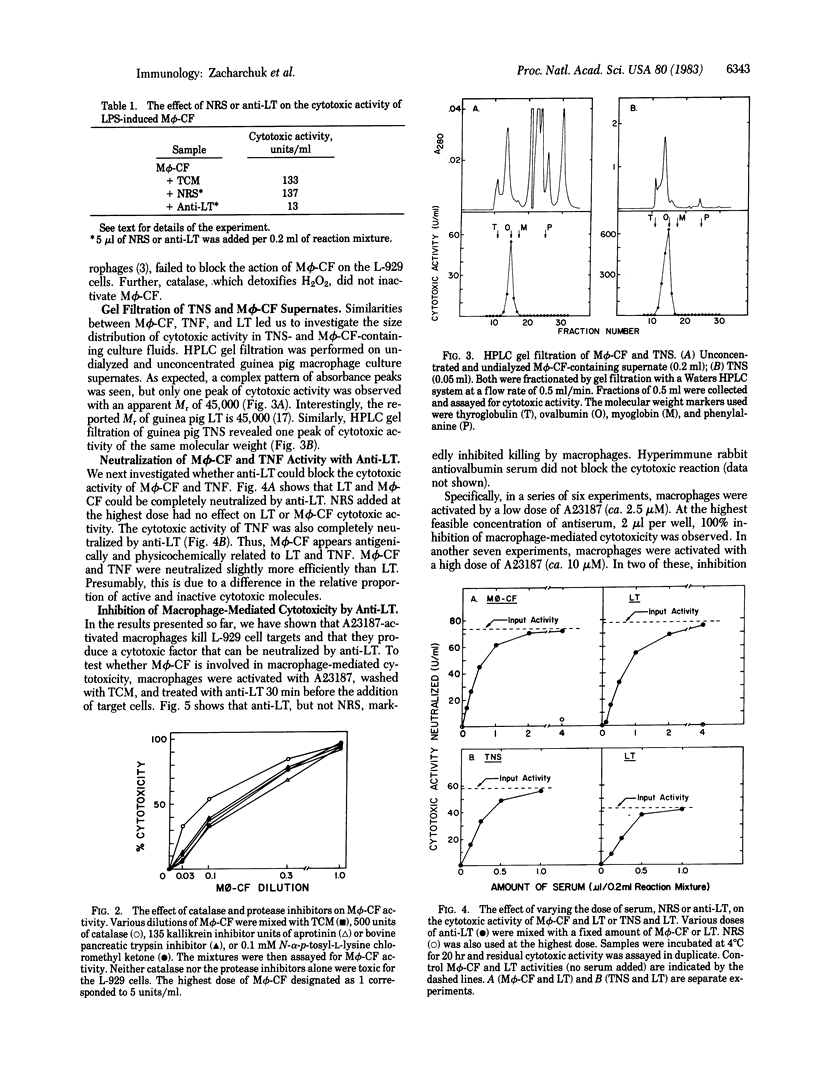
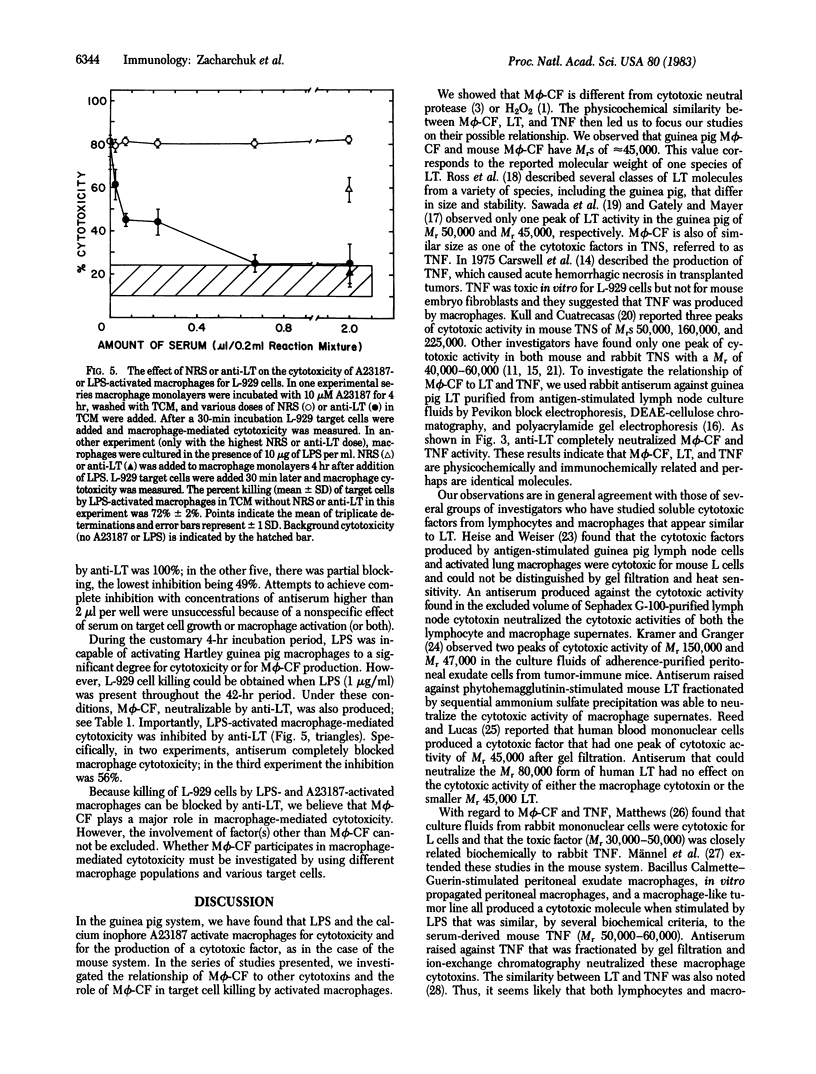
Guinea pig peritoneal macrophages, when activated for cytotoxicity by the calcium ionophore A23187 or lipopolysaccharide, produce a cytotoxic factor [macrophage cytotoxic factor (M phi-CF)] that is not blocked by catalase or protease inhibitors. Fractionation of culture supernates containing M phi-CF by gel filtration revealed one peak of cytotoxic activity of Mr approximately 45,000, the same as guinea pig lymphotoxin (LT). Antiserum prepared against purified guinea pig LT completely neutralized the cytotoxic activity of M phi-CF. In addition, the cytotoxic factor in guinea pig tumor necrosis serum was found to have a Mr of 45,000 and was neutralized by anti-LT. Thus, M phi-CF is physicochemically and immunochemically similar to LT and tumor necrosis factor, if not identical. To investigate the role of M phi-CF in macrophage-mediated cytotoxicity, anti-LT was added to A23187- or lipopolysaccharide-activated macrophages before addition of L-929 target cells. In 10 of 16 experiments, the inhibition of macrophage-mediated cytotoxicity was 100%. In the others, cytotoxicity was blocked partially, the lowest inhibition being 49%. The effectiveness of inhibition appeared to be inversely related to the intensity of macrophage activation. These results indicate that M phi-CF plays a significant role in macrophage-mediated cytotoxicity but involvement of another mechanism cannot be excluded.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Drysdale B. E., Shin H. S. Activation of macrophages for tumor cell cytotoxicity: identification of indomethacin sensitive and insensitive pathways. J Immunol. 1981 Aug;127(2):760–765. [PubMed] [Google Scholar]
- Ferluga J., Schorlemmer H. U., Baptista L. C., Allison A. C. Production of the complement cleavage product, C3a, by activated macrophages and its tumorolytic effects. Clin Exp Immunol. 1978 Mar;31(3):512–517. [PMC free article] [PubMed] [Google Scholar]
- Fishman M. Functional heterogeneity among peritoneal macrophages. III. No evidence for the role of arginase in the inhibition of tumor cell growth by supernatants from macrophages or macrophage subpopulation cultures. Cell Immunol. 1980 Sep 15;55(1):174–184. doi: 10.1016/0008-8749(80)90148-3. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Gately C. L., Henney C. S., Mayer M. M. Studies on lymphokines: the production of antibody to guinea pig lymphotoxin and its use to distinguish lymphotoxin from migration inhibitory factor and mitogenic factor. J Immunol. 1975 Sep;115(3):817–826. [PubMed] [Google Scholar]
- Gately M. K., Mayer M. M., Henney C. S. Effect of anti-lymphotoxin on cell-mediated cytotoxicity. Evidence for two pathways, one involving lymphotoxin and the other requiring intimate contact between the plasma membranes of killer and target cells. Cell Immunol. 1976 Nov;27(1):82–93. doi: 10.1016/0008-8749(76)90156-8. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Mayer M. M. The molecular dimensions of guinea pig lymphotoxin. J Immunol. 1974 Jan;112(1):169–177. [PubMed] [Google Scholar]
- Goodman M. G., Weigle W. O., Hugli T. E. Inability of the C3a anaphylatoxin to promote cellular lysis. Nature. 1980 Jan 3;283(5742):78–80. doi: 10.1038/283078a0. [DOI] [PubMed] [Google Scholar]
- Heise E. R., Weiser R. S. Factors in delayed sensitivity: lymphocyte and macrophage cytotoxins in the tuberculin reaction. J Immunol. 1969 Sep;103(3):570–576. [PubMed] [Google Scholar]
- Hiserodt J. C., Granger G. A. Inhibition of human lymphocyte-mediated mitogen-induced cytotoxicity of murine L-929 cells by heterologous anti-human lymphotoxin antisera in vitro. J Immunol. 1977 Aug;119(2):374–380. [PubMed] [Google Scholar]
- Kramer J. J., Granger G. A. The in vitro induction and release of a cell toxin by immune C57B1-6 mouse peritoneal macrophages. Cell Immunol. 1972 Jan;3(1):88–100. doi: 10.1016/0008-8749(72)90229-8. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Possible requirement of internalization in the mechanism of in vitro cytotoxicity in tumor necrosis serum. Cancer Res. 1981 Dec;41(12 Pt 1):4885–4890. [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Preliminary characterization of the tumor cell cytotoxin in tumor necrosis serum. J Immunol. 1981 Apr;126(4):1279–1283. [PubMed] [Google Scholar]
- Leopardi E., Rosenau W. Human T-cell mediated cytotoxicity: role of subsets and neutralization of cytotoxicity by anti-alpha-lymphotoxin serum. Cell Immunol. 1982 Jun;70(1):148–159. doi: 10.1016/0008-8749(82)90140-x. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M., Ayala E. Active and passive immunity against Pseudomonas aeruginosa with a ribosomal vaccine and antiserum in C3H/HeJ mice. J Immunol. 1983 Jul;131(1):1–3. [PubMed] [Google Scholar]
- Matthews N. Effect of human monocyte killing of tumour cells of antibody raised against an extracellular monocyte cytotoxin. Immunology. 1983 Feb;48(2):321–327. [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978 Aug;38(2):310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Falk W., Meltzer M. S. Inhibition of nonspecific tumoricidal activity by activated macrophages with antiserum against a soluble cytotoxic factor. Infect Immun. 1981 Jul;33(1):156–164. doi: 10.1128/iai.33.1.156-164.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Meltzer M. S., Mergenhagen S. E. Generation and characterization of a lipopolysaccharide-induced and serum-derived cytotoxic factor for tumor cells. Infect Immun. 1980 Apr;28(1):204–211. doi: 10.1128/iai.28.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Mayer M. M. Studies on the mechanism of action of guinea pig lymphotoxin. I. Membrane active substances prevent target cell lysis by lymphotoxin. J Immunol. 1978 Jan;120(1):272–278. [PubMed] [Google Scholar]
- Reed W. P., Lucas Z. J. Cytotoxic activity of lymphocytes. V. Role of soluble toxin in macrophage-inhibited cultures of tumor cells. J Immunol. 1975 Aug;115(2):395–404. [PubMed] [Google Scholar]
- Rosenau W., Tsoukas C. D. Lymphotoxin. A review and analysis. Am J Pathol. 1976 Sep;84(3):580–596. [PMC free article] [PubMed] [Google Scholar]
- Ross M. W., Tiangco G. J., Horn P., Hiserodt J. C., Granger G. A. The LT system in experimental animals. III. Physicochemical characteristics and relationships of lymphotoxin (LT) molecules released in vitro by activated lymphoid cells from several animal species. J Immunol. 1979 Jul;123(1):325–331. [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Sawada J. I., Shioiri-Nakano K., Osawa T. Purification and characterization of guinea pig lymphotoxin produced by lymph node cells stimulated by phytohemagglutinin. Transplantation. 1975 Apr;19(4):335–342. doi: 10.1097/00007890-197504000-00009. [DOI] [PubMed] [Google Scholar]
- Sawada J., Osawa T. Cellular cytotoxicity induced by various lectins and mitogens. Transplantation. 1978 Nov;26(5):319–324. doi: 10.1097/00007890-197811000-00011. [DOI] [PubMed] [Google Scholar]
- Sorrell T. C., Lehrer R. I., Cline M. J. Mechanism of nonspecific macrophage-mediated cytotoxicity: evidence for lack of dependence upon oxygen. J Immunol. 1978 Feb;120(2):347–352. [PubMed] [Google Scholar]
- Walker S. M., Lucas Z. J. Role of soluble cytotoxins in cell-mediated immunity. Transplant Proc. 1973 Mar;5(1):137–140. [PubMed] [Google Scholar]
- Ware C. F., Granger G. A. Mechanisms of lymphocyte-mediated cytotoxicity. I. The effects of anti-human lymphotoxin antisera on the cytolysis of allogeneic B cell lines by MLC-sensitized human lymphocytes in vitro. J Immunol. 1981 May;126(5):1919–1926. [PubMed] [Google Scholar]


