Abstract
Background
Immune recognition of vascular endothelial cells (EC) has been implicated in allograft rejection, protection against pathogens, and lymphocyte recruitment. However, EC pervade nearly all tissues and predominate in none, complicating any direct test of immune recognition. Here, we examined antigen presentation by EC in vivo by testing immune responses against E. coli β-galactosidase (β-gal) in two lines of transgenic mice that express β-gal exclusively in their EC. TIE2-lacZ mice express β-gal in all EC and VWF-lacZ mice express β-gal in heart and brain microvascular EC.
Results
Transgenic and congenic wild type FVB mice immunized with β-gal expression vector DNA or β-gal protein generated high titer, high affinity antisera containing comparable levels of antigen-specific IgG1 and IgG2a isotypes, suggesting equivalent activation of T helper cell subsets. The immunized transgenic mice remained healthy, their EC continued to express β-gal, and their blood vessels showed no histological abnormalities. In response to β-gal in vitro, CD4+ and CD8+ T cells from immunized transgenic and FVB mice proliferated, expressed CD25, and secreted IFN-γ. Infection with recombinant vaccinia virus encoding β-gal raised equivalent responses in transgenic and FVB mice. Hearts transplanted from transgenic mice into FVB mice continued to beat and the graft EC continued to express β-gal. These results suggested immunological ignorance of the transgene encoded EC protein. However, skin transplanted from TIE2-lacZ onto FVB mice lost β-gal+ EC and the hosts developed β-gal-specific antisera, demonstrating activation of host immune effector mechanisms. In contrast, skin grafted from TIE2-lacZ onto VWF-lacZ mice retained β-gal+ EC and no antisera developed, suggesting a tolerant host immune system.
Conclusion
Resting, β-gal+ EC in transgenic mice tolerize specific lymphocytes that would otherwise respond against β-gal expressed by EC within transplanted skin. We conclude that EC effectively present intracellular "self" proteins to the immune system. However, antigen presentation by EC does not delete or anergize a large population of specific lymphocytes that respond to the same protein following conventional immunization with protein or expression vector DNA. These results clearly demonstrate striking context sensitivity in the immune recognition of EC, a subtlety that must be better understood in order to treat immune diseases and complications involving the vasculature.
Background
Endothelial cells (EC) form the inner lining of blood vessels and are therefore uniquely positioned between circulating lymphocytes and peripheral tissues. Vascular EC are thought to participate in the recruitment of T lymphocytes from the bloodstream into sites of infection and inflammation [1,2]. After transplantation of vascularized, allogeneic tissues, EC are the first graft cells encountered by the host lymphocytes and may be key initiators and targets of allograft rejection [3,4]. T cells also protect against intracellular pathogens that infect EC, such as cytomegalovirus, Chlamydia pneumoniae, rickettsia and hantavirus. Immune responses within the blood vessel wall are particularly important because chronic inflammation can lead to vascular remodeling and the development of arteriosclerosis [5].
T cell antigen recognition can lead to tolerance or aggression, depending on the developmental stage of the T cell and the precise interactions between the T cell and the antigen presenting cell (APC). T cell antigen receptors recognize specific peptides bound to MHC molecules on the surface of the APC. Professional APC provide certain additional activities, collectively termed costimulation, that regulate and strengthen T cell responses. Cell surface molecules and soluble proteins (chemokines, cytokines, and lymphokines) mediate costimulation. Professional APC express CD80 (B7-1) and related molecules, which act through CD28-related molecules on T cells. Professional APC also express CD40, which acts through CD154 (CD40 ligand) and is an essential mediator of collaboration between T cells [6-8].
EC have been termed "semiprofessional" APC because they costimulate certain T cell responses in vitro and because they are thought to stimulate alloresponses in vivo [9-13]. EC cultured from different vascular beds can act as APC in vitro. For example, EC cultured from human umbilical veins (HUVEC), pulmonary arteries, iliac arteries and veins, and murine lung EC all costimulated T cells treated with mitogens, generating stronger proliferation and IL-2 secretion [9,14-20]. In contrast, vascular smooth muscle cells do not provide costimulation [20]. Human EC express CD40, CD58 (LFA-3), CD134 (OX40) ligand and ICOS ligand, which can contribute to T cell activation in vitro [21-23]. HUVEC do not express CD80 and they cannot stimulate naïve T cells in vitro [24]. Although murine lung microvascular EC do express CD80, they costimulated only memory T cells in vitro [25]. Mouse liver sinusoidal EC express CD80 and other costimulatory molecules but they are reported to induce antigen specific tolerance in vitro [26]. In contrast, murine aortic EC can stimulate in vitro naive T cells expressing alloreactive antigen receptor transgenes [27]. Thus, EC costimulation properties in vitro may differ depending upon the antigen, the species, the maturity of the T cell, and the vascular bed of origin.
EC activities as APC in vitro may be poor indicators of their activities in vivo because cells change upon isolation and culture. For example, although human EC strongly express MHC class I and class II molecules in vivo, MHC class I expression is reduced and class II expression is lost in vitro [28,29]. Murine EC also express elevated levels of MHC class I molecules in vivo, which is dependent on IFN-γ [30]. Static cocultures of leukocytes and EC may be misleading because shear forces promote the migration of lymphocytes and other leukocytes across the endothelium [31-33]. In addition, efficient T cell activation can require transient, not prolonged, stimulation by APC [34]. Thus, gene expression and cellular interactions are profoundly altered by culture conditions in vitro.
Transgenic mice are ideal for investigating antigen presentation by particular cell types. Cells remain in their physiological setting and transgene expression avoids the inflammation caused by other means of gene targeting, such as transfection or infection. Tissue specific gene promoters have been used to test the immune consequences of a restricted protein expression. Transgene expression within the primary lymphoid organs usually leads to tolerance. For example, lymphocytes specific for E. coli β-galactosidase (β-gal) were tolerized in transgenic mice expressing β-gal under the control of a B lymphocyte promoter [35]. When these mice were immunized with β-gal protein, only low titer, low affinity antisera were raised. In contrast, astrocytes or vascular smooth muscle cells expressing β-gal were initially ignored [36,37]. However, β-gal immunization of these mice induced a strong humoral and cellular response that led to lymphocytic infiltrates and pathology. Miller and colleagues noted that EC in an intact vasculature usually shield circulating lymphocytes from peripheral transgene expression [38]. Immune responses against transgene-encoded antigens expressed in the periphery often occurred only after disruption of the tissue and vasculature exposed the parenchymal cells to circulating lymphocytes [39].
To investigate the role of EC in presenting antigens in vivo, we tested the immune responses against β-gal in two different lines of transgenic mice that express β-gal exclusively in their EC. In VWF-lacZ mice, a portion of the promoter and the first intron of the gene encoding von Willebrand factor (VWF), a procoagulant EC protein, drives the expression of β-gal in microvascular EC of the heart and brain [40]. In TIE2-lacZ mice, a portion of the promoter and first intron of the gene encoding TIE2, the receptor for the vascular differentiation factor angiopoietin-1, drives the expression of β-gal in many EC in all organs [41]. We compared the responses of these transgenic mice to wild type, congenic FVB mice following β-gal immunization and tissue transplantation. We found that transgenic mice can mount strong, β-gal-specific cellular and humoral responses without causing any detectable injury to the endogenous β-gal+ EC. Transplanted skin, but not hearts, containing β-gal+ EC triggered β-gal-specific immune responses in FVB mice although both TIE2-lacZ and VWF-lacZ mice accepted the grafts. The failure of the VWF-lacZ and TIE2-lacZ mice to respond against the β-gal+ EC in TIE2-lacZ skin demonstrates that the immune system recognizes and accommodates proteins presented by EC.
Results
Transgenic mice respond to immunization with β-gal
To determine the state of β-gal specific lymphocytes in transgenic mice expressing β-gal in their vascular EC, VWF-lacZ transgenic and wild type mice were immunized with a β-gal expression vector DNA using a gene gun. DNA immunization can induce both humoral and cellular responses [42-44] and B lymphocytes require specific T cell help to form a high titer antiserum against β-gal [45]. Thus, antiserum titers demonstrated the activity of antigen specific B cells and also provided valuable surrogate markers for assessing the state of antigen specific T cell populations. The antiserum titer and antibody composition were determined by an Ig isotype specific ELISA for antibodies to β-gal.
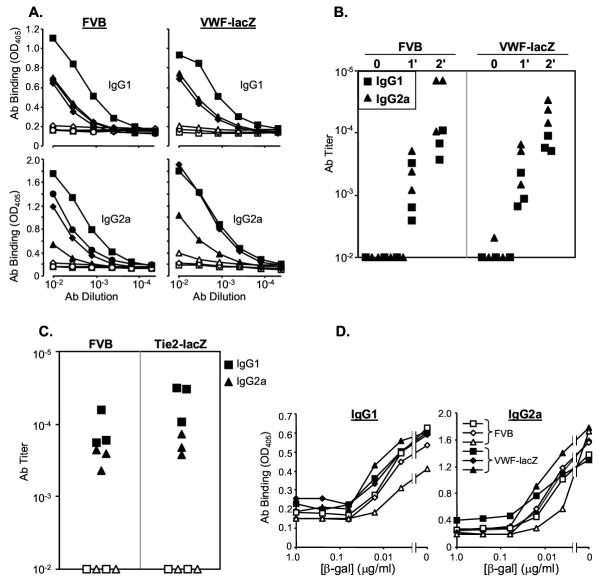
Immunization raised high titer, β-gal specific antisera in both transgenic VWF-lacZ and congenic, wild type FVB mice (Figure 1A, filled symbols). Ig isotypes characteristic of both Th1-like responses (IgG2a) and Th2-like responses (IgG1) were detected at equivalent levels in transgenic and wild type mice (Figure 1A). Serum collected from these mice before immunization (preimmune) or from mice immunized with a control expression vector (CMV-GFP) did not contain β-gal specific antibodies (open symbols, Figure 1A), demonstrating that the antisera were formed in response to the immunization. Both VWF-lacZ and FVB mice generated primary antisera that were much stronger than sera from non-immune mice and the secondary responses were even stronger, demonstrating maturation of the immune response in both transgenic and wild type mice (Figure 1B). Similarly, immunized TIE2-lacZ transgenic mice also generated strong antisera against β-gal (Figure 1C). Thus, expression of β-gal in the EC of these transgenic mice did not appear to alter their immune responses against β-gal.
Figure 1.
Genetic immunization with a β-gal expression vector induces strong humoral responses in wild type mice (FVB) and transgenic mice (VWF-lacZ and TIE2-lacZ) that express β-gal in their endothelial cells A. Equivalent β-gal specific antisera are generated in FVB and VWF-lacZ mice. ELISAs were performed on antisera from mice immunized 6 weeks earlier with a gene gun. Closed markers represent sera from mice immunized with the expression vector CMV-β gal. Open markers represent preimmune sera (VWF-lacZ) or sera from mice immunized with a control expression vector (CMV-GFP, FVB). Similar results were obtained in two independent experiments. B. The humoral response matures in wild type and transgenic mice. Titers of antibodies in control sera (0) and sera from mice immunized once (1') or twice (2') with the CMV-β-gal expression vector DNA are shown. Similar results were obtained in two independent experiments. C. TIE2-lacZ mice respond against β-gal. Antisera of wild type (FVB) and TIE2-lacZ mice immunized once 6 weeks previously are shown. Closed symbols represent sera assayed on plates coated with β-gal, open symbols represent assays on control, BSA-coated plates. Similar results were obtained in two independent experiments. Titers of sera from pre- or un-immunized mice were <10-2 (not shown). D. Soluble β-gal competes for antibodies in sera from FVB and transgenic mice equivalently. Sera from immunized and boosted mice were mixed with soluble β-gal at the concentrations indicated and incubated overnight at 4°C. Free antibodies were then detected by adding the mixture to β-gal coated wells.
B lymphocytes secreting high affinity antibodies can be the most susceptible to tolerance induction [35]. To compare the affinities of the β-gal specific antisera from transgenic and wild type mice, we incubated the sera with soluble β-gal before adding them to the coated wells. Once bound to antigen, high affinity antibodies are less likely to dissociate and bind the plated antigen, providing a good measure of affinity [46]. Equivalent levels of soluble β-gal inhibited sera from wild type and transgenic mice, suggesting that they have equivalent affinities for β-gal (Figure 1D). Plate binding was halved (IC50) between 10–100 pM free β-gal, demonstrating the generation of high affinity antisera in both strains. Surface plasmon resonance assays also suggested equivalent antibody affinities in wild type and transgenic mouse sera (Biacore, data not shown).
We also compared immune responses to immunization with β-gal protein. Transgenic and wild-type mice were immunized with β-gal protein emulsified in complete Freund's adjuvant (CFA). Both strains of transgenic mice and the wild type mice responded with high titer antisera containing both IgG1 and IgG2a isotype antibodies (Figure 2).
Figure 2.
Immunization with β-gal protein in adjuvant raises strong antibody responses in wild type (FVB) and transgenic (VWF-lacZ and TIE2-lacZ) mice. Serum samples were taken 2 weeks after boosting with soluble β-gal and specific antibodies were measured by ELISA. Closed symbols represent sera assayed on β-gal coated plates and open symbols represent sera assayed on control, BSA-coated plates.
Specific T cells in TIE2-lacZ and VWF-lacZ mice respond against "self" β-gal
To analyze directly the T cell responses in transgenic mice, we measured the responses of lymph node T cells from immunized mice in vitro. Proliferation was measured by tritiated ([3H]) thymidine incorporation. The expansion of CD4+ (helper) and CD8+ (cytotoxic) T cell populations was measured by immunostaining and FACS analysis. Activated T cells were detected by their expression of CD25 (IL-2 growth factor receptor) and effector functions were assessed by measuring IFN-γ secretion.
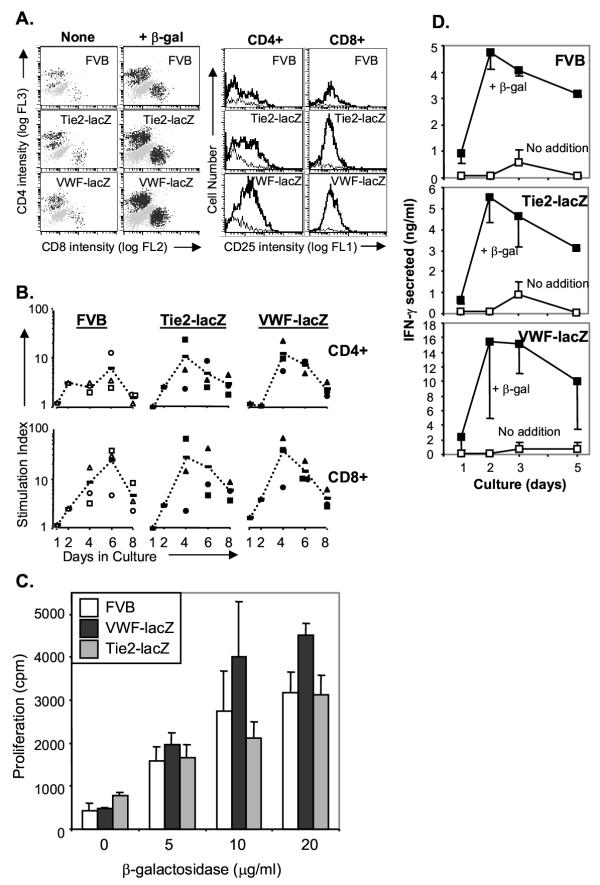
In cultures that received β-gal protein, both CD4+ and CD8+ T cells from wild type and transgenic mice were activated (CD25+) on day 4 in vitro (Figure 3A). Cultures that received β-gal protein antigen contained many more cells than those that did not. We compared the kinetics of activation in the three mouse strains. The data compiled from several independent experiments revealed that activation of CD4+ and CD8+ T cells from transgenic and FVB mice peaked between days 4 and 6 (Figure 3B). To determine the antigen presentation pathways, monoclonal antibodies that bind FVB (H-2q) MHC class I or class II molecules were added at the beginning of the culture (34-5-3S or 15-1-5P hybridoma supernatants, ATCC). Each antibody partially blocked T cell proliferation, suggesting that β-gal peptides are presented by both MHC class I and class II molecules (data not shown).
Figure 3.
T cells from immunized FVB and transgenic VWF-lacZ and TIE2-lacZ mice respond to β-gal in vitro. A. CD4+ and CD8+ lymph node T cells proliferate and express IL-2 receptors (CD25+) in response to β-gal. Representative FACS profiles are shown. Lymph node cells were obtained from mice 7 days after in vivo priming in all the experiments shown. Lymph node cells (2 × 105) were cultured in flat bottom wells and stained for CD4, CD8, and CD25 on day 4. The histograms represent CD25 expression in cultures receiving β-gal (thick line) or not (thin line). B. CD25+ lymphocyte populations expand in cultures of lymph node cells from FVB and transgenic mice. The results of three independent FACS experiments are shown, each represented with a different symbol, and the mean response at each time is indicated with a dash. For the stimulation indices, the background proliferation of cultures receiving no addition or ovalbumin additions were averaged. C. Wild type and transgenic T cells display equivalent avidity for β-gal. Lymph node cells (105) were cultured with irradiated (1500 R) splenocytes (106) in flat bottom wells with the indicated concentrations of β-gal. Results for day 4 are shown. Similar results were obtained in more than 4 independent dose-response proliferation assays. D. FVB and transgenic T cells secrete IFN-γ in response to β-gal. Lymph node cells (2 × 105) were cultured in round bottom wells with β-gal (closed squares) or without (open squares). Culture superantants were recovered at the times indicated, one sample per culture, three cultures per point, and IFN-γ was measured by ELISA.
Tolerance may be most evident in the T cell populations with highest avidity for the antigen [47]. T cell avidity can be estimated by reducing the antigen dose, which limits antigen more rapidly for lower avidity T cells. T cells from transgenic and FVB mice showed equivalent dose-response relationships (Figure 3C), suggesting that T cells with similar avidities respond against β-gal in wild type and transgenic mice.
We next tested the effector functions of β-gal specific T cells. IFN-γ is an important antiviral cytokine produced by T cells. Recently, IFN-γ was shown to also induce blood vessel remodeling in the absence of leukocytes, suggesting that IFN-γ may link pathological changes in blood vessels to immune responses occurring within the vessel wall [48]. To test IFN-γ secretion, lymph node cells were restimulated immediately ex vivo with β-gal. Equivalent amounts of IFN-γ induced by β-gal were detected in the supernatants of cultured lymph node cells from transgenic and wild type mice (Figure 3D). This was true at all times after the initiation of culture, with the peak accumulation occurring between days 2 and 3. However, substantial variation was observed in the responses of the TIE2-lacZ mice. Whereas lymph node cells from FVB and transgenic VWF-lacZ mice responded to β-gal by secreting equivalent amounts of IFN-γ in 5 out of 5 experiments, lymph node cells from TIE2-lacZ mice produced equivalent or higher amounts of IFN-γ twice, lower amounts twice, and were unresponsive once (not shown). Although the reason for variation in the TIE2-lacZ responses is uncertain, it is clear that effector T cell populations from the transgenic mice are capable of responding normally following immunization with β-gal.
Transgenic mice respond to infection with vaccinia virus encoding β-gal
It was possible that conventional immunization protocols did not efficiently induce mature T cells capable of responding aggressively against intracellular proteins. Immune responses against microbial products can produce autoimmunity when they cross-react with self-proteins [49]. We attempted to initiate self-reactivity by infecting transgenic mice with a recombinant vaccinia virus encoding β-gal (β-gal-VV).
Wild type FVB, VWF-lacZ, and TIE2-lacZ mice all responded to β-gal-VV infection with antisera specific for β-gal (8 mice from each strain, 2 independent experiments, data not shown). Lymphocyte responses in a small number of mice were also tested ex vivo. One week after infection, splenocytes from wild type and transgenic mice proliferated and secreted IFN-γ in response to cells infected with β-gal-VV and cells expressing a β-gal transgene but not uninfected cells alone (data not shown). All of the mice remained healthy for over two months post-infection.
EC continue to express β-gal in immune, transgenic mice
Transgenic mice remained apparently healthy for over 9 months after immunization with DNA or protein. Antiserum specific for β-gal would not be expected to bind EC in transgenic mice because the enzyme is expressed in the cytoplasm and not on the cell surface. However, specific T cells could perhaps respond against β-gal peptides presented on EC. To detect any immune effects on the vasculature, organs were harvested, fixed and sectioned, and blood vessels were stained and analyzed by morphometry. The heart (left ventricle), liver, lung, and spleen, the infrarenal aorta, ascending aorta, coronary artery, carotid artery, and iliac artery were examined in cross section. There were no histological abnormalities. No changes were observed in the areas of the lumen, intima, media or vessel (defined as within the external elastic lamina) of any of the large vessels examined (data not shown).
It was possible that mice reduced their expression of the lacZ transgene upon mounting an immune response. For example, transgenic mice expressing hepatitis B antigens were shown to reduce their expression of the viral transgenes upon infection with virus [50].
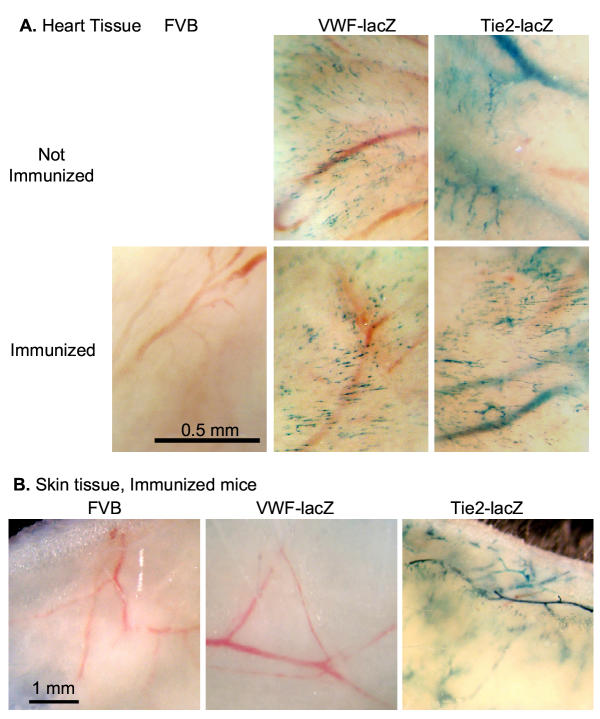
To compare overall lacZ expression in immunized and unimmunized mice, whole tissues were stained with the chromogenic β-gal substrate 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal). Cardiac EC expressed equivalent, easily detectable amounts of β-gal in immunized and unimmunized TIE2-lacZ and VWF-lacZ mice but not in control FVB mice (Figure 4A). Dermal EC also continued to express β-gal at comparable levels in immunized and unimmunized TIE2-lacZ mice (Figure 4B). Therefore, EC continued to express β-gal despite the strong humoral and cellular immune responses specific for β-gal.
Figure 4.
EC continue to express β-gal in transgenic VWF-lacZ and TIE2-lacZ mice mounting strong immune responses against β-gal. A. EC in cardiac vessels of immunized, transgenic mice continue to make β-gal. Control, unimmunized mice and mice immunized and boosted with β-gal protein were sacrificed and their hearts were harvested and stained with X-gal. Representative fields are shown. Similar results were obtained in four independent experiments, two analyzing mice immunized with β-gal protein and two analyzing mice immunized with β-gal expression vectors. B. Dermal EC of immunized, transgenic TIE2-lacZ mice continue to express β-gal. Mice were sacrificed and full-thickness portions of their skins were stained with X-gal. The underside is shown. Unimmunized TIE2-lacZ mice expressed equivalent levels of β-gal, as assessed by X-gal staining (not shown).
Transgenic heart transplants are accepted and retain β-gal+ EC
Transplantation offers an in vivo test of immune recognition. To test immune responses against cardiac EC expressing β-gal, we performed heterotopic heart transplants from TIE2-lacZ or VWF-lacZ mice into wild type FVB mice. Graft survival was monitored by palpation and the strength of the graft heartbeat was noted.
In three series of transplantations, only 9 out of 35 transplanted hearts or hosts did not survive the procedure, failing within 1 week for technical reasons. Nearly all transgenic donor hearts that survived 1 week continued to beat for over 60 days after transplantation into FVB mice (11 out of 12 TIE2-lacZ; 10 out of 12 VWF-lacZ). Two control FVB transplants showed similar graft survival. β-gal specific antisera were not induced in FVB hosts of transgenic hearts (data not shown). Transgenic grafts survived even when the recipients were sensitized before transplantation by TIE2-lacZ skin graft or by immunization with β-gal protein in adjuvant (data not shown).
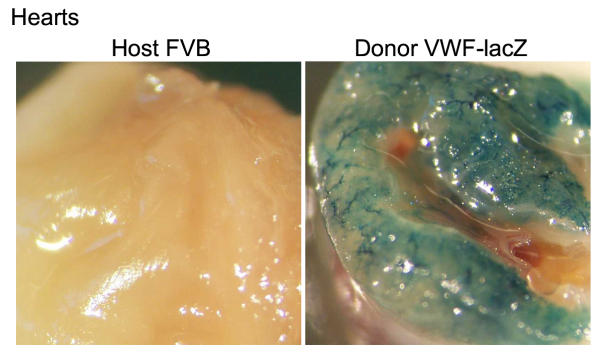
It was possible that the donor EC were replaced or stopped making β-gal. To test β-gal expression in the donor EC, hearts from long-term graft survivors were harvested and stained for β-gal expression. The vascular EC within the donor hearts continued to express β-gal (Figure 5) and the graft vessels were histologically normal (data not shown). Therefore, the transplanted hearts survived despite the continued expression of β-gal by their EC.
Figure 5.
Transplanted hearts from transgenic mice are tolerated and continue to express β-gal. Heterotopic transplants were harvested 3 months after transplantation and stained with X-gal. Similar results were obtained in 2 additional experiments.
β-gal+ EC in skin grafts trigger immunity and are cleared from wild-type FVB but not transgenic VWF-lacZ hosts
Skin grafting is a more rigorous test of immunological tolerance, capable of detecting very small differences between host and donor tissues. We performed full thickness skin transplants between TIE2-lacZ mice and wild type FVB mice. Full thickness grafts included the blood vessels underlying the muscle layer.
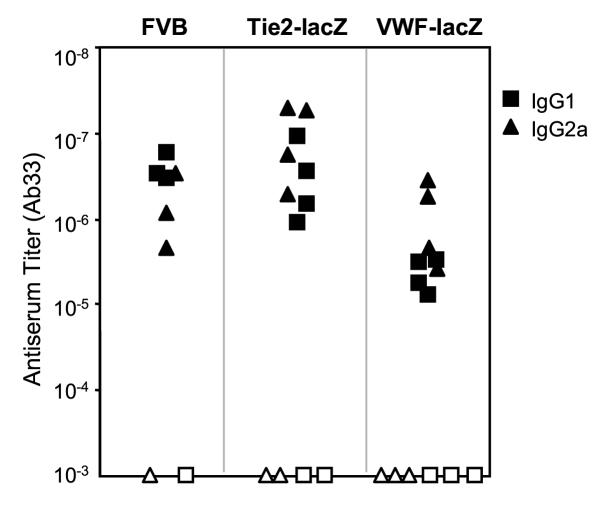
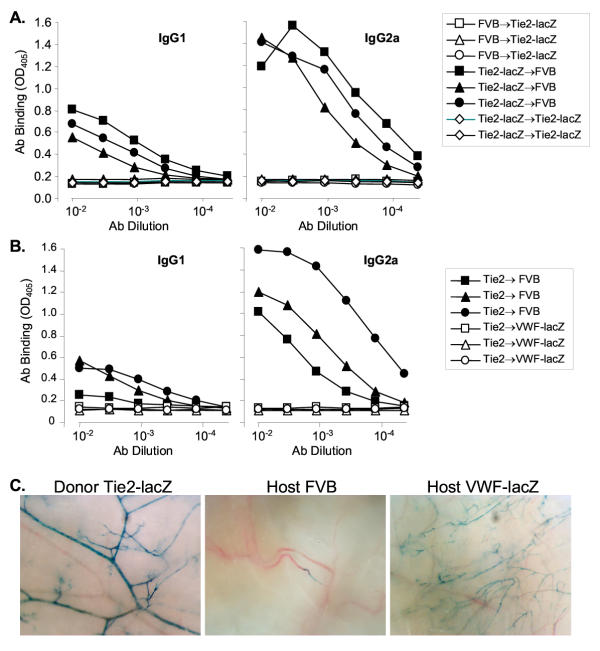
Skin grafts between transgenic TIE2-lacZ and wild type FVB mice appeared to survive equivalently on both hosts (5 separate experiments, totaling over 20 host mice of each strain). When skin grafts were reversed before placement and stapling, the direction of the re-grown fur also indicated that both hosts accepted the grafts. However, β-gal specific antibodies were detected in the serum of FVB mice engrafted with TIE2-lacZ skin (Figure 6A, representative of 5 separate experiments). The antibodies were largely of the IgG2a isotype, which predominate in cellular, Th1-mediated responses. No β-gal specific antibodies were detected in TIE2-lacZ mice engrafted with FVB skin or TIE2-lacZ mice engrafted with TIE2-lacZ skin.
Figure 6.
β-gal+ EC trigger immune responses and are cleared from TIE2-lacZ skin grafts onto wild type FVB but not VWF-lacZ hosts. A. ELISA of sera from reciprocal donor-host skin transplants detects β-gal specific antibodies in FVB but not TIE2-lacZ hosts. Similar results were obtained in 3 separate experiments, in which 7 out of 8 wild type mice generated strong IgG2a isotype antibodies to β-gal following TIE2-lacZ skin grafts. B. VWF-lacZ mice do not raise β-gal-specific antisera following grafting with TIE2-lacZ skin. Grafting and antisera were analyzed as described above.C. EC expressing β-gal are cleared from TIE2-lacZ skin grafts onto host wild type FVB but not VWF-lacZ mice. Photomicrographs of the underside of X-gal stained donor TIE2-lacZ skin and TIE2-lacZ skin grafts from FVB or VWF-lacZ hosts are shown.
To test whether β-gal expression by cardiac EC had any effect on the host immune response, VWF-lacZ mice were grafted with TIE2-lacZ skin. In 3 separate experiments, all but one of the control FVB mice (7 out of 8) and none of the VWF-lacZ mice (8 out of 8) developed antibodies, suggesting the VWF-lacZ mice accepted the β-gal+ EC in the donor TIE2-lacZ skin grafts (Figure 6B). In addition, the β-gal expression by EC within the skin grafts was analyzed by X-gal staining. All TIE2-lacZ skin grafts transplanted onto VWF-lacZ mice contained many β-gal+ EC whereas virtually no β-gal+ EC were observed in the grafts transplanted onto FVB mice (Figure 6C). The organization of the β-gal+ vessels within the TIE2-lacZ graft was disrupted, suggesting that the dermal vasculature had undergone remodeling but not replacement during engraftment (Figure 6C, compare TIE2-lacZ donor vs. VWF-lacZ host). The continued presence of β-gal+ EC in grafts on VWF-lacZ mice demonstrates that EC normally remain in the graft and suggests that EC were cleared from the grafts on FVB mice, probably by the host immune response.
We also tested the effect of EC activation on lymphocyte recognition by denuding endothelium from femoral arteries by wire injury in TIE2-lacZ mice immunized against β-gal. Vessels were allowed to repair for 4 weeks and then they were analyzed by morphometry and staining for β-gal. The EC within the repaired vessels continued to express β-gal and there were no changes in the intimal, medial or overall vessel areas (Jun Yu and William Sessa, personal communication), suggesting that activated EC in large vessels are not able to activate specific T cells in vivo.
Discussion
Transgenic mice have been widely used to analyze immune recognition of particular cell types in vivo (reviewed in [51]). Previous studies have found that transgenes expressed in the periphery were usually ignored initially, perhaps in part because naïve T cells do not circulate through peripheral tissues [36,37]. Other studies showed that autoimmunity could result if lymphocytes specific for transgene-encoded proteins trafficked into the parenchyma following either tissue disruption or immunization [38,39]. Similarly, immunization with self proteins in adjuvant can produce autoimmunity against antigens that are normally sequestered from the naive immune system (e.g., thyroglobulin and thyroiditis, myelin basic protein and encephalopathy).
EC have been called "semiprofessional" APC because they do not express certain costimulatory molecules yet they enhance T cell responses in vitro and are thought to stimulate alloresponses in vivo [9-12,52]. To determine the ability of EC to act as APC in vivo, we analyzed the behavior of β-gal-specific lymphocytes in transgenic mice that express β-gal exclusively in their EC. We reasoned that if EC normally presented antigen to circulating T cells, then β-gal-specific T cells would be anergized (rendered unresponsive) or deleted to maintain self-tolerance. In this case, immune responses against β-gal would be reduced or absent. Alternatively, if EC did not normally act as APC, then specific T cells would be responsive and help to generate strong immune responses. Once activated by professional APC, memory T cells might be subsequently stimulated by, and target, EC expressing the transgene. Our results indicate that EC are capable of presenting antigen, but this presentation does not result in anergy or deletion of β-gal-specific T cells, and does not prevent the generation of a strong immune response.
VWF-lacZ mice and TIE2-lacZ immunized with β-gal protein, β-gal-DNA, or vaccinia virus expressing β-gal mounted strong humoral and cellular immune responses against β-gal but showed no evidence of EC damage (Figures 1, 2, 3, 4). One definition of tolerance is the failure to mount a destructive immune response (Ehrlich's "horror autotoxicus", [53,54]). By this definition, these transgenic mice are tolerant of β-gal, despite generating specific antisera and T cells, because they do not appear to injure their own EC that express the antigen. Alternatively, tolerance has also been defined as unresponsiveness to an (otherwise) immunogenic antigen [54]. By this definition, tolerance is not induced because a strong immune response is detected. While the strong β-gal specific antisera would not be expected to damage EC expressing intracellular β-gal [54], it is unclear why the activation of β-gal specific T cells did not lead to vascular pathology.
One possibility, that the β-gal expressed by EC is ignored by the immune system, is not supported by the findings that skin containing β-gal expressing EC triggered an antigen-specific immune response when transplanted onto wild type, but not TIE2-lacZ mice (Figure 6). In addition, the inability of VWF transgenic mice to respond to these skin grafts provides evidence that β-gal expressed by heart and or brain EC is not ignored, but is presented, albeit in a manner that does not render the host completely unresponsive. It is possible that the inflamed setting of the skin graft activated graft EC to become better APC or that dead EC were cross presented by professional APC. However, the observation that VWF-lacZ mice did not raise immune responses against β-gal+ EC in TIE2-lacZ skin grafts (Figure 6B) strongly suggests that specific lymphocytes directly recognize and are rendered unresponsive by resting, endogenous, β-gal+ cardiac EC within the intact vasculature. Proinflammatory stimuli present in the skin grafts were not able to cause VWF-lacZ mice to generate β-gal-specific antibody responses. Furthermore, even though EC at sites of inflammation adopt an activated phenotype and may be better APC, T cells activated by professional APC in the course of an immune response in vivo should be able to recognize quiescent EC. For example, brain EC and HUVEC are good CTL targets in vitro despite being inefficient APC [55-57].
A second explanation for the lack of vascular pathology in the presence of circulating β-gal-specific T cells is that EC in transgenic mice could express a slightly different form of β-gal than the protein or DNA-encoded forms we used for immunization. We sequenced the PCR-amplified lacZ gene from both transgenic strains and found it is identical to the E. coli gene contained in the CMV-lacZ expression construct. Cell-specific post-translational modifications could add or mask epitopes. However, the antibody assays and the T cell proliferation assays demonstrated that many epitopes are common between the purified protein and the forms encoded by the transgenes or the expression vector.
A third possibility is that the destructive immune response is actively controlled by regulatory mechanisms. Others have shown that cytotoxic T cells (CTL) specific for transgene-encoded cytoplasmic proteins expressed in peripheral tissues could be generated from tolerant mice only after extended culture in vitro [38,58-61]. No killing was detectable in vivo when CTL were adoptively transferred to transgenic animals. Such dominant tolerance of many peripheral proteins appears to be established and maintained by regulatory or suppressor T cells, which have been demonstrated in a number of systems in vivo (reviewed in [62]). CTLA-4+ T cells can suppress self-reactivity following weak immunization with a transgene-encoded protein [63]. To examine the possibility that aggressive T cells responded against β-gal in wild-type FVB mice but were controlled in the transgenic mice, we adoptively transferred lymphocytes from immunized, wild type FVB mice into transgenic mice. Immunization was performed with protein in adjuvant or by skin grafting. The adoptive VWF-lacZ and TIE2-lacZ hosts remained healthy for more than 6 months following the transfer of primed spleen cells or lymph node cells (data not shown), suggesting that either aggressive lymphocytes were not primed in wild type mice or their activity was controlled in transgenic mice.
Central tolerance is caused by protein expression within the central immune system and lymphoid organs, principally within the thymic epithelium, usually resulting in complete unresponsiveness against the protein [35]. The investigators who generated the VWF-lacZ and TIE2-lacZ mice thoroughly examined β-gal expression patterns and concluded that in post-embryonic mice, vascular EC alone expressed β-gal [40,41]. We could not detect β-gal mRNA in the thymus of VWF-lacZ mice when we analyzed expression by reverse-transcription, PCR (RT-PCR), although we could easily detect β-gal mRNA in VWF-lacZ hearts and TIE2-lacZ thymus tissues (not shown). However, it is possible that undetectable levels of expression in the thymus or elsewhere in VWF-lacZ and TIE2-lacZ mice cause an incomplete central tolerance that is responsible for their acceptance of TIE2-lacZ skin.
Immune tolerance is usually a consequence of T cell tolerance, which is achieved through ignorance, clonal anergy, clonal exhaustion, suppression, or a combination of these mechanisms [58,64,65]. The restricted circulation of naïve T cells through only the lymphoid organs helps maintain ignorance of many peripheral proteins. However, any circulation would expose T cells to antigens expressed by vascular EC. Unlike some cell types such as neurons, which express almost no MHC proteins, murine and human EC express easily detectable levels of MHC class I proteins in vivo [29,30]. The EC in these transgenic mice also express significant amounts of β-gal (Figure 4), suggesting that MHC-peptide complexes are relatively abundant. Very few MHC-peptide complexes are necessary to trigger mature T cells [66]. Tolerance can result from anergy caused by inadequate costimulation [67]. However, many β-gal specific T cells in the TIE2-lacZ mice proliferate in vitro after priming in vivo (Figure 3), demonstrating that they were not anergized in the many different vascular beds containing the β-gal+ EC.
Conclusions
We conclude that EC effectively present intracellular "self" proteins to the immune system, resulting in a form of tolerance. However, antigens presented by EC do not anergize naïve lymphocytes that are activated by conventional immunization protocols or by vaccination. EC are also not effective targets of these lymphocytes activated by conventional immunization. Restricted antigen presentation by EC may contribute to the inability of the immune response to clear certain chronic infections by pathogens that target EC, such as Chlamydia pneumonia and cytomegalovirus.
Methods
Mice
VWF-lacZ transgenic mice were provided for this study by Dr. William Aird (Beth Israel Deaconess Medical Center, Boston) [40]. TIE2-lacZ transgenic mice were generated by Sato and colleagues [41] and purchased from the Jackson Laboratories (Bar Harbor, ME). Both transgenic strains were generated and maintained on the FVB background. The lines were maintained by backcrossing hemizygous transgenic mice to FVB/NJ mice (Jackson Laboratories). Transgenic offspring were identified from ear punches at approximately 6 weeks of age. The punches were digested (100 μl, 10 μg proteinase K, 10 mM KCl, 10 mM Tris pH 8, 2.5 mM MgCl2, 0.45% NP-40, 0.45% Tween-20) for 3 h at 55°C then heated for 10 min. at 95°C. PCR was performed using 1 μl of the digest with primers for lacZ (Bgal.51: GCGTTGGCAATTTAACCG and Bgal.31: TGGTAATGGTAGCGACCG, each at 0.5 μM) and a control sequence tagged site (STS) from the X chromosome (M37335, each primer at 0.1 μM). The cycling conditions were: 1 min at 94°C, then 30 cycles of 30 s at 94°C, 30 s at 53°C, and 30 s at 72°C, then 10 min. at 72°C. The PCR products were separated on an agarose gel and detected by staining with ethidium bromide (788 bp β-gal, 200 bp M37335).
Antiserum generation and assay (ELISA)
For generating antisera, mice were immunized by intraperitoneal (i.p.) injection of 50 μg β-gal protein emulsified in complete Freund's adjuvant (CFA, 50 μl final volume) and boosted 4 weeks later with 50 μg β-gal, i.p., in phosphate buffered saline. β-gal (CAS 9031-11-2) protein was purchased from Sigma (recombinant, G3153) and Calbiochem (345788). These preparations were used interchangeably and no differences were observed. Alternatively, mice were immunized with a gene gun. Briefly, an expression vector encoding β-gal (100 μg, CMV-β-gal, Clontech, CA) was precipitated with CaCl2 onto gold beads (21 mg, 2.6 μm diameter, Aldrich or 1 μm BioRad), which were dried onto plastic tubing (1/8" × 3/32" Tefzel, Bio-Rad). The tubing was cut (25 × 1 cm) and loaded into a high pressure gun (Agracetus, Middleton, WI). With a burst of gas (300 psi), the beads were delivered into the skin on the backs of mice whose fur had been clipped. Mice were boosted with a second gene gun immunization after 6 weeks.
Serum samples were taken before and after immunizations, as noted in the figures. Blood was allowed to clot at room temperature then it was placed at 4°C for 2–12 h. The clots were spun down at 10,000 × g and the sera were collected and stored frozen at -20°C.
Wells of a 96-well plate (Microtest III, Becton-Dickinson) were coated with β-gal protein (2 μg/ml PBS, 50 μl/well, overnight), blocked (50 μl 1% BSA/PBS, 2 h), and rinsed with wash buffer (0.05% Tween 20, 138 mM NaCl, 2.7 mM KCl, 50 mM Tris, pH 8.0). Sera were diluted in dilution buffer (1% BSA/PBS) either 1:200 initially and 1:3 serially or 1:1,000 initially and 1:5 serially then added (50 μl). After incubation (2 h), the wells were washed and secondary antibodies were added (50 μl, 1:2,000 diluted alkaline phosphatase conjugated goat anti-mouse IgG1 or IgG2a, ICN Biochemicals). The specificity of these secondary antibodies was confirmed in a sandwich ELISA on plates coated with a capture antibody (goat anti-mouse Fc, Jackson Immunoresearch) and using the mAbs WT31 (IgG1) and M9144 (IgG2a) as positive and negative controls. After incubation (1 h), wells were washed and substrate was added (50 μl p-nitrophenyl phosphate, Sigma). After color developed (<1 h), the absorption at 405 nm wavelength was measured on a plate reader (Bio-Rad). Antiserum titers were determined from a curve fit to the data.
T cell culture and analysis (FACS, proliferation, IFN-γ)
Mice were immunized by subcutaneous injection in the base of the tail with 25 μg of β-gal emulsified in CFA in a total volume of 50 μl. The draining inguinal lymph nodes were harvested on day 7. Lymph node cells were cultured either alone or with irradiated filler cells (0.2 ml, 96-well plate (Microtest, Falcon) in complete medium (RPMI 1640, 10% FBS, 100 Units/ml penicillin, 100 μg/ml streptomycin, 2.5 mM L-glutamine, 1 mM Sodium pyruvate, 100 μM non-essential amino acids, 50 μM 2-mercaptoethanol, 5 mM HEPES (GIBCO, Grand Island, NY)) with antigen additions at 10 μg/ml or as noted in the Results. For flow cytometric analysis, cells from triplicate wells were harvested, pooled, and incubated with biotin-conjugated rat anti-mouse CD25 (clone PC 615.3, Caltag, Burlingame, CA), washed and then incubated with fluorescein-conjugated streptavidin (Amersham), Quantum Red-conjugated rat anti-mouse CD4 (clone H129.19, Sigma) and R-Phycoerythrin-conjugated rat-anti-mouse CD8a (clone 53-6.7, Sigma or Pharmingen) then analyzed by flow cytometry (FACS Scan, Becton-Dickinson, CA). For proliferation studies, triplicate wells were pulsed with 3H-thymidine 16 hours prior to harvesting. For measurement of secreted IFN-γ, supernatants from triplicate cultures were harvested, centrifuged to remove residual cells, and frozen until sandwich immunoassay (R & D Systems, Minneapolis, MN). Replicates supernatants were assayed individually and the results were averaged. For all T cell cultures, purified protein derivative (PPD, Mycobacterium tuberculosis H37Rv, Mycos Research LLC, Fort Collins, CO) was used as a positive control and ovalbumin (OVA) was used as a negative control. Responses to OVA were not significantly different from the responses of unstimulated cultures (data not shown).
Blood vessel analyses
A portion of skin (~4 cm2) and the heart were harvested from freshly sacrificed mice. Skin sections and the heart base were placed into neutral buffered formalin (10%, Sigma) for 5 min. then washed 4 times in PBS. Staining solution was added (0.25 ml, 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal), 1 mM MgCl2, 10 mM K3Fe(CN)6, 10 mM K4Fe(CN)6 in PBS) and the reaction proceeded at room temperature overnight. Digital photographs were taken (DC290, Kodak, Rochester NY) through a microscope (SMZ1000, Nikon).
Blood vessel morphometry was performed on animals that were perfusion fixed immediately after sacrifice. Vessels were dissected, embedded in OCT, and flash frozen. Sections were cut, stained with hematoxalin and eosin or Elastic van Giesen, and scored under a microscope (Optiphot2, Nikon).
β-gal vaccinia virus infection
Recombinant vaccinia virus containing the lacZ gene (β-gal-VV) was generously provided for these studies by Dr. Nicholas Restifo (NCI, Bethesda, MD). Virus was grown and titered using HeLa cells. Virus stock was prepared by scrape harvesting in PBS, freeze thawing three times, and passing the suspension through a 70 μm sieve. Mice were infected with 107 PFU (0.25–0.35 ml, i.p.).
Skin transplantation
Donor and recipient mice were anaesthetised (0.2 ml xylazene/ketamine per 20 g body weight i.p.). Ophthalmic balm was applied to protect their eyes. Effective anaesthesia was tested before proceeding by pinching. More anaesthetic (~0.05 ml) was administered if the mouse flinched. Fur was clipped off the upper back and a full thickness, ~1 cm square patch of skin was cut from the upper backs. The host skin patch was removed and replaced with the donor skin patch, which was held in place by surgical staples. Mice were kept warm and under observation until they recovered, then single housed until they were healed (~2 w).
Heart transplantation
Heterotopic vascularized cardiac transplantation was performed as previously described [68]. The donor heart was arrested by infusion of 0.5 ml of University of Wisconsin (UW) solution (DuPont, Wilmington, Delaware, USA) into the inferior vena cava and the aortic root was flushed with 0.1 ml of UW solution to expel intracoronary blood thoroughly. The heart was removed from the chest and placed into UW solution on ice. After the recipient abdominal aorta and inferior vena cava were isolated, the donor aorta and pulmonary artery were joined end-to-side to the recipient aorta and vena cava, respectively, using 10-0 nylon suture. After the completion of the anastomoses, the abdomen was closed with a single running suture to all layers. The recipient mouse was then warmed for a few hours during recovery from anesthesia and had free access to water and food. The function of the transplanted hearts was assessed daily by abdominal palpation
Abbreviations
EC, endothelial cells; HUVEC, human umbilical vein endothelial cells; β-gal, E. coli β-galactosidase; X-gal, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside; VWF, von Willebrand factor
Authors' contributions
All authors provided helpful and critical comments. AR helped plan and perform the cellular assays. YW performed the heterotopic heart transplants. JS performed the skin transplantation. BM-K assisted in cellular and molecular assays. WA provided the VWF-lacZ mice. JP and GT suggested the heart transplantation experiments. DJ conceived the study and participated in its design and execution. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Dr. Janet Brandsma and Dr. Mark Shlyankevich for their assistance in using the gene gun and Dr. Deepti Pradhan for conducting the plasmon resonance affinity assay. We thank Ms. Faiya Wang for her assistance in skin transplantation and Ms. Lisa Grass for her assistance in sampling mouse sera. We thank Dr. Jun Yu and Dr. William Sessa for their analysis of the response to vessel injury. This work was supported by grants from the American Heart Association (Heritage Affiliate) and the NIH (HL62188, HL51014).
Contributor Information
Annette L Rothermel, Email: arothermel@niaid.nih.gov.
Yinong Wang, Email: yw66@biomed.med.yale.edu.
Jeffrey Schechner, Email: jeffrey.schechner@yale.edu.
Barry Mook-Kanamori, Email: barry@mook.org.
William C Aird, Email: waird@bidmc.harvard.edu.
Jordan S Pober, Email: jordan.pober@yale.edu.
George Tellides, Email: george.tellides@yale.edu.
David R Johnson, Email: drjohnson@niaid.nih.gov.
References
- Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- de Waal RM, Bogman MJ, Maass CN, Cornelissen LM, Tax WJ, Koene RA. Variable expression of Ia antigens on the vascular endothelium of mouse skin allografts. Nature. 1983;303:426–429. doi: 10.1038/303426a0. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T- helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Marelli-Berg FM, Scott D, Bartok I, Peek E, Dyson J, Lechler RI. Activated murine endothelial cells have reduced immunogenicity for CD8+ T cells: a mechanism of immunoregulation? J Immunol. 2000;165:4182–4189. doi: 10.4049/jimmunol.165.8.4182. [DOI] [PubMed] [Google Scholar]
- Kosaka H, Surh CD, Sprent J. Stimulation of mature unprimed CD8+ T cells by semiprofessional antigen- presenting cells in vivo. J Exp Med. 1992;176:1291–1302. doi: 10.1084/jem.176.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert T, Lipsky P. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J Immunol. 1985;135:3750–3762. [PubMed] [Google Scholar]
- Vora M, Yssel H, de Vries J, Karasek M. Antigen presentation by human dermal microvascular endothelial cells. Immunoregulatory effect of IFN-γ and IL-10. J Immunol. 1994;152:5734–5741. [PubMed] [Google Scholar]
- Briscoe DM, Henault LE, Geehan C, Alexander SI, Lichtman AH. Human endothelial cell costimulation of T cell IFN-gamma production. J Immunol. 1997;159:3247–3256. [PubMed] [Google Scholar]
- Hirschberg H, Evensen S, Henriksen T, Thorsby E. The human mixed lymphocyte-endothelium culture interaction. Transplantation. 1975;19:495–504. doi: 10.1097/00007890-197506000-00008. [DOI] [PubMed] [Google Scholar]
- Ashida E, Johnson A, Lipsky P. Human Endothelial Cell-Lymphocyte Interaction: Endothelial cells fuction as accessory cells necessary for mitogen-induced human T lymphocyte activation in vitro. J Clin Invest. 1981;67:1490–1499. doi: 10.1172/JCI110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan EC, Smith BR, Doukas JT, Miller RA, Pober JS. Vascular endothelial cells enhance T cell responses by markedly augmenting IL-2 concentrations. Cell Immunol. 1989;118:166–177. doi: 10.1016/0008-8749(89)90366-3. [DOI] [PubMed] [Google Scholar]
- Hughes C, Savage C, Pober JS. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J Exp Med. 1990;171:1453–1467. doi: 10.1084/jem.171.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Louis J, Lederer J, Lichtman A. Costimulator deficient antigen presentation by an endothelial cell line induces a nonproliferative T cell activation response without anergy. J Exp Med. 1993;178:1597–1605. doi: 10.1084/jem.178.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci U S A. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, Hauser IA, Voll RE, Emmrich F. Arterial and venular endothelial cell costimulation of cytokine secretion by human T cell clones. J Leukoc Biol. 1998;63:612–619. doi: 10.1002/jlb.63.5.612. [DOI] [PubMed] [Google Scholar]
- Karmann K, Min W, Fanslow WC, Pober JS. Activation and homologous desensitization of human endothelial cells by CD40 ligand, tumor necrosis factor, and interleukin 1. J Exp Med. 1996;184:173–182. doi: 10.1084/jem.184.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayyamian S, Hutloff A, Buchner K, Grafe M, Henn V, Kroczek RA, Mages HW. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc Natl Acad Sci U S A. 2002;99:6198–6203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitomi A, Hori T, Imura A, Uchiyama T. Vascular endothelial cells provide T cells with costimulatory signals via the OX40/gp34 system. J Leukoc Biol. 2000;68:111–118. [PubMed] [Google Scholar]
- Ma W, Pober JS. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J Immunol. 1998;161:2158–2167. [PubMed] [Google Scholar]
- Perez VL, Henault L, Lichtman AH. Endothelial antigen presentation: stimulation of previously activated but not naive TCR-transgenic mouse T cells. Cell Immunol. 1998;189:31–40. doi: 10.1006/cimm.1998.1362. [DOI] [PubMed] [Google Scholar]
- Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984;38:287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984;38:293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Goes N, Urmson J, Hobart M, Halloran P. The unique role of interferon-γ in the regulation of MHC expression on arterial endothelium. Transplantation. 1996;62:1889–1894. doi: 10.1097/00007890-199612270-00036. [DOI] [PubMed] [Google Scholar]
- Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- Cuvelier SL, Patel KD. Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: a role for endothelium-associated eotaxin-3. J Exp Med. 2001;194:1699–1709. doi: 10.1084/jem.194.12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M, Tohka S, Jalkanen S. Human vascular adhesion protein-1 (VAP-1) plays a critical role in lymphocyte-endothelial cell adhesion cascade under shear. Circ Res. 2000;86:1245–1251. doi: 10.1161/01.res.86.12.1245. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/S1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Theopold U, Kohler G. Partial tolerance in beta-galactosidase-transgenic mice. Eur J Immunol. 1990;20:1311–1316. doi: 10.1002/eji.1830200617. [DOI] [PubMed] [Google Scholar]
- Borrow P, Cornell JL, Ruppe MD, Mucke L. Immunization-induced inflammatory infiltration of the central nervous system in transgenic mice expressing a microbial antigen in astrocytes. J Neuroimmunol. 1995;61:133–149. doi: 10.1016/0165-5728(95)00082-D. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Freigang S, Jaggi M, Kurrer MO, Pei YC, Vlk L, Odermatt B, Zinkernagel RM, Hengartner H. Linking immune-mediated arterial inflammation and cholesterol-induced atherosclerosis in a transgenic mouse model. Proc Natl Acad Sci U S A. 2000;97:12752–12757. doi: 10.1073/pnas.220427097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan G, Allison J, Miller JF. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 1989;339:622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- Lo D, Freedman J, Hesse S, Brinster RL, Sherman L. Peripheral tolerance in transgenic mice: tolerance to class II MHC and non-MHC transgene antigens. Immunol Rev. 1991;122:87–102. doi: 10.1111/j.1600-065x.1991.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Aird W, Edelberg J, Weiler-Guettler H, Simmons W, Smith T, Rosenberg R. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol. 1997;138:1117–1124. doi: 10.1083/jcb.138.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci U S A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Johnston SA, Riedy M, DeVit MJ, McElligott SG, Sanford JC. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc Natl Acad Sci U S A. 1991;88:2726–2730. doi: 10.1073/pnas.88.7.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca F, Kunkl A, Fenoglio D, Fowler A, Sercarz E, Celada F. Constraints in T-B cooperation related to epitope topology on E. coli beta-galactosidase. I. The fine specificity of T cells dictates the fine specificity of antibodies directed to conformation-dependent determinants. Eur J Immunol. 1985;15:345–350. doi: 10.1002/eji.1830150408. [DOI] [PubMed] [Google Scholar]
- Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. Measurements of the true affinity constant in solution of antigen- antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985;77:305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Cordaro TA, Kioussis D, Haanen JB, Schumacher TN, Kruisbeek AM. Tracing and characterization of the low-avidity self-specific T cell repertoire. Eur J Immunol. 2000;30:1458–1468. doi: 10.1002/(SICI)1521-4141(200005)30:5<1458::AID-IMMU1458>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol. 2001;2:797–801. doi: 10.1038/ni0901-797. [DOI] [PubMed] [Google Scholar]
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- Tindle RW. Peripheral T-cell tolerance defined through transgenic mouse studies. Autoimmunity. 2001;33:135–149. doi: 10.3109/08916930108995998. [DOI] [PubMed] [Google Scholar]
- Pober J, Orosz C, Rose M, Savage C. Can graft endothelial cells initiate a host anti-graft immune response? Transplantation. 1996;61:343–349. doi: 10.1097/00007890-199602150-00001. [DOI] [PubMed] [Google Scholar]
- Silverstein AM. The History of Immunology. In: Paul WE, editor. Fundamental Immunology. 4. Philadelphia: Lippincott-Raven; 1999. pp. 19–35. [Google Scholar]
- Schwartz R. Immunological Tolerance. In: Paul WE, editor. Fundamental Immunology. 4. Philadelphia: Lippencott-Raven Press; 1999. pp. 701–739. [Google Scholar]
- Risau W, Engelhardt B, Wekerle H. Immune function of the blood-brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 1990;110:1757–1766. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler TJ, Johnson DR, Pober JS. Human Vascular Endothelial Cells Stimulate a Lower Frequency of Alloreactive CD8(+) Pre-CTL and Induce Less Clonal Expansion than Matching B Lymphoblastoid Cells: Development of a Novel Limiting Dilution Analysis Method Based on CFSE Labeling of Lymphocytes. J Immunol. 2001;166:3846–3854. doi: 10.4049/jimmunol.166.6.3846. [DOI] [PubMed] [Google Scholar]
- Biedermann BC, Pober JS. Human vascular endothelial cells favor clonal expansion of unusual alloreactive CTL. J Immunol. 1999;162:7022–7030. [PubMed] [Google Scholar]
- Nugent CT, Morgan DJ, Biggs JA, Ko A, Pilip IM, Pamer EG, Sherman LA. Characterization of CD8+ T lymphocytes that persist after peripheral tolerance to a self antigen expressed in the pancreas. J Immunol. 2000;164:191–200. doi: 10.4049/jimmunol.164.1.191. [DOI] [PubMed] [Google Scholar]
- Ma H, Ke Y, Li Q, Kapp JA. Bovine and human insulin activate CD8+-autoreactive CTL expressing both type 1 and type 2 cytokines in C57BL/6 mice. J Immunol. 2000;164:86–92. doi: 10.4049/jimmunol.164.1.86. [DOI] [PubMed] [Google Scholar]
- Jones-Youngblood SL, Wieties K, Forman J, Hammer RE. Effect of the expression of a hepatocyte-specific MHC molecule in transgenic mice on T cell tolerance. J Immunol. 1990;144:1187–1195. [PubMed] [Google Scholar]
- Wieties K, Hammer RE, Jones-Youngblood S, Forman J. Peripheral tolerance in mice expressing a liver-specific class I molecule: inactivation/deletion of a T-cell subpopulation. Proc Natl Acad Sci U S A. 1990;87:6604–6608. doi: 10.1073/pnas.87.17.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- Walker L, Ausube L, Chodos A, Bekarian N, Abbas A. CTLA-4 Differentially Regulates T Cell Responses to Endogenous Tissue Protein Versus Exogenous Immunogen. J Immunol. 2002;169:6202–6209. doi: 10.4049/jimmunol.169.11.6202. [DOI] [PubMed] [Google Scholar]
- Maeda H, Fujimoto S, Greene MI. Suppressor T cells regulate the nonanergic cell population that remains after peripheral tolerance is induced to the Mls-1 antigen in T cell receptor Vbeta 8.1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:13257–13262. doi: 10.1073/pnas.230449097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein S, Pritchard-Briscoe H, Anderson TA, Crosbie J, Gammon G, Loblay RH, Basten A, Goodnow CC. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991;251:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- Gimmi C, Freeman G, Gribben J, Gray G, Nadler L. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci (USA) 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qin L, Bai Y, Bielinska AU, Kukowska-Latallo JF, JRB , Jr, Bromberg JS. DNA/dendrimer complexes mediate gene transfer into murine cardiac transplants ex vivo. Molecular Therapy. 2000;1:602–608. doi: 10.1006/mthe.2000.0201. [DOI] [PubMed] [Google Scholar]