Abstract
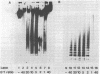
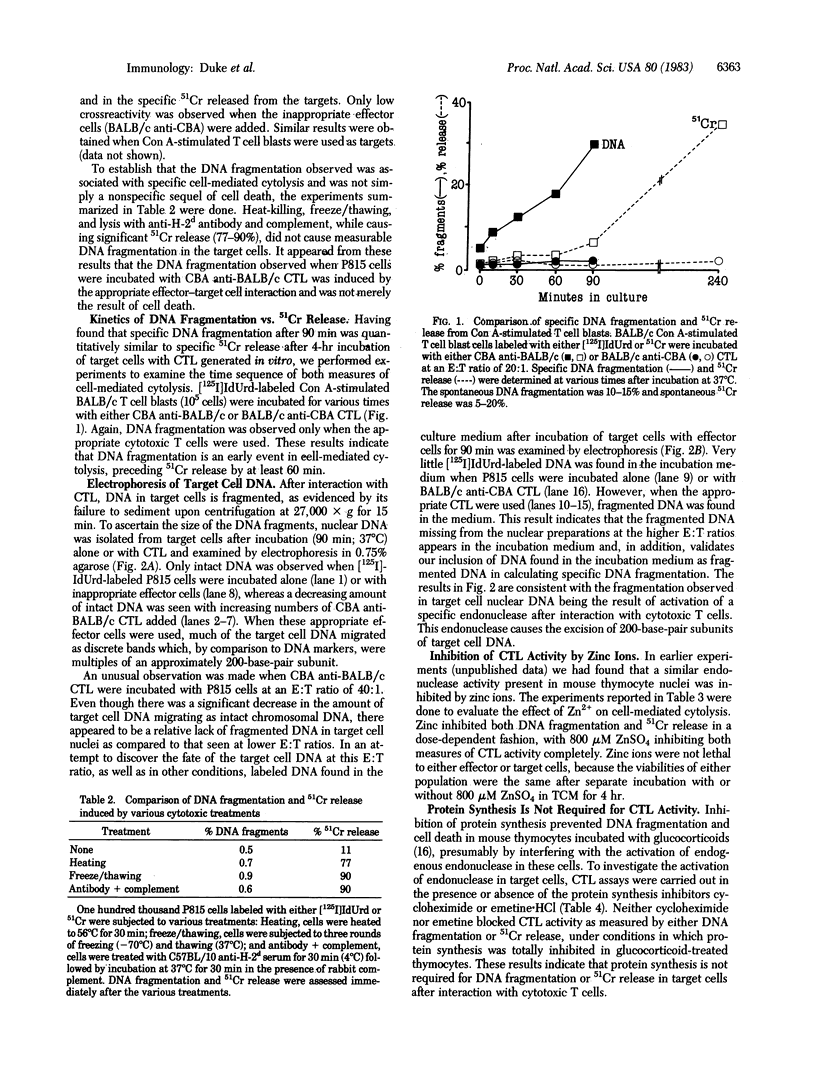
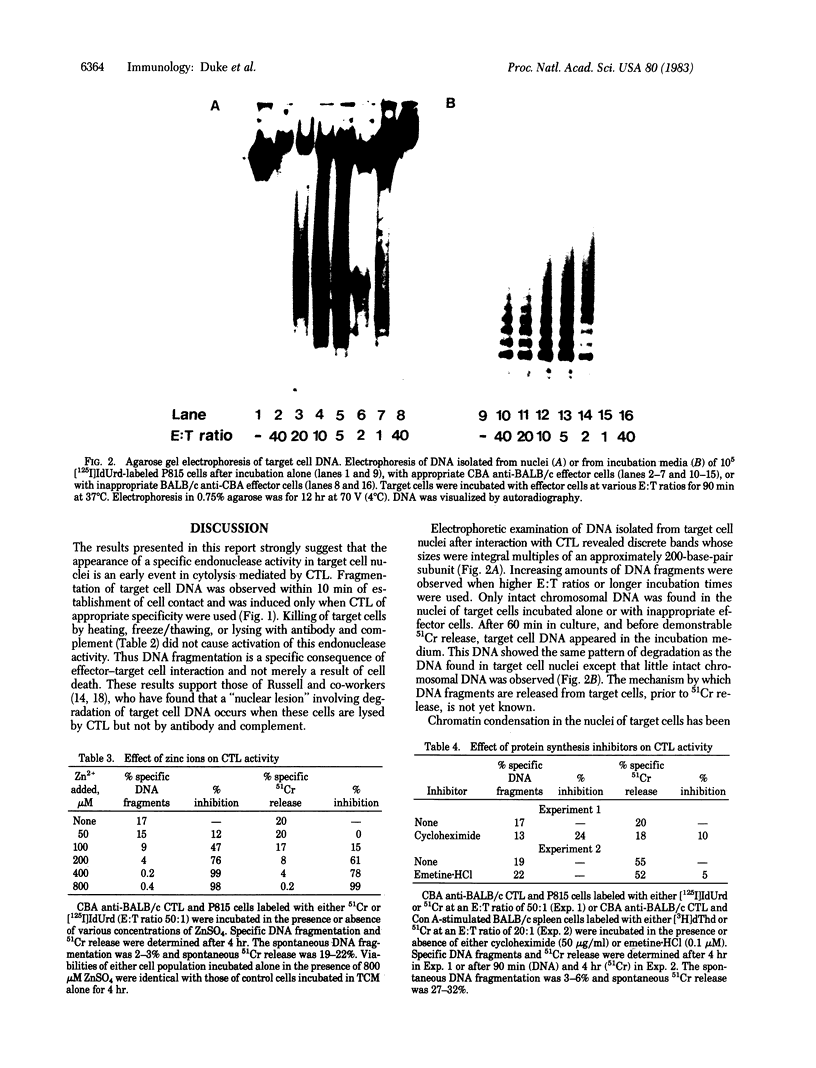
Within minutes of exposure of target cells to cytotoxic T lymphocytes, their nuclear DNA begins to be fragmented. This phenomenon precedes 51Cr release by at least an hour. DNA fragmentation occurs only when appropriately sensitized cytotoxic T cells are used and is not merely a result of cell death because killing of target cells by heating, freeze/thawing, or lysing with antibody and complement did not yield DNA fragments. Agarose gel electrophoresis of target cell DNA showed discrete multiples of an approximately 200-base-pair subunit, suggesting that fragmentation was the result of activation of a specific endonuclease. A similar pattern of DNA fragments is observed during glucocorticoid-induced killing of mouse thymocytes. The endonuclease in that case is inhibited by zinc ions, and we find that Zn2+ also inhibits DNA fragmentation and 51Cr release induced by cytotoxic T cells, suggesting a final common biochemical pathway for both types of cell death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby D. W., Modak S. P. DNA degradation in terminally differentiating lens fiber cells from chick embryos. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5579–5583. doi: 10.1073/pnas.74.12.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M. M., Ablett G., Bishop C. J., Bundesen P. G., Donald K. J., Searle J., Kerr J. F. Death of cells by apoptosis following attachment of specifically allergized lymphocytes in vitro. Aust J Exp Biol Med Sci. 1977 Aug;55(4):407–417. doi: 10.1038/icb.1977.38. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Martz E. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. V. Evidence that manganese inhibits a calcium-dependent step in programming for lysis. Cell Immunol. 1981 Jun;61(1):78–89. doi: 10.1016/0008-8749(81)90355-5. [DOI] [PubMed] [Google Scholar]
- Golstein P., Smith E. T. The lethal hit stage of mouse T and non-T cell-mediated cytolysis: differences in cation requirements and characterization of an analytical "cation pulse" method. Eur J Immunol. 1976 Jan;6(1):31–37. doi: 10.1002/eji.1830060108. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Hiserodt J. C., Bonavida B. Studies on the induction and expression of T cell-mediated immunity. XI. Inhibition of the "lethal hit" in T cell-mediated cytotoxicity by heterologous rat antiserum made against alloimmune cytotoxic T lymphocytes. J Immunol. 1981 Jan;126(1):256–262. [PubMed] [Google Scholar]
- Kaiser N., Edelman I. S. Calcium dependence of glucocorticoid-induced lymphocytolysis. Proc Natl Acad Sci U S A. 1977 Feb;74(2):638–642. doi: 10.1073/pnas.74.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz E. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. I. Resolution and characterization. J Immunol. 1975 Jul;115(1):261–267. [PubMed] [Google Scholar]
- Martz E. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. II. Electrolyte permeability increase in the target cell membrane concomitant with programming for lysis. J Immunol. 1976 Sep;117(3):1023–1027. [PubMed] [Google Scholar]
- Matter A. Microcinematographic and electron microscopic analysis of target cell lysis induced by cytotoxic T lymphocytes. Immunology. 1979 Feb;36(2):179–190. [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Rudolf H., Chapuis B., Brunner K. T. Studies of allograft immunity in mice. II. Mechanism of target cell inactivation in vitro by sensitized lymphocytes. Immunology. 1970 Apr;18(4):517–535. [PMC free article] [PubMed] [Google Scholar]
- Miovic M. L., Pizer L. I. Characterization of RNA synthesized in isolated nuclei of herpes simplex virus type 1-infected KB cells. J Virol. 1980 Jan;33(1):567–571. doi: 10.1128/jvi.33.1.567-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa T., Anai M., Urabe H. Degradation of deoxyribonucleic acid by guinea pig epidermal extracts. Arch Dermatol Res. 1975 Nov 14;254(1):79–85. doi: 10.1007/BF00561538. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Sakaki Y., Watanabe N., Takagi Y. Purification and characterization of the Ca2+ plus Mg2+-dependent endodeoxyribonuclease from calf thymus chromatin. J Biochem. 1981 Jan;89(1):143–152. doi: 10.1093/oxfordjournals.jbchem.a133175. [DOI] [PubMed] [Google Scholar]
- Nakayama J., Fujiyoshi T., Nakamura M., Anai M. Purification and properties of an endodeoxyribonuclease from nuclei of bovine small intestinal mucosa. J Biol Chem. 1981 Feb 25;256(4):1636–1642. [PubMed] [Google Scholar]
- Russell J. H., Dobos C. B. Mechanisms of immune lysis. II. CTL-induced nuclear disintegration of the target begins within minutes of cell contact. J Immunol. 1980 Sep;125(3):1256–1261. [PubMed] [Google Scholar]
- Russell J. H., Masakowski V., Rucinsky T., Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [PubMed] [Google Scholar]
- Sanderson C. J., Glauert A. M. The mechanism of T-cell mediated cytotoxicity. VI. T-cell projections and their role in target cell killing. Immunology. 1979 Jan;36(1):119–129. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J. The mechanism of T cell mediated cytotoxicity. II. Morphological studies of cell death by time-lapse microcinematography. Proc R Soc Lond B Biol Sci. 1976 Jan 20;192(1107):241–255. doi: 10.1098/rspb.1976.0011. [DOI] [PubMed] [Google Scholar]
- Thorn R. M., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. VI. A reappraisal of the requirement for protein synthesis during T cell-mediated lysis. J Immunol. 1976 Jan;116(1):146–149. [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]
- Williamson R. Properties of rapidly labelled deoxyribonucleic acid fragments isolated from the cytoplasm of primary cultures of embryonic mouse liver cells. J Mol Biol. 1970 Jul 14;51(1):157–168. doi: 10.1016/0022-2836(70)90277-9. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]