Abstract
Food allergy is a common disease that is rapidly increasing in prevalence for reasons that remain unknown. Current research efforts are focused on understanding the immune basis of food allergy, identifying environmental factors that may contribute to its rising prevalence, and developing immunotherapeutic approaches to re-establish immune tolerance to foods. Technological advances such as peptide microarray and MHC class II tetramers have begun to provide a comprehensive profile of the immune response to foods. The burgeoning field of mucosal immunology has provided intriguing clues to the role of the diet and the microbiota as risk factors in the development of food allergy. The purpose of this review is to highlight significant gaps in our knowledge that need answers in order to stem the progression of this disorder that is reaching epidemic proportions.
Keywords: IgE, anaphylaxis, mucosal immunology, microbiota, Th2, Treg, immunotherapy
Food allergies: a growing clinical problem
Food allergies encompass a broad spectrum of disorders secondary to abnormal immunologic responses to food antigens. IgE-mediated reactions are most common and induce a variety of symptoms that are rapid in onset and may manifest as itchy flushing of the skin and/or urticaria, nausea, abdominal pain and/or vomiting, mild to severe bronchospasm and respiratory distress, hypotension, cardiovascular collapse and/or death, with cutaneous and abdominal symptoms by far the most common [1]. Although there are a number of non-IgE-mediated food allergic reactions such as eosinophilic esophagitis and food-protein-induced enterocolitis syndrome, we will focus on IgE-mediated reactions in this review. The exact prevalence of food allergy is difficult to ascertain due to the imprecision of laboratory tests, but recent reviews of the literature estimate that food allergy affects greater than 2% and less than 10% of the US population [2]. The prevalence of food allergy peaks at 6% – 8% during the first few years of life and is most often due to milk, egg, peanut, fish and shellfish, although any food may induce allergic reactions. Most children “outgrow” their allergy to milk, egg, wheat and soy during the first decade, but allergies to peanut, tree nuts, fish and shellfish are often retained for life [1]. Limited data suggest that the prevalence of food allergy has increased in industrialized countries world-wide [3–5], e.g. estimates of peanut and tree nut allergy tripled in American children between 1997 and 2008 [6], but the reason for this increase remains unknown [7]. Similar increases have been seen in the UK and Australia [4, 8]. The “standard of care” for managing food allergies involves proper diagnosis, including a detailed clinical history, laboratory studies (skin prick tests and/or quantification of food-specific IgE, and often oral food challenge), education about strict dietary avoidance, and provision of an emergency plan and medications, e.g. self-injectable epinephrine, for the treatment of accidental ingestions [9]. In recent years the field of food allergy has gone through considerable growth and technical advances have allowed for a growing understanding of the immune basis of clinical reactivity to food allergens. The field has begun to focus on the role of environmental risk factors, including diet and the microbiota that will be reviewed here. A number of immunotherapeutic approaches are currently under investigation, including different routes of immunotherapy (oral, sublingual and epicutaneous), immunotherapy with modified recombinant proteins, and use of anti-IgE monoclonal antibodies combined with immunotherapy [10]. By taking advantage of recent advances at the intersection of immunology, nutrition and microbiome, the field is poised to make significant inroads to the prevention and treatment of food allergy.
Immune profile of food allergy
Humoral responses to food proteins
By definition, IgE-mediated food allergy is characterized by the presence of IgE specific for antigens within the triggering foods. IgE antibodies are commonly found in healthy controls, but for several foods the level of IgE is predictive of clinical reactivity, and probability curves have been established relating the likelihood of tolerating a food based on levels of allergen-specific IgE [11] [12]. The lack of clinical reactivity in those with IgE antibodies to foods may relate to the ratio of allergen-specific to total IgE, the ratio of specific IgE to antibodies of blocking isotypes such as IgG4 or IgA, or may relate to the affinity or clonality of IgE antibodies. The ratio of specific to total IgE has been called the specific activity of IgE, and higher specific activity has been shown to be associated with higher levels of basophil activation [13]. The relevance of specific activity of IgE to clinical reactivity and efficacy of anti-IgE therapy for other allergic diseases has been reviewed by Hamilton et al [14]. However, food allergen specific-to-total IgE ratios have not been found to be more predictive measures of clinical reactivity than food allergen-specific IgE levels alone [15]. Clinical reactivity may reflect the presence or absence of IgE to relevant components of the food. For example, testing of IgE against components of peanut have shown that IgE against the allergen Ara h 2 is predictive of clinical reactivity, while IgE against the allergen Ara h 8 (cross-reactive with the birch pollen allergen Bet v 1) in the absence of IgE to other peanut allergens is predictive of clinical tolerance [16, 17]. These differences in reactivity to components of peanut would not be detected by measurement of specific IgE against the whole peanut extract. The clonality of the IgE response to foods can be measured using peptide microarrays [18, 19]. Clinical reactivity is associated with recognition of a greater number of epitopes, and for peanut, 4 informative epitopes were found to be effective in prediction of clinical reactivity [19]. It has been shown in mice that low and high affinity IgE are generated through distinct immune pathways, and only high affinity IgE can generate anaphylactic responses [20]. Measurement of IgE levels by standard techniques does not reflect affinity and it needs to be determined whether incorporating a measure of affinity [21] would increase the predictive value of IgE measurements. IgG and IgA antibodies to foods are commonly found in food allergic and healthy subjects [22] but do not relate to clinical reactivity. IgG4 and IgA are thought to be protective by functioning as blocking antibodies, and are increased in response to immunotherapy (e.g. oral immunotherapy for peanut [23, 24]), but these antibody levels are generally not predictive of tolerance in the absence of intervention.
Anaphylaxis in mice has been shown to be inducible in the absence of IgE, although generally only at high doses of antigen given systemically. These reactions are mediated by IgG1, and involve activation of macrophages, basophils or mast cells. Although IgG-mediated anaphylaxis has not been demonstrated in man, biomarkers of IgG-mediated anaphylaxis have been proposed [25] that may help to determine if IgG-mediated anaphylaxis contributes to human food allergy. Other candidates for alternative pathways of effector cell activation include immunoglobulin free light chains that are elevated in children with cow’s milk allergy [26]. Mechanism of allergen specificity and effector cell activation have not yet been established for this pathway.
T cell responses to food proteins
Class-switching to IgE is dependent on T cell help, and therefore the T cell response to food antigens in food allergic subjects compared to healthy controls is of particular interest. Early studies used T cell lines grown from peripheral blood of food allergic subjects and found a predominant Th2 phenotype. CFSE-based detection of cells proliferating in response to culture with antigen confirmed a Th2 profile in allergen-responsive T cells from food allergic subjects, and a Th1 profile in healthy controls [27]. More recently, CD154-based detection of antigen-specific T cells after short-term stimulation was used to compare the T cell phenotype of patients with IgE-mediated food allergy, non-IgE-mediated food allergy, and healthy controls [28]. Healthy controls had few allergen-specific T cells that expressed low levels of IFN-γ and TNFα. Food allergic subjects had significantly greater frequencies of allergen specific T cells. IL-4 and IL-13 that drive IgE-class switching were elevated in both IgE-mediated and non-IgE-mediated food allergy, therefore other factors beyond production of Th2 cytokines must be present to allow for IgE sensitization to occur. This may relate to homing properties of the T cells. Human T follicular helper (Tfh) cells provide better help for IgE class-switching than non-Tfh cells that express IL-4 [29]. Tfh cells can traffic to B cell follicles through their expression of the chemokine receptor CXCR5, where they are optimally located to provide help for B cell isotype switching. Therefore, this subset of T helper cells may be most critical in regulating inappropriate IgE responses to foods, but the contribution of Tfh cells remains to be addressed in the context of IgE-mediated food allergy. MHC class II tetramers have also been used to identify food allergen-specific T cells in healthy controls versus allergic subjects without the need for re-stimulation of cells [30]. An epitope from the peanut allergen Ara h 1 was used, and again the frequency of allergen-specific T cells was substantially higher in food allergic subjects compared to healthy controls, with the number in healthy controls being too low to reliably phenotype. In comparison to the CD4+ population as a whole, these tetramer-positive cells were enriched for the memory marker CD45RA, CD25, and the skin-homing chemokine receptor CCR4, but not the skin-homing receptor CLA. The antigen specific T cells expressed lower levels of the gut-homing addressin β7 than the general pool of CD4+ T cells, indicating that they are not likely to have originated from the gut. This may relate to sensitization by routes other than the gastrointestinal tract, as will be discussed in more detail later. In peanut allergic subjects, the range of antigen-specific CD4+ T cells detectable with this method was in the range of 10 cells per million CD4+ T cells. This low frequency underscores the difficulty of obtaining sufficient allergen-specific T cells for study, particularly when studying pediatric populations. The even lower frequency of antigen-specific T cells in healthy controls makes them difficult to phenotype, so the question has not yet been answered if an active regulatory phenotype consistent with experimentally induced mucosal immune tolerance is the normal immune response to antigens in the diet. A subset of patients with a deletion in a non-coding region of Foxp3 that results in low Foxp3 protein expression develop a severe allergic phenotype associated with hyper IgE, eosinophilia, villous atrophy, and elevated Th2 cytokines in the intestinal mucosa [31]. Mice lacking Tregs induced in the periphery (but having normal level of thymus-derived natural Tregs) develop a spontaneous Th2-biased inflammation at mucosal sites, and develop antibodies to components of the mouse chow (the isotype of these antibodies was not reported) [32]. These data show that Tregs have a role in the suppression of inappropriate immune responses to food antigens. More data are required to determine if food allergy is really a consequence of an impaired regulatory response to foods, which if true, would suggest that immune tolerance by oral allergen immunotherapy may be unlikely to develop in the absence of additional therapeutic targeting.
Immune mechanisms of food-induced anaphylaxis
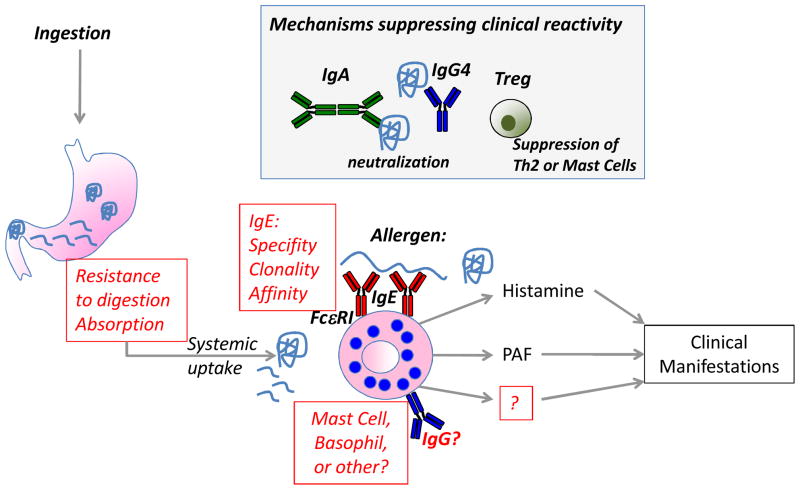
In a sensitized individual, re-exposure to the food allergen by the oral route can lead to clinical manifestations at local and systemic sites. Figure 1 illustrates mechanisms contributing to the development of clinical reactivity versus tolerance to foods. The skin is the most commonly affected site following a food challenge, followed by symptoms involving the gastrointestinal and respiratory tracts [33]. In mice it has been shown that antigen must be absorbed from the gut into the systemic circulation in order to trigger reactions [34]. In mice, anaphylaxis is almost entirely mast cell-dependent, with only marginal contributions from basophils [35]. The general absence of elevated serum tryptase in food allergic reactions, and studies in peanut-allergic patients on the kinetics of clinical protection using the anti-IgE antibody omalizumab [36] point to a more significant contribution of basophils rather than mast cells in man. Clinical protection in response to omalizumab treatment was observed at a time-point when basophil reactivity was reduced but mast cell activation (measured by skin test reactivity) was not. However, it should be noted that these results are open to interpretation – at the first post-treatment challenge, skin tests were reduced but did not reach the level of statistical significance. Basophil activation was reduced, but only when looking at thresholds of activation and not when looking at the degree of activation. Nevertheless, this raises an important issue about the relative role of basophils and mast cells in food protein-induced anaphylaxis. Accurately identifying the major effector cell of anaphylactic reactions to food proteins is necessary for rational therapeutic design for the prevention of allergic reactions to foods.
Figure 1. Factors contributing to clinical reactivity to foods.
The presence of food-specific IgE is not sufficient to predict clinical reactivity to foods, therefore other co-factors are needed. In a sensitized individual, allergen ingestion by the oral route can lead to clinical manifestations secondary to activation of systemic allergic effector cells by cross-linking IgE bound to effector cells through the FcεRI receptor. Highlighted in red are open questions about mechanisms contributing to the clinical reactivity to foods. Factors altering the absorption of food allergens from the intestine (e.g. food matrix, food processing such as heating), or preventing the proteolytic digestion of food allergens (e.g. antacid, food matrix, food processing) may determine whether clinical reactivity to foods occurs. The nature of the IgE response (affinity, clonality, linear versus conformational epitopes, and to which antigens within a food) will determine if activation of effector cells will occur. Questions remain about the relative contribution of mast cells and basophils in human food allergy, and the possible contribution of other effector cells or antibodies that have been shown to play a role in the mouse. In the grey box are mechanisms that are thought to suppress clinical reactivity to foods, and may play a role in the development of clinical tolerance. These include IgA and IgG4 that function as blocking antibodies and prevent IgE-mediated effector cell degranulation or IgE-facilitated antigen presentation, and Tregs that may suppress food allergy by blocking the generation of IgE (through blocking Th2 cells) or directly suppressing allergic effector cells.
Histamine is one major mediator released from basophils and mast cells after IgE-mediated activation, and antihistamines are often used in the treatment of mild reactions to foods (although epinephrine is the first-line treatment for anaphylaxis). In mice, blockade of both histamine and platelet activating factor (PAF) are necessary to prevent anaphylactic reactions to peanut [37]. Plasma PAF is elevated in patients presenting in the emergency department with acute allergic reactions including those triggered by foods, and is related to reaction severity [38]. It is not yet known if targeting PAF would be of therapeutic benefit in addition to epinephrine and antihistamines for treating acute reactions to foods in man.
Triggering of allergic effector cells by IgE cross-linking is the end of a long line of immune events that must occur to result in clinical food allergy. At the first exposure to a food allergen, there is an immune decision that must be made at the level of the antigen presenting cell, which will influence the developmental fate of the allergen-specific T cell to become a Th2 cell and the allergen-specific B cell to become an IgE-producing plasma cell. If we understand the factors that tip that immune decision to sensitization rather than tolerance, we may be able to intervene and develop prevention strategies to stem the increase in food allergy.
Contributing Factors to Food Sensitization
Genetics
While the rapid increase in food allergy prevalence speaks against a genetic etiology, both genetic and epigenetic factors have been implicated in the development of food allergy [7]. One twin study found a significantly higher concordance rate of peanut allergy among monozygotic twins (64%) as compared to dizygotic twins (7%) [39], suggesting a strong genetic influence. There are conflicting reports on the role of HLA haplotype in food allergy [40, 41]. Loss-of-function gene mutations in the skin barrier protein filaggrin have been associated with peanut allergy [42]. This association was recently reported to be associated with sensitization to peanut rather than clinical reactivity to peanut [43], but suggests that food allergy like other atopic disorders may be result of an insufficient barrier to allergens.
Exposure during pregnancy and lactation
It has been noted that the majority of children who react to peanut or tree nuts will react on their first known ingestion of allergen. This suggests that primary sensitization occurred either through non-oral environmental routes, as will be discussed, or by exposure due to maternal ingestion during pregnancy and/or breastfeeding. There is currently insufficient clinical evidence on the impact of maternal ingestion of food allergens during pregnancy and lactation to make any dietary recommendations [44]. There are conflicting reports of allergen exposure during pregnancy and/or lactation having no effect or being a risk factor [45] for the development of food sensitization. Studies in mice indicate that exposure to allergens through breast milk promotes tolerance in the offspring, which is enhanced in the context of maternal antibodies to the allergen [46]. Mechanisms of tolerance induction in the neonate are different depending on the maternal immune status: in the absence of maternal antibodies, tolerance is mediated by antigen being delivered together with milk-derived TGF-β [47]. In the presence of maternal antibodies, tolerance is dependent on IgG-antigen immune complexes modifying the adaptive immune response to antigen through the neonatal Fc receptor [46]. Although it was believed that oral tolerance could not be effectively induced in neonates, epidemiologic studies suggest that early exposure of infants to food allergens may in fact be protective against food allergy [48, 49]. The open question of the role of early oral exposure to allergenic foods in the development of tolerance or allergy will be addressed by the “Learning Early About Peanut Allergy (LEAP) Study”. The LEAP study is an interventional study that will directly test the impact of early introduction of peanut into the diet on the development of peanut allergy [50]. Similar prospective studies are needed to determine the impact of maternal allergen ingestion on the development of food allergy in infants.
Routes of exposure
In studies on risk factors associated with development of peanut allergy in infants, household exposure to peanut has been shown to be a significant risk factor independent of maternal ingestion [51]. The relevant site of exposure has been proposed to be the skin, and this is supported by the finding that peanut-responsive T cells in peanut-allergic patients can be found in the subset defined as skin-homing (CLA+), but not those defined as gut-homing (integrin β7+) [52]. Ara h 1-specific T cells identified by tetramer staining express high levels of the chemokine receptor CCR4, indicative of skin-homing potential [30]. The site of initial immune priming imprints homing receptor usage on the lymphocyte, and therefore these results support the hypothesis that initial priming occurred via the skin. Furthermore, as discussed previously, mutations in the gene encoding for the epithelial barrier protein filaggrin are associated with peanut allergy or sensitization [42], independent of atopic dermatitis, which is also a risk factor for food allergy [50]. Filaggrin is expressed in the skin, and its mutation or absence results in the upregulation of pro-inflammatory cytokines in skin [53]. However, filaggrin is also expressed in the oral mucosa [54] and therefore the contribution of filaggrin mutations to risk of peanut allergy does not necessarily point to a role for skin exposure over oral exposure. Mice can be readily sensitized by skin exposure to allergens, but this is dependent on tissue damage (induced by tape stripping) or presence of adjuvant [55–57]. Thus, the data suggest that skin exposure is not inherently sensitizing, but may be a potent site of sensitization in a genetically susceptible host, in children with inflammatory skin disorders, e.g. atopic dermatitis (many of whom have enterotoxin-producing Staphylococcus aureus strains colonizing their skin), or in the presence of other environmental co-factors. The observation that the skin is the most frequent site of manifestations of food allergy upon oral food challenge [33] highlights the need for a better understanding of the immune communication between gut and skin during sensitization and allergen re-exposure.
Nutritional Factors
The rapid rise in incidence of food allergy suggests an important environmental influence, which may be derived from factors such as nutrition, the gut microbiome, or a combination of the two. Figure 2 summarizes intrinsic and extrinsic factors that may influence the development of allergic sensitization to foods. Vitamin A has immunomodulatory effects on the mucosal immune system and affects T and B cell homing to the gut as well as the generation of both regulatory and pathogenic effector responses in the gut [58] [59]. The role of vitamin A in food allergy remains to be determined. Vitamin D, like Vitamin A, can modulate mucosal immunity [60] and low vitamin D levels are associated with food allergic sensitization [61]. Food allergy rates also vary with latitude, which has been hypothesized to be due to sun exposure and synthesis of vitamin D through the skin [62]. Obesity is associated with food sensitization [63], and a high fat diet can profoundly influence both innate responses in the gastrointestinal mucosa and the composition of the microbiota [64]. Dietary changes in the US over the past 30 years have indicated that energy consumption from fats/oils/lipids has increased approximately 30% (http://www.ers.usda.gov/Data/FoodConsumption). Medium-chain trigylcerides but not long-chain trigylcerides can promote allergic sensitization to co-administered food antigens in mice [65].
Figure 2. Potential factors contributing to sensitization to foods.
Environmental factors may provide an increased or decreased risk for the development of allergic sensitization to foods. The gut microbiota has been shown to suppress allergy by decreasing IgE, decreasing allergic effector cells (basophils), and increasing Tregs in the intestine. Nutritional factors may either suppress or promote allergy. Data show that vitamin A and D may promote tolerance or suppress allergy, as do long chain fatty acids (FA) and aryl hydrocarbon receptor (AHR) ligands. In contrast, high fat diet and medium chain trigylcerides (TG) promote allergy. Exposure to food allergens through non-oral routes such as the skin may predispose to sensitization, particularly in the context of genetic barrier defects or inflamed skin. Inflamed skin produces cytokines that change the phenotype of the dendritic cell and promote Th2 polarization. Allergens themselves can activate innate immune responses in dendritic cells to promote Th2 polarization.
In addition to a potential role of dietary factors in susceptibility to food allergy, supplementation with dietary factors may provide a means for preventing food allergy. There are mixed data both supporting and refuting a protective role of long-chain fatty acid supplementation in the development of clinical food allergy [66, 67]. Administration of non-digestible carbohydrates reduces allergen-induced skin reactions in mice orally sensitized to milk [68]. The diet is an important source of aryl hydrocarbon receptor (AHR) ligands that support the maintenance of innate lymphoid cells in the gut with regulatory activity [69, 70]. Exogenous AHR ligands can suppress autoimmunity and food allergy in mice by modulating the adaptive immune response [71, 72]. It remains to be determined if specific changes in the diet could be protective against the development of allergic disease, but manipulation of the diet could potentially be a feasible and inexpensive approach to wide-scale disease prevention.
Microbiome
The human body is heavily colonized with a commensal flora that profoundly influences our immune and metabolic status [73]. The hygiene hypothesis is based on the idea that reduced exposure to microbial products (with or without pathogenic potential) alters the immune milieu and predisposes to the development of inappropriate immune responsiveness to innocuous antigens such as food. There is no compelling human data to show an association between antibiotic usage and food allergy, but in mice treatment with wide-spectrum antibiotics to reduce the gut flora predisposes mice to sensitization to peanut [74]. In addition, a recent study demonstrated that mice with food allergy exhibit a specific gut microbiota signature and these bacteria were capable of transmitting disease susceptibility [75]. In humans there are a few studies using culture-based techniques showing evidence of a dysbiosis in children with food allergy, and a recent study used 16S-based sequencing to show a reduced microbial diversity in children with atopic eczema, including food allergy [76]. There is a need to perform profiling of the microbiome, ideally in a large prospective birth cohort study in order to determine if there are microbial signatures that are predictive of the development of food allergy. Such a finding could be followed up with mechanistic studies in mice as it has been shown that germ-free mice can be colonized with defined human flora [77, 78].
Characteristics of Food Allergens
Food proteins that are water-soluble, and stable to heat and digestive enzymes tend to be responsible for most allergic reactions, although a carbohydrate moiety, galactose-alpha-1,3-galactose, was recently reported to be responsible for a delayed form of anaphylaxis [79]. In general, proteins with greater than 62% homology to human proteins are unlikely to be allergenic [80]. The majority of animal food allergens can be classified into 3 protein groups: caseins, EF-hand proteins (primarily parvalbumins) and tropomyosins; and the majority of plant food allergens into 4 families, Bet v 1-related, cupin and prolamin superfamilies, and profilins [81]. Processing may affect the allergenicity of certain foods; e.g. high roasting temperatures make peanuts more allergenic [82] whereas high baking temperatures make milk and egg less allergenic, the latter due to the destruction of conformational epitopes [83, 84] as well as a change in gastrointestinal uptake of the heated antigens [85, 86]. Food processing may alter the structure of the food antigens such that they are recognized and bound by innate pattern receptors on antigen presenting cells [87], thereby altering the nature of the immune response to the antigen. Binding of relevant food allergens to innate receptors such as DC-SIGN has been reported [88, 89], suggesting that recognition by the innate immune system may contribute to allergenicity of food proteins as has been shown for clinically important aeroallergens such as dust mite [90, 91].
Establishment of Immune Tolerance
Naturally acquired tolerance in food allergy
The majority of children with allergy to milk or egg will outgrow their food allergy in childhood, whereas this occurs in the minority of children with allergy to peanut or tree nuts. It is not known whether persistent food allergy is an immunologically distinct phenotype from food allergy that is outgrown. IgE reactivity to sequential rather than conformational epitopes is associated with persistent egg and milk allergy, perhaps suggesting an important role for B cell antigen presentation and T cell help in the maintenance of sensitization to foods. In a study comparing the milk-specific IgE response in milk-sensitized children who had outgrown their milk allergy or were tolerant to extensively heated milk versus children who were reactive to all forms of milk, it was found that children who had outgrown their milk allergy lost their high-affinity IgE and had primarily low-affinity IgE antibodies to milk [21]. In a small study examining milk-specific serum antibody responses in children with persistent as compared to resolved milk allergy, milk-specific IgG4 levels rose in those with resolved milk allergy, but not in those with persistent milk allergy [92]. There was an overlap in epitope binding between IgE and IgG4, and resolution was associated with a shift from IgE binding to IgG4 binding. However, in other studies the levels of milk-specific IgG4 are not increased in those who outgrow milk allergy, although IgG4 levels do rise upon inclusion of milk into the diet [83]. A rise in IgG4 levels may suggest that outgrowth is an active immune process, whereas a loss of IgE sensitization may be a passive process. Is there any evidence that natural outgrowth of food allergy is an active process mediated by regulatory T cells? Two studies have specifically addressed the role of regulatory T cells in the outgrowth of milk allergy. The first showed that an in vivo oral milk challenge expanded the number of CD4+ CD25+ putative regulatory cells in milk-tolerant but not milk-reactive children [93]. Furthermore, depletion of CD25+ cells prior to re-stimulation of cells with allergen in vitro showed the significant presence of regulatory activity only in the tolerant children after the in vivo challenge. The second study investigated patients with IgE sensitization to milk who were reactive to all forms of milk, tolerant to extensively heated milk, or tolerant to all forms of milk. The frequency of CD4+ CD25+ CD27+ cells proliferating to milk was significantly higher in those who were heated-milk tolerant, and intermediate in those who had outgrown their milk allergy [94]. This suggested that a transient expansion of allergen-specific T cells was associated with the process of outgrowing milk allergy. Although these data are suggestive of a role for regulatory T cells in the natural outgrowth of milk allergy, more comprehensive immunoprofiling needs to be performed on this subset of patients who naturally develop tolerance to foods. This should be ideally performed in a prospective manner to determine if outgrowth can be predicted. Understanding the mechanisms of natural outgrowth of food allergy may provide an understanding of immune pathways that could be targeted therapeutically in those with persistent food allergy.
Immunotherapy-induced desensitization or tolerance
There has been an increased emphasis on developing immunotherapeutic approaches to treat food allergy in the past decade. A number of therapeutic strategies are being investigated for the treatment of food allergy [10]. Standard subcutaneous immunotherapy traditionally used to treat pollen and insect allergies was found to provoke severe adverse reactions with food allergens [95]. Oral immunotherapy (OIT) has been most intensively investigated over the past decade, and in small, mostly uncontrolled trials has been shown to induce “desensitization,” a reversible state following short-term exposure to incremental doses of an allergen, in the majority of patients [96]. Desensitization renders effector cells less reactive or non-reactive, but once regular administration of the allergen is discontinued, clinical reactivity returns. Whether OIT will induce “tolerance,” i.e. the relatively long-lasting effects presumably due to alteration in B cell and T cell reactivity, which persist for prolonged periods even after the treatment is discontinued, remains to be established. OIT in combination with anti-IgE antibodies (omalizumab) or various adjuvants to activate more effective regulatory responses are being explored. While OIT shows promise as an effective form of therapy, the high rate of adverse reactions and uncertainty of long-term outcome require further study [96].
Concluding remarks
Food allergy has reached near epidemic proportions in the US and other westernized countries, and now represents the leading cause of anaphylaxis seen in US emergency departments (or about 1 in 800 food-allergic American patients each year) [97] and has resulted in a 3.5 fold increase in hospitalizations for children < 18 years of age [3]. The reason for this increase remains enigmatic, but clearly environmental factors are at play since it has occurred over a relatively brief period of time and is largely confined to westernized countries. The diagnosis and management of food allergy has improved remarkably over the past two decades, but more specific diagnostic tests and more effective forms of therapy are still needed. With a better understanding of basic immunologic mechanisms involved in the development of oral tolerance to food antigens and in re-establishment of tolerance, and a better understanding in the basic pathways involved in food allergic responses, it is likely that new biomarkers will be discovered that accurately reflect clinical sensitivity and reactivity, and that more effective forms of therapy will be developed.
Highlights.
The presence of IgE to food proteins is not sufficient to generate clinical reactivity; IgE clonality, reactivity to food components, and IgE affinity are important criteria to determine immune reactivity.
Food allergy is associated with Th2-skewed CD4+ T cells that express skin-homing markers, suggesting skin exposure as a risk factor for the development of food allergy.
Although food allergy has an important genetic component, environmental factors such as dietary factors (e.g. vitamin D) and the intestinal microbiota appear to play a critical role in disease susceptibility.
Immunotherapy by oral and other routes, together with the use of anti-IgE therapy, is being explored as a means of inducing desensitization or tolerance to foods.
References
- 1.Wang J, Sampson HA. Food allergy. J Clin Invest. 2011;121:827–835. doi: 10.1172/JCI45434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chafen JJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848–1856. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 3.Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008:1–8. [PubMed] [Google Scholar]
- 4.Venter C, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 5.Osborne NJ, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–676. e661–662. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer SH, et al. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22:155–160. doi: 10.1111/j.1399-3038.2011.01145.x. [DOI] [PubMed] [Google Scholar]
- 8.Mullins RJ, et al. Characteristics of childhood peanut allergy in the Australian Capital Territory, 1995 to 2007. J Allergy Clin Immunol. 2009;123:689–693. doi: 10.1016/j.jaci.2008.12.1116. [DOI] [PubMed] [Google Scholar]
- 9.Boyce JA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127:558–573. doi: 10.1016/j.jaci.2010.12.1098. quiz 574–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 12.Komata T, et al. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007;119:1272–1274. doi: 10.1016/j.jaci.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 13.Christensen LH, et al. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton RG, et al. IgE antibody-specific activity in human allergic disease. Immunol Res. 2010;47:273–284. doi: 10.1007/s12026-009-8160-3. [DOI] [PubMed] [Google Scholar]
- 15.Mehl A, et al. Utility of the ratio of food-specific IgE/total IgE in predicting symptomatic food allergy in children. Allergy. 2005;60:1034–1039. doi: 10.1111/j.1398-9995.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 16.Kattan JD, Wang J. Allergen component testing for food allergy: ready for prime time? Curr Allergy Asthma Rep. 2013;13:58–63. doi: 10.1007/s11882-012-0311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asarnoj A, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130:468–472. doi: 10.1016/j.jaci.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, et al. Development of a novel peptide microarray for large-scale epitope mapping of food allergens. J Allergy Clin Immunol. 2009;124:315–322. 322 e311–313. doi: 10.1016/j.jaci.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, et al. A bioinformatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129:1321–1328. e1325. doi: 10.1016/j.jaci.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong H, et al. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, et al. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J Allergy Clin Immunol. 2010;125:695–702. 702 e691–702 e696. doi: 10.1016/j.jaci.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shek LP, et al. Humoral and cellular responses to cow milk proteins in patients with milk-induced IgE-mediated and non-IgE-mediated disorders. Allergy. 2005;60:912–919. doi: 10.1111/j.1398-9995.2005.00705.x. [DOI] [PubMed] [Google Scholar]
- 23.Kulis M, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vickery BP, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131:128–134. e121–123. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khodoun MV, et al. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc Natl Acad Sci U S A. 2011;108:12413–12418. doi: 10.1073/pnas.1105695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schouten B, et al. Contribution of IgE and immunoglobulin free light chain in the allergic reaction to cow’s milk proteins. J Allergy Clin Immunol. 2010;125:1308–1314. doi: 10.1016/j.jaci.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 27.Turcanu V, et al. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–1072. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prussin C, et al. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(−) T(H)2 responses. J Allergy Clin Immunol. 2009;124:1326–1332. e1326. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLong JH, et al. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127:1211–1218. e1213. doi: 10.1016/j.jaci.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torgerson TR, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–1717. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahrens B, et al. Organ-specific symptoms during oral food challenge in children with food allergy. J Allergy Clin Immunol. 2012;130:549–551. doi: 10.1016/j.jaci.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 34.Strait RT, et al. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol. 2011;127:982–989. e981. doi: 10.1016/j.jaci.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arias K, et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J Allergy Clin Immunol. 2011;127:1552–1561. e1551. doi: 10.1016/j.jaci.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Savage JH, et al. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130:1123–1129. e1122. doi: 10.1016/j.jaci.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arias K, et al. Concurrent blockade of platelet-activating factor and histamine prevents life-threatening peanut-induced anaphylactic reactions. J Allergy Clin Immunol. 2009;124:307–314. 314 e301–302. doi: 10.1016/j.jaci.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Vadas P, et al. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Sicherer SH, et al. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol. 2000;106:53–56. doi: 10.1067/mai.2000.108105. [DOI] [PubMed] [Google Scholar]
- 40.Shreffler WG, et al. Lack of association of HLA class II alleles with peanut allergy. Ann Allergy Asthma Immunol. 2006;96:865–869. doi: 10.1016/S1081-1206(10)61351-8. [DOI] [PubMed] [Google Scholar]
- 41.Howell WM, et al. HLA class II DRB1, DQB1 and DPB1 genotypic associations with peanut allergy: evidence from a family-based and case-control study. Clin Exp Allergy. 1998;28:156–162. doi: 10.1046/j.1365-2222.1998.00224.x. [DOI] [PubMed] [Google Scholar]
- 42.Brown SJ, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–667. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan HT, et al. Filaggrin loss-of-function mutations do not predict food allergy over and above the risk of food sensitization among infants. J Allergy Clin Immunol. 2012;130:1211–1213. e1213. doi: 10.1016/j.jaci.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Greer FR, et al. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–191. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 45.Sicherer SH, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010;126:1191–1197. doi: 10.1016/j.jaci.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosconi E, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010;3:461–474. doi: 10.1038/mi.2010.23. [DOI] [PubMed] [Google Scholar]
- 47.Verhasselt V, et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14:170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 48.Du Toit G, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Katz Y, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010;126:77–82. e71. doi: 10.1016/j.jaci.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Du Toit G, et al. Identifying infants at high risk of peanut allergy: The Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131:135–143. e112. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Fox AT, et al. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–423. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Chan SM, et al. Cutaneous lymphocyte antigen and alpha4beta7 T-lymphocyte responses are associated with peanut allergy and tolerance in children. Allergy. 2012;67:336–342. doi: 10.1111/j.1398-9995.2011.02765.x. [DOI] [PubMed] [Google Scholar]
- 53.Kezic S, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol. 2012;129:1031–1039. e1031. doi: 10.1016/j.jaci.2011.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Benedetto A, et al. Filaggrin expression in oral, nasal, and esophageal mucosa. J Invest Dermatol. 2008;128:1594–1597. doi: 10.1038/sj.jid.5701208. [DOI] [PubMed] [Google Scholar]
- 55.Strid J, et al. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011;334:1293–1297. doi: 10.1126/science.1211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunkin D, et al. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. 2011;128:1251–1258. e1252. doi: 10.1016/j.jaci.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oyoshi MK, et al. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126:976–984. 984 e971–975. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall JA, et al. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DePaolo RW, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang JH, et al. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharief S, et al. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1195–1202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osborne NJ, et al. Prevalence of eczema and food allergy is associated with latitude in Australia. J Allergy Clin Immunol. 2012;129:865–867. doi: 10.1016/j.jaci.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 63.Visness CM, et al. Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009;123:1163–1169. 1169 e1161–1164. doi: 10.1016/j.jaci.2008.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upadhyay V, et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol. 2012;13:947–953. doi: 10.1038/ni.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, et al. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J Allergy Clin Immunol. 2013;131:442–450. doi: 10.1016/j.jaci.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer DJ, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. BMJ. 2012;344:e184. doi: 10.1136/bmj.e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furuhjelm C, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505–514. doi: 10.1111/j.1399-3038.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 68.Schouten B, et al. Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J Nutr. 2009;139:1398–1403. doi: 10.3945/jn.109.108514. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 70.Kiss EA, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 71.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 72.Schulz VJ, et al. Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model. Toxicol Sci. 2011;123:491–500. doi: 10.1093/toxsci/kfr175. [DOI] [PubMed] [Google Scholar]
- 73.Hooper LV, et al. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bashir ME, et al. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 75.Noval Rivas M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abrahamsson TR, et al. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–440. 440 e431–432. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 77.Turnbaugh PJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faith JJ, et al. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333:101–104. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Commins SP, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jenkins JA, et al. Evolutionary distance from human homologs reflects allergenicity of animal food proteins. J Allergy Clin Immunol. 2007;120:1399–1405. doi: 10.1016/j.jaci.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 81.Radauer C, et al. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008;121:847–852. e847. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 82.Beyer K, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107:1077–1081. doi: 10.1067/mai.2001.115480. [DOI] [PubMed] [Google Scholar]
- 83.Nowak-Wegrzyn A, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122:342–347. 347 e341–342. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 84.Lemon-Mule H, et al. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122:977–983. e971. doi: 10.1016/j.jaci.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Roth-Walter F, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy. 2008;63:882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 86.Martos G, et al. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011;127:990–997. e991–992. doi: 10.1016/j.jaci.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hilmenyuk T, et al. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. 2010;129:437–445. doi: 10.1111/j.1365-2567.2009.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shreffler WG, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 89.Hsu SC, et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J Biol Chem. 2010;285:7903–7910. doi: 10.1074/jbc.M109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barrett NA, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trompette A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Savilahti EM, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol. 2010;125:1315–1321. e1319. doi: 10.1016/j.jaci.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karlsson MR, et al. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199:1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shreffler WG, et al. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43–52. e47. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 95.Cox L, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 96.Sampson HA. Peanut Oral Immunotherapy: Is It Ready for Clinical Practice? Journal of Allergy and Clinical Immunology: In Practice. 2013;1:15–21. doi: 10.1016/j.jaip.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Ross MP, et al. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. 2008;121:166–171. doi: 10.1016/j.jaci.2007.10.012. [DOI] [PubMed] [Google Scholar]