Abstract
Aims
To review three key and controversial comorbidities of cannabis use – other illicit drug use, psychosis and depression as well as suicide, from a genetically informed perspective.
Design
Selective review.
Results
Genetic factors play a critical role in the association between cannabis use, particularly early-onset use and use of other illicit drugs, psychosis and depression as well as suicide, albeit via differing mechanisms. For other illicit drugs, while there is strong evidence for shared genetic influences, residual association that is attributable to causal or person-specific environmental factors cannot be ruled out. For depression, common genetic influences are solely responsible for the association with cannabis use but for suicidal attempt, evidence for person-specific factors persists. Finally, even though rates of cannabis use are inordinately high in those with psychotic disorders, there is no evidence of shared genetic etiologies underlying this comorbidity. Instead, there is limited evidence that adolescent cannabis use might moderate the extent to which diathesis influences psychosis.
Conclusions
Overlapping genetic influences underlie the association between early-onset cannabis use and other illicit drug use as well as depression and suicide. For psychosis, mechanisms other than shared genetic influences might be at play.
Keywords: cannabis, comorbidity, twin, genetics, psychosis, gateway
Well recognized as the most ubiquitously used illicit drug in developed nations and, currently, the center of considerably attention in the United States for its evanescing illicit status, cannabis has long been the eye of a great deal of controversy. Worldwide, rates of cannabis use remain high, particularly in youth (1–3). For instance, the 2012 Monitoring the Future Survey noted that 45.2% of U.S. 12th graders reported a lifetime history of cannabis use (2). Likewise, according to the European Monitoring Center for Drugs and Drug Addiction’s 2013 report, 0.8–40% of those aged 15–24 years report lifetime cannabis use (1). Cumulative incidence is equally high in Oceania (42%) (4). Further, while not amongst the most addictive of psychoactive substances (5), a modest but significant proportion (10–20%) of chronic cannabis users develop cannabis use disorders (6).
The acute effects of cannabis intoxication increase risk for unintentional injury and motor vehicle accidents (7) in particular when the drug is used in conjunction with alcohol (8). However, what has, perhaps, elicited a greater degree of scientific and public scrutiny is the alleged relationships between cannabis use and comorbid mental health conditions across the lifespan. Three key outcomes have dominated this debate:
Is cannabis use a gateway to the use of harder illicit drugs?
Is cannabis use a risk factor for psychotic illness?
Does cannabis use increase the risk of affective disorders and suicide?
In this review, we examine these three outcomes from a genetically informed perspective. This approach has considerable potential to expand our understanding of the relationship between cannabis use, misuse and each of these outcomes for three principal reasons. First, there is substantial evidence that cannabis use and use disorders are heritable with genetic influences explaining approximately 40–48% and 51–59% for use and problem use respectively (9). Second, there is also substantial evidence for the role of heritable variation in the outcomes under review (other illicit drug use: h2=50–70% (10,11); psychosis: h2=80% (12); depression: h2=30% (13); suicide: h2=40–50% (14)). Third, and importantly, increasing acceptance of gene-environment interplay in shaping the etiology and developmental course of behavior and psychopathology implicates genetically informed processes as key contributors to comorbidity (15–17).
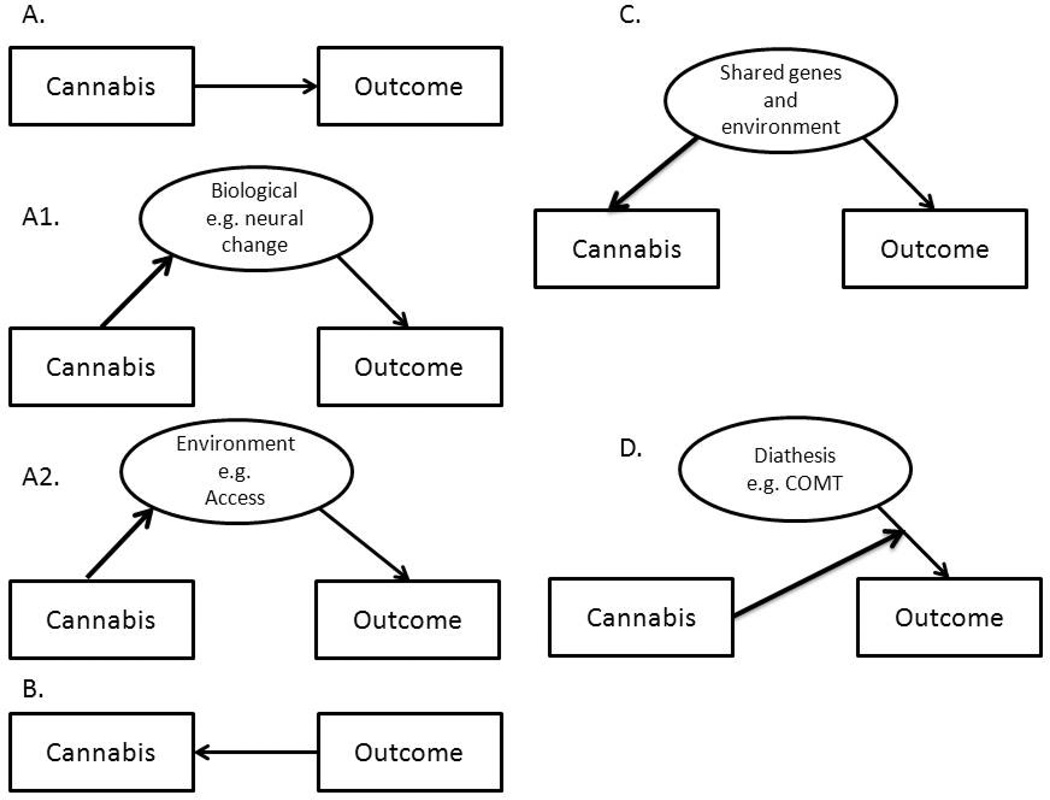
Regardless of the biological slant of this review, the mechanisms proposed in explanation of these (see Figure 1), and as such all, comorbidities can be broadly categorized as:
FIGURE 1.
Multiple possible mechanisms that may link cannabis use and misuse to mental health outcomes, including other illicit drug use, depression and suicide as well as psychosis. Panel A: Cannabis use directly causes outcome; A1 depicts how this causal effect may be exerted via alterations in biological pathways (such as receptor cross-tolerance) while A2 demonstrates a similar causal effect mediated by environmental exposures (such as access to drug supplier). Panel B demonstrates reverse causation, such as self-medication. Panel C shows how cannabis use and outcomes might be related via common genetic and environmental underpinnings. Panel D illustrates stress-diathesis (or gene-environment interaction) – cannabis use modifies the extent to which diathesis (such as select genotypes in the catechol-o-methyltransferase gene) influences the outcomes.
Causal
A prominent, albeit controversial explanation of the comorbidity between cannabis use and psychiatric morbidity and other behavioral outcomes is that cannabis use directly increase risks of these conditions through causal mechanisms (Figure 1A) that may include the pharmacological properties of the drug itself (Figure 1A1.). For instance, as some of the acute effects of tetrahydrocannabinol (THC), the psychoactive component in cannabis, include psychotic symptoms, some have argued that cannabis use may cause psychosis via enduring alterations in brain regions that are activated during acute cannabis-intoxication (18; 19). However, investigators have argued that a more plausible causal pathway may involve socio-environmental factors (20)(Figure 1A2).
Reverse causation
Alternatively, it is possible that psychiatric conditions cause cannabis (or other drug use, Figure 1B) as has been proposed, for example, in the self-medication hypothesis (21) which posits that drug use develops in an attempt to ameliorate the negative states associated with common mental health problems such as depression (22).
Correlated Liabilities
That cannabis use shares risk and protective influences with the outcomes listed in (a)-(c) is evident (Figure 1C). For example, adolescent exposure to cannabis use often co-aggregates with other putatively deviant or precocious behaviors, such as conduct problems and early consensual sex, which in turn, are also associated with use of other illicit drugs (23–25). There are environmental factors, such as neighborhood characteristics (e.g. easy access to cannabis in a neighborhood that also promotes early delinquent activity), that bind these behaviors together (26; 27) and, importantly, also genetic influences. For example, numerous studies have shown that underlying early drug use and other problem behaviors is a general, highly heritable predisposition to externalizing behaviors (28; 29).
Activation of Diathesis
In this instance (Figure 1D), cannabis might be viewed as an environmental trigger of genetic vulnerability to an outcome, say psychotic illness (30; 31). Also referred to as a gene-environment interaction, a few studies have found that a variant in the gene encoding catechol-o-methyltransferase (COMT) is associated with a higher likelihood of psychotic features during adulthood but only if individuals had used cannabis during adolescence (32). As the variant itself did not correlate with the use of cannabis during adolescence, this effect was not due to correlated liabilities nor was the variant alone associated with psychotic illness.
Disentangling causal mechanisms and correlated liabilities in human studies
The etiological explanations supporting the role of adolescent exposure to cannabis use on later occurrence of (a) – (c) are amongst the most controversial and elusive. There are other outcomes (e.g. educational achievement, cognition and working memory) that have been addressed to a limited degree with genetic methodologies and hence, are not included (some of that research and a significantly extended description of the research presented here is reviewed elsewhere (33)). Several prospective methodologies have been brought to bear on these hypotheses, however, the unequivocal demonstration of causality is a challenge even in well-crafted longitudinal studies that allow for the statistical control of multiple confounding factors (34). In particular, genetic influences that might contribute to, for instance, both early cannabis use and subsequent illicit hard drug use need to be accounted for.
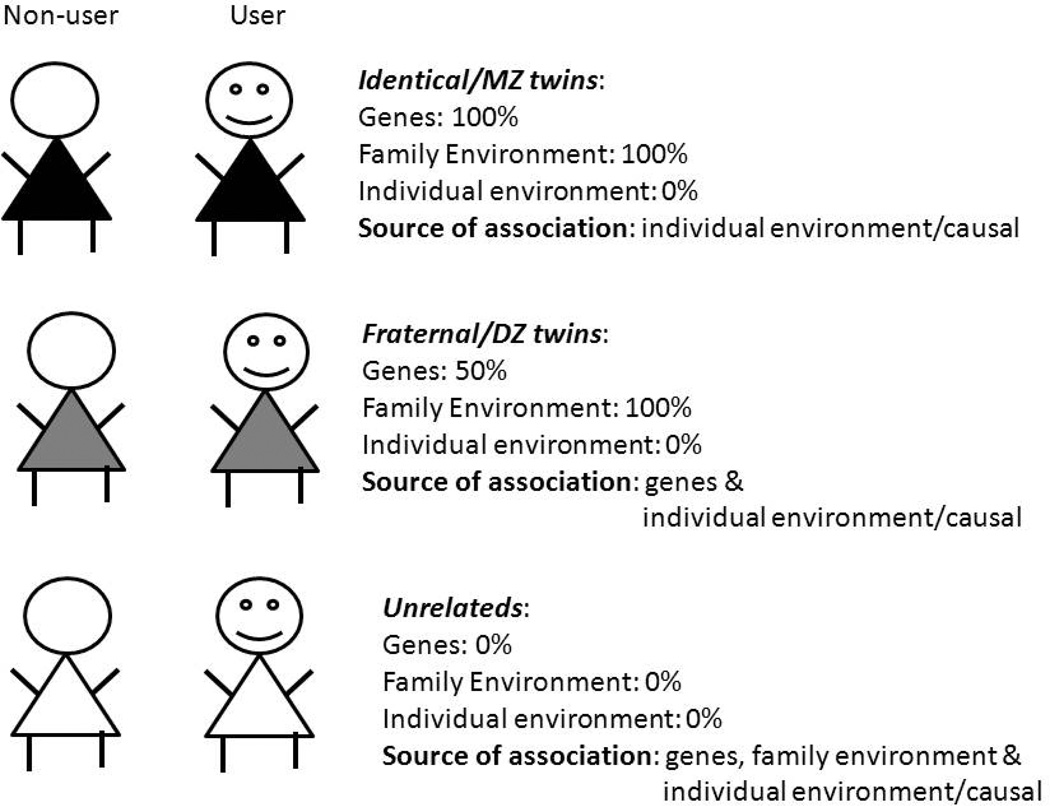
While causation cannot be proved with any degree of certainty in human subject research, pairs of twins discordant for cannabis exposure afford one elegant framework for disproving causation (35; 36) as illustrated in Figure 2. We use the example of the association between cannabis use and use of hard drugs. The classical twin design capitalizes on differences between identical or monozygotic (MZ) twins, who share all their segregating genes and fraternal or dizygotic (DZ) twins who only share 50% of them. It is also assumed that both MZ and DZ twins reared together are equally matched for familial environment (the equal environments assumption) (37). As all MZ twin pairs, including those discordant for cannabis use, are perfectly correlated for their segregating genes and for familial environment, any residual association between cannabis exposure and, say, subsequent hard drug use, within the pairs of discordant twins, can be attributed to non-familial sources, such as individual-specific environments and causal processes (Figure 2). Only those pairs of twins where one twin uses cannabis but the other does not are selected. Within these discordant pairs, if the MZ twin who uses cannabis is also more likely to report use of hard drugs, relative to their identical co-twin who does not use cannabis (i.e. a significant odds-ratio when comparing the risk within the pair of twins), then even after accounting for shared genetic and familial influences, cannabis use is associated with use of hard drugs. The mechanisms that contribute to this residual association are person-specific (i.e. experienced by the cannabis-using twin but not their co-twin) and might include socio-environmental factors (affiliations with drug-using peers or exposure to adverse neighborhoods) or pharmacological (e.g. receptor cross-sensitization) and epigenetic modifications, which may act via putatively causal or non-causal pathways. Therefore, such a residual association in pairs of MZ twins is necessary but not sufficient evidence in favor of causation. On the other hand, absence of such a residual association provides strong support for the lack of causal inference. In addition, the pattern of association in discordant twins, when compared with association in pairs of unrelated individuals provides considerable insight into contributors to the association – these are illustrated in Figure 3.
FIGURE 2.
The discordant twin design is illustrated. Pairs of twins (MZ=monozygotic/identical; DZ=dizygotic/fraternal) discordant for cannabis use are matched to varying degrees for genetic and environmental factors allowing for residual associations to be explained by unshared factors. Pairs of unrelated individuals are shown for comparison.
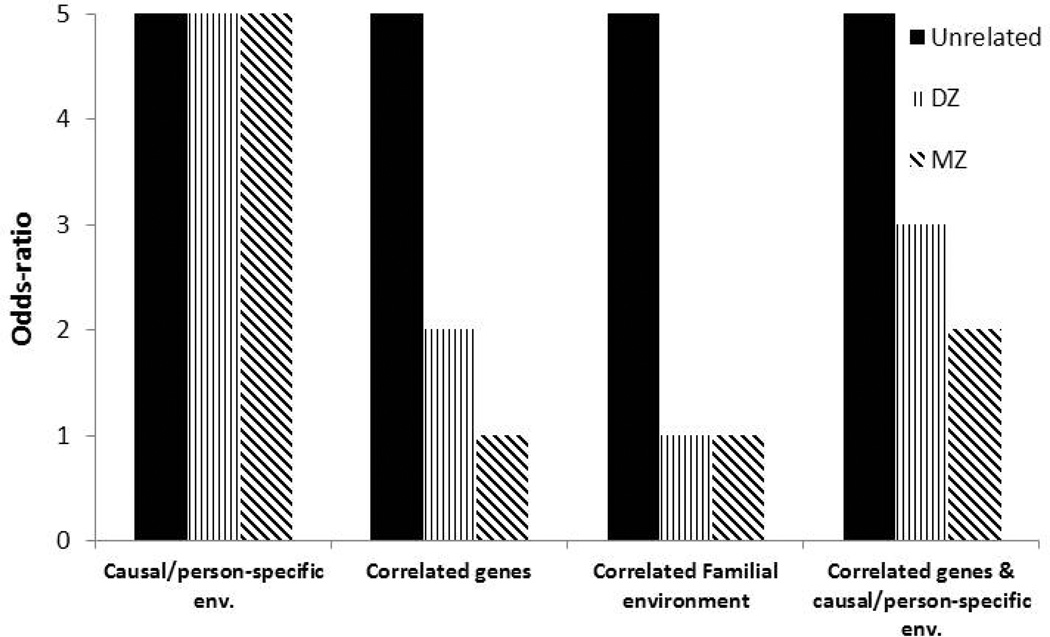
FIGURE 3.
Hypothetical results expected from a discordant twin study, for discordant pairs of MZ, DZ and unrelated individuals, which can be used to infer the nature of the association between cannabis use and outcomes. If the association is entirely due to causal or individual-specific factors then the magnitude of association is invariant to degree of relatedness. In contrast, if genes or family environment play a role, then matching discordant pairs of individuals for these factors results in an attenuation of the association.
Cannabis & the Gateway Theory
In 1975, Kandel posited that cannabis use, particularly with adolescent onset, was a “gateway” to the use of other illicit or hard drugs (38). The gateway process involves sequence (onset of gateway drug prior to use of hard drugs), association (increased likelihood of hard drug use in those who use cannabis) and, controversially, causation (39). Accordingly, researchers have demonstrated that cannabis use does occur prior to use of harder drugs, such as cocaine and heroin (40) and that, relative to non-users, cannabis users are considerably more likely to subsequently report use of hard drugs (41). However, the evidence for causation, or that cannabis use exerts a causal influence on the likelihood of using other illicit drugs, has been less unequivocal. Evidence for causation draws primarily from prevention and intervention studies that have noted that delaying or ceasing the onset of cannabis use has notable effects on use of harder drugs (42; 43). However, these studies also note that these prevention strategies are not drug-specific and hence, generally reduce exposure to risk influences that correlate with early use of cannabis and later experimentation with harder drugs. While prevention studies rely on social mechanisms of causation (i.e. early cannabis use causes use of hard drugs via exposure to deviance-prone environments), a rodent study has noted that adolescent exposure to cannabis, even when controlling for environmental similarity, leads to increased preference for other drugs (e.g. opiates)(44), implicating biological mechanisms, such as cross-sensitization. Although widely cited in support of the gateway hypothesis, such preclinical studies focus on the process of drug sensitization, which occurs after onset of use, rather than initiation of use and therefore are not applicable to the gateway hypothesis.
Using the discordant twin method, Lynskey and colleagues (36) selected pairs of MZ and DZ twins where one twin had used cannabis prior to age 17 and the other had used it later or not at all. They found that regardless of zygosity, individuals who used cannabis prior to age 17, when compared to their MZ or DZ cotwin (who was a never or late-onset user) were more likely to report use and misuse of harder drugs as well as cannabis use disorders. Even after adjustment for covariates such as tobacco and early alcohol use, conduct disorder, major depression and social anxiety, early onset cannabis users were at 2.4 to 3.9 increased odds of use of drugs like cocaine, hallucinogens, sedatives and opioids and twice as likely as their co-twins to meet criteria for dependence on hard drugs and alcohol. This excess risk in MZ twins indicated that causal explanations could not be excluded.
Since that initial publication, 4 studies (45–48) have systematically replicated this association. In particular, in a Dutch sample, Lynskey and colleagues (47) found that rates of party and hard drug use were considerably elevated (18 vs. 4%) in the cannabis-using twin relative to their co-twin and even after adjustment for confounders, the residual association between cannabis use and use of hard/party drugs persisted. The authors suggested that, as this association remained in a sample for whom cannabis was legal, the previously observed associations between cannabis and other drug use could not be explained by the legal status of cannabis. Similarly, utilizing all twins (not just those from discordant pairs), Agrawal and colleagues (48) found that this correlation could be partially ascribed to person-specific factors that influenced both early-onset of cannabis use and the subsequent use of hard drugs. In particular, Cleveland & Wiebe attribute this effect to the natural developmental course of substance use, which is genetically influenced (49).
Cannabis and Psychosis
Rates of cannabis use and misuse are particularly high in those with psychotic disorders, especially those with early onset of these illnesses. For instance, Koskinen and colleagues (50) report a mean lifetime rate of 27.1% for cannabis use disorders in a meta-analysis of schizophrenia patients while another systematic meta-analysis (51) revealed that cannabis users, on average, had an onset of psychotic illness 2.7 years prior to non-users. Prospective studies have been remarkably informative as well, showing that the pooled (meta-analysis) odds of developing psychosis in cannabis users is 2.1 (52). The association is particularly strong in those who start to use cannabis at an early age – for example, Arseneault and colleagues (53) found that rates of schizophreniform disorder in those who had used cannabis by age 15 was more than threefold greater than in others.
In positing mechanisms that link cannabis use to psychosis, the role of reverse causation has been largely eliminated (31; 54–56). In contrast, the causal hypothesis has received significant attention. Causation has been strongly asserted on account of longitudinal studies showing a persisting association between cannabis use and psychosis even after accounting for confounders as well as preexisting psychotic symptoms (57–60). Unfortunately, these studies have had limited ability to control for genetic factors as twin studies adequately powered to study psychotic illness are rare. Additionally, the causal mechanisms that underpin this relationship are likely to be complex. Persuasive support for this arises from the observation that during periods of increasing cannabis use, rates of psychosis have remained fairly constant (61; 62). If cannabis use caused psychosis, a significantly higher rate of the latter would be expected.
An appealing alternative to direct causation is that cannabis use precipitates psychosis in individuals with pre-existing vulnerabilities. In support of this hypothesis, Caspi and colleagues (63) examined whether individuals that carried the Valine (Val) allele of a missense polymorphism (rs4680) in the catechol-O-methyltransferase (COMT) gene are at heightened risk for developing psychotic disorders. COMT codes for an enzyme that is instrumental in the degradation of endogenous amines, including dopamine, which is well known to mediate the psychoactive effects of THC. Preliminary evidence supports the role of reduced enzymatic activity and slower dopamine metabolism in Val carriers (64). Furthermore, genetic (e.g. knock-out) and pharmacological attenuation of COMT activity has been noted to modify cannabis-induced effects on endophenotypes related to schizoprenia (e.g. sensorimotor gating (65)). In the Caspi et al study of 803 individuals, cannabis use prior to age 17 was examined as the activator of diathesis, as indexed by the Val158Met polymorphism in COMT to predict psychosis outcomes at age 26. For schizophreniform disorder, the odds-ratios reflecting the association with adolescent cannabis use were 10.9, 2.5 and 1.1 in the Val/Val, Val/Met and Met/Met (Met for Methionine) individuals respectively. Similar effects were seen for hallucinations and delusions, however when adult-onset cannabis use was examined, no significant associations were noted for any of the psychotic outcomes. Furthermore, presence of the Val allele was not associated with increased or decreased likelihood of adolescent cannabis exposure.
While intuitively appealing, the above genotype x environment interaction model has witnessed only limited replication. For instance, Zammit and colleagues (66), examined whether cannabis use at age 14 was associated with psychotic symptoms at age 16 in the Avon Longitudinal Study of Parents and Children (N=2630). There was no support for an interaction between cannabis exposure and COMT genotype and sensitivity analyses indicated a high degree of variability in the interaction findings based on the outcomes and exposure definition under study. However, in a smaller sample of patients with psychotic disorder (N=31) and healthy controls (N=25), carriers of the Val allele showed hallucinations after cannabis exposure (recorded via experience sampling) but only if they had reported a prior history of psychosis proneness (67). Similarly, in another psychiatric patient population (N=157), decreasing copies of the Val allele were associated with decreasing age at onset of psychotic disorder in lifetime cannabis users whereas the reverse was noted (decreasing age at onset with decreasing copies of Val allele) for lifetime nonusers (68). In addition, a recent study (N=533) posits that the predictive effect of the interaction between cannabis use and COMT genotype on psychosis is only exerted in those with a history of childhood abuse (69). Finally, contradictory evidence from a recent study (N=748 patients) of COMT haplotypes in two Spanish samples indicates a higher degree of association between lifetime cannabis use and schizophrenia in Met (not Val) carriers (70).
It appears likely that if cannabis exposure activates diathesis to psychosis (via COMT) then this effect is limited to individuals with additional vulnerabilities (e.g. psychosis proneness) or adverse exposures (e.g. childhood abuse). Alternatively, it is also possible that overlapping genetic (and environmental) factors link cannabis use and psychosis. However, gene association studies with COMT, as well as other dopaminergic (e.g. DRD2), cannabinoid (e.g. CNR1) and cholinergic (e.g. CHRNA7) polymorphisms have failed to show consistent associations with cannabis use and psychosis (71). Overall, the small sample size of a number of these studies limits their generalizability and meta-analysis across studies is needed. Adequately powered samples of psychotic disorders (e.g. Schizophrenia and Bipolar Disorder in the Psychiatric Genomics Consortium) with genomewide association data (72) might provide the most promising search for all variants whose influence on psychosis is moderated by early cannabis exposure.
Cannabis, depression and suicide
The links between cannabis and depression as well as suicide have been noted in both cross-sectional and longitudinal studies (22; 73; 74) – for instance, a recent meta-analysis notes an odds-ratio of 1.62 for the likelihood of depression in heavy cannabis users (73). Another meta-analysis of four Australasian datasets found that, even upon adjustment for confounders, weekly cannabis use was modestly associated with depression, particularly during adolescence (75). Similarly, for suicide, van Ours et al (76) found that frequent cannabis use (several times per week) was associated with suicidal ideation, particularly in males. In contrast, Price and colleagues, using a cohort of 50,087 Swedish male conscripts reported no association between repeated cannabis use (> 50 times) and completed suicide (odds-ratio 1.04) after adjustment for confounders (77).
As with psychosis, there is limited evidence that depression and suicidal ideation cause an increase in cannabis use, putatively via mechanisms of self-medication (78). While some researchers have found support for cannabis use exerting a causal influence on depression and suicide (79), others posit that confounding factors, which lead to a network of correlated risk influences is responsible (80). We are aware of three studies that have explored the genetic overlap between cannabis involvement and depression or suicide. Using a sample of adopted and non-adopted offspring, Marmorstein and colleagues (81) found that parental history of cannabis use disorder was associated, at a trend level, with major depressive disorder in non-adopted offspring even after accounting for offspring cannabis use disorder, indicating evidence for familial co-aggregation. Specifically examining their genetic overlap, Fu et al (82) noted that antisocial personality disorder was a complete mediator of the genetic overlap between cannabis use disorder and major depressive disorder in male twins. Finally, one study (83) has utilized the discordant twin design to explore whether the links between cannabis involvement, including early use and dependence, and depression as well as suicidal ideation and attempt are explained by individual-specific/causal factors. Even after accounting for covariates, such as childhood trauma exposure, use of cannabis prior to age 17 was associated with suicidal attempt (odds-ratio=3.49; but not with ideation or major depression). This association could not be equated to 1.0 even in genetically-identical/MZ twin pairs indicating that while shared genetic influences may be solely responsible for the association between early cannabis use and depression, potential causal or individual-specific non-causal factors played a role in the relationship between early cannabis use and suicidal attempt, over and above their shared genetic underpinnings.
An attractive candidate gene system for the links between cannabis use, depression and suicide is the endogenous cannabinoid system. In particular, the gene encoding the cannabinoid receptor 1 (CNR1) to which endocannabinoids and THC bind, is an excellent target. There is well-documented evidence for the role of the endocannabinoid system in the regulation of mood, particularly in the context of stress adaptation for which it interfaces with the Hypothalamic-Pituatary-Adrenal axis (84; 85). For instance, rodents administered a cannabinoid receptor 1 (CB1) antagonist engage in more depressive and anhedonic behaviors upon administration of chronic mild and unpredictable stress (86). In humans as well, clinical trials for the anti-obesity medication Rimonabant, also a CB1 antagonist, found a high rate of serious adverse events related to suicidality that consequently led to its discontinuation (87; 88). In addition, studies have documented that variants in CNR1 in conjunction with stress exposure are related to low mood (89; 90). While THC binds to CB1 to exert its psychotropic effects, and this is well documented in rodent models, human association studies with variants in CNR1 have yielded equivocal findings (10).
Considerations and caveats
Genetically informed methods provide a powerful tool to unpack comorbidity and co-occurrence. However, even elegant methods such as the discordant twin design have their caveats. Recently, O’Brien and colleagues (91) concluded that the origins of twin discordance (for early onset cannabis use) are as pertinent as the sequelae of this discordance (e.g. other illicit drug use or low academic achievement). They advocate for a case-crossover (person-as-own-control) as an attractive alternative, however note the limitation of unmeasured covariates (92).
Another caveat of the twin design is that genetic relatedness is indexed by matching for segregating genes which should not be confused with all biological mechanisms that could be in play. Of note, epigenetic mechanisms are not controlled for in the twin design and indeed, it is not uncommon to find epigenetic differences within pairs of MZ twins (93). Epigenetics, broadly defined, refers to heritable variation that cannot be solely attributed to modifications in the sequence of DNA (94). Epigenetic change is a key contributor to psychiatric illnesses (95) and that such mechanisms underlie substance use is becoming more widely accepted (96–99). The extent to which such epigenetic modification can be implicated in discordant MZ correlations (those that reflect causal or shared individual-specific factors) remains to be investigated. For instance, Mill and colleagues have found evidence for discordance in methylation (increased methylation typically indexes gene silencing) signatures for the COMT gene in MZ twin pairs (100). Furthermore, there is evidence for stress-related methylation of the Val158 (rs4680) allele, which in turn, predicts aspects of human cognition, such as working memory (101).
In instances where causation has been implied (e.g. the gateway hypothesis), Mendelian randomization is an intriguing alternative possibility. Relying on the Mendelian law of random and independent assortment of alleles, this conceptualization of epidemiological causation suggests that the functional alleles that modify the likelihood of early cannabis use (i.e. exposure) should predict later outcomes to which early cannabis use is causally related (e.g. illicit drug use) (102). The allele-outcome association is considered a sound test of causation as it is unencumbered by confounding social factors. For instance, Irons et al (103) reported that the non-protective allele of the functional variant (rs671) in ALDH2, which reduces alcohol use due to reduced acetaldehyde clearance and consequently, flushing, was not associated with increased likelihood of other substance use in Asian Americans (i.e. a test of the alcohol-drug gateway). While intuitively appealing, tests of this nature are challenging in studies of cannabis exposure. First, no genomic variants have been robustly and causally implicated in the etiology of early cannabis use (or any cannabis-related measure for that matter)(104; 105). Secondly, those that have been studied are likely to relate to cannabis as much as they do to putative confounders (e.g. CNR1 variation is associated with cannabis exposure but also with alcohol involvement, a putative confounder (106)).
Finally, that cannabinoids might exert a causal influence at a cellular level cannot be ruled out. For instance, a recent study shows that, in rodents, administration of THC was associated with reduced spatial working memory via activation of astroglial cannabinoid 1 receptors, which, in turn, activated glutamate receptors that led to long-term depression in synaptic strength in the hippocampus (107). While there are questions regarding how the levels of THC administered to the rodents compare with typical patterns of human cannabis use, this study (and others) hints at possible causal mechanisms that operate with such a degree of biological intricacy so as to remain entirely undetected in genetic epidemiological experiments.
Cannabis controversies – a redux
In the United States, the legal standing of cannabis is at a watershed with two states passing legislation permitting large scale commercialization (108) while in many other countries there is ongoing controversy surrounding the appropriate legal approach to recreational cannabis use (109). That cannabis use can be implicated in the (co)-occurrence of major psychiatric disorders (e.g. depression, psychosis), reduced life opportunities (e.g. low academic achievement) and death (e.g. suicide) are often cited as barriers to its legalization. A review of the literature quickly notes that while the evidence for a straightforward causal link between cannabis and these outcomes is tenuous at best (34), any rational public health policy regarding the regulation of cannabis cannot ignore these potential harms (110–112). What genetic studies do is shed light on these mechanisms. There is overwhelming support for a cluster of risk and protective influences, including familial, genetic and environmental factors that contribute to their co-occurrence. Thus, while genetic research may never put to bed the controversy surrounding the legality of cannabis use, nor should it, we believe that such studies have made and continue to substantially impact our understanding of the manner in which cannabis use and misuse produces societal and personal harm.
Acknowledgments
Funding: AA is supported by R01DA23668 and K02DA32573.
Disclosure: AA has previously received peer-reviewed grant funding from ABMRF/Foundation for Alcohol Research that receives contributions from the alcohol industry.
Reference List
- 1.European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2013: Trends and developments. Lisbon: EMCDDA; 2013. [PubMed] [Google Scholar]
- 2.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2012. Ann Arbor: Institute for Social Research: The University of Michigan; 2013. [Google Scholar]
- 3.Australian Institute of Health and Welfare. 2010 National Drug Strategy Household Survey Report. Cat no. PHE. Vol. 145. Canberra: AIHW; 2011. Report No.: Data statistics series no. 25. [Google Scholar]
- 4.Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008 Jul 1;5(7):e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007 Mar 24;369(9566):1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- 6.Anthony JC, Helzer JE. Syndromes of drug abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric Disorders in America: The Epidemiological Catchment Area Study. New York: Free Press; 1991. [Google Scholar]
- 7.Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013 Mar;59(3):478–492. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010 Mar;105(3):417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, et al. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007 Mar;102(3):413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rijsdijk FV, Gottesman II, McGuffin P, Cardno AG. Heritability estimates for psychotic symptom dimensions in twins with psychotic disorders. Am J Med Genet B Neuropsychiatr Genet. 2011 Jan;156B(1):89–98. doi: 10.1002/ajmg.b.31145. [DOI] [PubMed] [Google Scholar]
- 13.Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med. 2001 May;31(4):605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- 14.Statham DJ, Heath AC, Madden PA, Bucholz KK, Bierut L, Dinwiddie SH, et al. Suicidal behaviour: an epidemiological and genetic study. Psychol Med. 1998 Jul;28(4):839–855. doi: 10.1017/s0033291798006916. [DOI] [PubMed] [Google Scholar]
- 15.Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006 Mar;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 16.Plomin R, Koeppen-Schomerus G. Beyond Heritability. Psychosom Med. 2002;64:204–205. [Google Scholar]
- 17.Bookman EB, McAllister K, Gillanders E, Wanke K, Balshaw D, Rutter J, et al. Gene-environment interplay in common complex diseases: forging an integrative model-recommendations from an NIH workshop. Genet Epidemiol. 2011 Feb 9; doi: 10.1002/gepi.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossong MG, Jansma JM, van Hell HH, Jager G, Oudman E, Saliasi E, et al. Effects of delta9-tetrahydrocannabinol on human working memory function. Biol Psychiatry. 2012 Apr 15;71(8):693–699. doi: 10.1016/j.biopsych.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Bossong MG, Jager G, Bhattacharyya S, Allen P. Acute and non-acute Effects of Cannabis on Human Memory Function: a Critical Review of Neuroimaging Studies. Curr Pharm Des. 2013 Jun 14; doi: 10.2174/13816128113199990436. [DOI] [PubMed] [Google Scholar]
- 20.Kenkel D, Mathios AD, Pacula RL. Economics of youth drug use, addiction and gateway effects. Addiction. 2001 Jan;96(1):151–164. doi: 10.1046/j.1360-0443.2001.96115111.x. [DOI] [PubMed] [Google Scholar]
- 21.Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985 Nov;142(11):1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- 22.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003 Nov;98(11):1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 23.Fergusson DM, Horwood LJ, Lynskey MT. The comorbidities of adolescent problem behaviors: a latent class model. J Abnorm Child Psychol. 1994 Jun;22(3):339–354. doi: 10.1007/BF02168078. [DOI] [PubMed] [Google Scholar]
- 24.Hays RD, Ellickson PL. Associations between drug use and deviant behavior in teenagers. Addict Behav. 1996 May;21(3):291–302. doi: 10.1016/0306-4603(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 25.Donovan JE, Jessor R, Costa FM. Adolescent health behavior and conventionality-unconventionality: an extension of problem-behavior theory. Health Psychol. 1991;10(1):52–61. [PubMed] [Google Scholar]
- 26.Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull. 2000 Mar;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- 27.Galea S, Ahern J, Tracy M, Vlahov D. Neighborhood income and income distribution and the use of cigarettes, alcohol, and marijuana. Am J Prev Med. 2007 Jun;32(6 Suppl):S195–S202. doi: 10.1016/j.amepre.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002 Aug;111(3):411–424. [PubMed] [Google Scholar]
- 29.Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav Genet. 2011 Jul;41(4):459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006 Jul;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 31.Henquet C, Di FM, Morrison P, Kuepper R, Murray RM. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008 Nov;34(6):1111–1121. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the Catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction. Biol Psychiatry. 2005 May 15;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal A, Lynskey M. Have the genetics of cannabis involvement gone to pot?; Report No.: 61st Annual Nebraska Symposium on Motivation; 2013. Springer. In Press. [DOI] [PubMed] [Google Scholar]
- 34.Macleod J, Oakes R, Copello A, Crome I, Egger M, Hickman M, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004 May 15;363(9421):1579–1588. doi: 10.1016/S0140-6736(04)16200-4. [DOI] [PubMed] [Google Scholar]
- 35.Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Arch Gen Psychiatry. 1993 Jan;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 36.Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003 Jan 22;289(4):427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 37.Neale MC, Cardon LR. Methodology for Genetic studies of Twins and Families. Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 38.Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- 39.Kandel DB. Does marijuana use cause the use of other drugs? JAMA. 2003 Jan 22;289(4):482–483. doi: 10.1001/jama.289.4.482. [DOI] [PubMed] [Google Scholar]
- 40.Kandel D, Yamaguchi K. From beer to crack: developmental patterns of drug involvement. Am J Public Health. 1993 Jun;83(6):851–855. doi: 10.2105/ajph.83.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000 Apr;95(4):505–520. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- 42.Hays RD, Ellickson PL. Associations between drug use and deviant behavior in teenagers. Addict Behav. 1996 May;21(3):291–302. doi: 10.1016/0306-4603(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 43.Botvin GJ, Griffin KW, Diaz T, Scheier LM, Williams C, Epstein JA. Preventing illicit drug use in adolescents: long-term follow-up data from a randomized control trial of a school population. Addict Behav. 2000 Sep;25(5):769–774. doi: 10.1016/s0306-4603(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 44.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007 Mar;32(3):607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 45.Grant JD, Lynskey MT, Scherrer JF, Agrawal A, Heath AC, Bucholz KK. A cotwin-control analysis of drug use and abuse/dependence risk associated with early-onset cannabis use. Addict Behav. 2010 Jan;35(1):35–41. doi: 10.1016/j.addbeh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lessem JM, Hopfer CJ, Haberstick BC, Timberlake D, Ehringer MA, Smolen A, et al. Relationship between Adolescent Marijuana Use and Young Adult Illicit Drug Use. Behav Genet. 2006 Mar;:25. doi: 10.1007/s10519-006-9064-9. [DOI] [PubMed] [Google Scholar]
- 47.Lynskey M, Vink JM, Boomsma DI. Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behav Genet. 2006;36(2):195–200. doi: 10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004 Oct;34(7):1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- 49.Cleveland HH, Wiebe RP. Understanding the association between adolescent marijuana use and later serious drug use: gateway effect or developmental trajectory? Dev Psychopathol. 2008;20(2):615–632. doi: 10.1017/S0954579408000308. [DOI] [PubMed] [Google Scholar]
- 50.Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010 Nov;36(6):1115–1130. doi: 10.1093/schbul/sbp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011 Jun;68(6):555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 52.Henquet C, Murray R, Linszen D, van OJ. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005 Jul;31(3):608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 53.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002 Nov 23;325(7374):1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Degenhardt L, Hall W. Cannabis and psychosis. Curr Psychiatry Rep. 2002 Jun;4(3):191–196. doi: 10.1007/s11920-002-0026-5. [DOI] [PubMed] [Google Scholar]
- 55.Degenhardt L, Hall W. Is cannabis use a contributory cause of psychosis? Can J Psychiatry. 2006 Aug;51(9):556–565. doi: 10.1177/070674370605100903. [DOI] [PubMed] [Google Scholar]
- 56.Macleod J, Davey SG, Hickman M, Egger M. Cannabis and psychosis. Lancet. 2007 Nov 3;370(9598):1539–1540. doi: 10.1016/S0140-6736(07)61651-1. [DOI] [PubMed] [Google Scholar]
- 57.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002 Nov 23;325(7374):1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012 Jun;42(6):1321–1328. doi: 10.1017/S0033291711002078. [DOI] [PubMed] [Google Scholar]
- 59.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004 Feb;184:110–117. doi: 10.1192/bjp.184.2.110. 110-7. [DOI] [PubMed] [Google Scholar]
- 60.Kuepper R, van OJ, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011 Mar 1;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Degenhardt L, Hall W, Lynskey M. Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend. 2003 Jul 20;71(1):37–48. doi: 10.1016/s0376-8716(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 62.Hickman M, Vickerman P, Macleod J, Lewis G, Zammit S, Kirkbride J, et al. If cannabis caused schizophrenia--how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009 Nov;104(11):1856–1861. doi: 10.1111/j.1360-0443.2009.02736.x. [DOI] [PubMed] [Google Scholar]
- 63.Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the Catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction. Biol Psychiatry. 2005 May ;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 64.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996 Jun;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 65.O’Tuathaigh CM, Clarke G, Walsh J, Desbonnet L, Petit E, O’Leary C, Genetic vs, et al. pharmacological inactivation of COMT influences cannabinoid-induced expression of schizophrenia-related phenotypes. Int J Neuropsychopharmacol. 2012 Oct;15(9):1331–1342. doi: 10.1017/S1461145711001581. [DOI] [PubMed] [Google Scholar]
- 66.Zammit S, Owen MJ, Evans J, Heron J, Lewis G. Cannabis, COMT and psychotic experiences. Br J Psychiatry. 2011 Nov;199(5):380–385. doi: 10.1192/bjp.bp.111.091421. [DOI] [PubMed] [Google Scholar]
- 67.Henquet C, Rosa A, Delespaul P, Papiol S, Fananas L, van OJ, et al. COMT ValMet moderation of cannabis-induced psychosis: a momentary assessment study of ‘switching on’ hallucinations in the flow of daily life. Acta Psychiatr Scand. 2009 Feb;119(2):156–160. doi: 10.1111/j.1600-0447.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- 68.Estrada G, Fatjo-Vilas M, Munoz MJ, Pulido G, Minano MJ, Toledo E, et al. Cannabis use and age at onset of psychosis: further evidence of interaction with COMT Val158Met polymorphism. Acta Psychiatr Scand. 2011 Jun;123(6):485–492. doi: 10.1111/j.1600-0447.2010.01665.x. [DOI] [PubMed] [Google Scholar]
- 69.Alemany S, Arias B, Fatjo-Vilas M, Villa H, Moya J, Ibanez MI, et al. Psychosis-inducing effects of cannabis are related to both childhood abuse and COMT genotypes. Acta Psychiatr Scand. 2013 Feb 28;:10. doi: 10.1111/acps.12108. [DOI] [PubMed] [Google Scholar]
- 70.Costas J, Sanjuan J, Ramos-Rios R, Paz E, Agra S, Tolosa A, et al. Interaction between COMT haplotypes and cannabis in schizophrenia: a case-only study in two samples from Spain. Schizophr Res. 2011 Apr;127(1-3):22–27. doi: 10.1016/j.schres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Zammit S, Spurlock G, Williams H, Norton N, Williams N, O’Donovan MC, et al. Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use. Br J Psychiatry. 2007 Nov;191:402–407. doi: 10.1192/bjp.bp.107.036129. [DOI] [PubMed] [Google Scholar]
- 72.Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013 Sep;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lev-Ran S, Roerecke M, Le FB, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2013 Jun 24;:1–14. doi: 10.1017/S0033291713001438. [DOI] [PubMed] [Google Scholar]
- 74.Wittchen HU, Frohlich C, Behrendt S, Gunther A, Rehm J, Zimmermann P, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. 2007 Apr;88(Suppl 1):S60–S70. doi: 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 75.Horwood LJ, Fergusson DM, Coffey C, Patton GC, Tait R, Smart D, et al. Cannabis and depression: an integrative data analysis of four Australasian cohorts. Drug Alcohol Depend. 2012 Dec 1;126(3):369–378. doi: 10.1016/j.drugalcdep.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 76.van Ours JC, Williams J, Fergusson D, Horwood LJ. Cannabis use and suicidal ideation. J Health Econ. 2013 May;32(3):524–537. doi: 10.1016/j.jhealeco.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Price C, Hemmingsson T, Lewis G, Zammit S, Allebeck P. Cannabis and suicide: longitudinal study. Br J Psychiatry. 2009 Dec;195(6):492–497. doi: 10.1192/bjp.bp.109.065227. [DOI] [PubMed] [Google Scholar]
- 78.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002 Nov 23;325(7374):1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002 Sep;97(9):1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 80.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Allebeck P. Cannabis use and depression: a longitudinal study of a national cohort of Swedish conscripts. BMC Psychiatry. 2012 Aug 16;12:112. doi: 10.1186/1471-244X-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marmorstein NR, Iacono WG, McGue M. Associations between substance use disorders and major depression in parents and late adolescent-emerging adult offspring: an adoption study. Addiction. 2012 Nov;107(11):1965–1973. doi: 10.1111/j.1360-0443.2012.03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, et al. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002 Dec;59(12):1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- 83.Lynskey MT, Glowinski AL, Todorov AA, Bucholz KK, Madden PA, Nelson EC, et al. Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Arch Gen Psychiatry. 2004 Oct;61(10):1026–1032. doi: 10.1001/archpsyc.61.10.1026. [DOI] [PubMed] [Google Scholar]
- 84.Hill MN, Gorzalka BB. Impairments in endocannabinoid signaling and depressive illness. JAMA. 2009 Mar 18;301(11):1165–1166. doi: 10.1001/jama.2009.369. [DOI] [PubMed] [Google Scholar]
- 85.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 2010 May 18;107(20):9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010 Aug;39(2):148–155. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 87.Kintscher U. The cardiometabolic drug rimonabant: after 2 years of RIO-Europe and STRADIVARIUS. Eur Heart J. 2008 Jul;29(14):1709–1710. doi: 10.1093/eurheartj/ehn255. [DOI] [PubMed] [Google Scholar]
- 88.STRADIVARIUS: a brave trial aimed at clarifying benefits of rimonabant therapy. Cardiovasc J Afr. 2008 May;19(3):158–159. [PubMed] [Google Scholar]
- 89.Juhasz G, Chase D, Pegg E, Downey D, Toth ZG, Stones K, et al. CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology. 2009 Jul;34(8):2019–2027. doi: 10.1038/npp.2009.19. [DOI] [PubMed] [Google Scholar]
- 90.Agrawal A, Nelson EC, Littlefield AK, Bucholz KK, Degenhardt L, Henders AK, et al. Cannabinoid Receptor Genotype Moderation of the Effects of Childhood Physical Abuse on Anhedonia and Depression. Arch Gen Psychiatry. 2012 Mar 5;69(7):732–740. doi: 10.1001/archgenpsychiatry.2011.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Brien MS, Comment LA, Liang KY, Anthony JC. Does cannabis onset trigger cocaine onset? A case-crossover approach. Int J Methods Psychiatr Res. 2012 Mar;21(1):66–75. doi: 10.1002/mpr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anthony JC. Steppingstone and gateway ideas: a discussion of origins, research challenges, and promising lines of research for the future. Drug Alcohol Depend. 2012 Jun;123(Suppl 1):S99–S104. doi: 10.1016/j.drugalcdep.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009 Feb;41(2):240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- 94.Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010 Jun 10;465(7299):721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- 95.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007 May;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 96.Wong CC, Mill J, Fernandes C. Drugs and addiction: an introduction to epigenetics. Addiction. 2011 Mar;106(3):480–489. doi: 10.1111/j.1360-0443.2010.03321.x. [DOI] [PubMed] [Google Scholar]
- 97.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008 Aug;14(8):341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011 Jan;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2013 Apr 30; doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mill J, Dempster E, Caspi A, Williams B, Moffitt T, Craig I. Evidence for monozygotic twin (MZ) discordance in methylation level at two CpG sites in the promoter region of the catechol-O-methyltransferase (COMT) gene. Am J Med Genet B Neuropsychiatr Genet. 2006 Jun 5;141B(4):421–425. doi: 10.1002/ajmg.b.30316. [DOI] [PubMed] [Google Scholar]
- 101.Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A, et al. Stress-related methylation of the catechol-O-methyltransferase Val 158 allele predicts human prefrontal cognition and activity. J Neurosci. 2011 May 4;31(18):6692–6698. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davey SG. Mendelian Randomization for Strengthening Causal Inference in Observational Studies: Application to Gene x Environment Interaction. Perspectives on Psychological Science. 2010;5:527–545. doi: 10.1177/1745691610383505. [DOI] [PubMed] [Google Scholar]
- 103.Irons DE, McGue M, Iacono WG, Oetting WS. Mendelian randomization: a novel test of the gateway hypothesis and models of gene-environment interplay. Dev Psychopathol. 2007;19(4):1181–1195. doi: 10.1017/S0954579407000612. [DOI] [PubMed] [Google Scholar]
- 104.Verweij KJ, Zietsch BP, Liu JZ, Medland SE, Lynskey MT, Madden PA, et al. No association of candidate genes with cannabis use in a large sample of Australian twin families. Addict Biol. 2011 Apr 20; doi: 10.1111/j.1369-1600.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction. 2009 Apr;104(4):518–532. doi: 10.1111/j.1360-0443.2009.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, et al. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008 Jul;65(7):841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012 Mar 2;148(5):1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 108.Hawken A, Caulkins J, Kilmer B, Kleiman M. Quasi-legal cannabis in Colorado and Washington: local and national implications. Addiction. 2013 May;108(5):837–838. doi: 10.1111/add.12156. [DOI] [PubMed] [Google Scholar]
- 109.Reuter P. Marijuana Legalization: What Can Be Learned from Other Countries. Santa Monica, CA: RAND: Drug Policy Research Center; 2010. Report No.: http://www.rand.org/content/dam/rand/pubs/working_papers/2010/RAND_WR771.pdf. [Google Scholar]
- 110.Macleod J, Hickman M. Response to commentaries: moving towards an evidence-based policy around cannabis use. Addiction. 2010 Aug;105(8):1337–1339. doi: 10.1111/j.1360-0443.2010.03038.x. [DOI] [PubMed] [Google Scholar]
- 111.Macleod J, Hickman M. How ideology shapes the evidence and the policy: what do we know about cannabis use and what should we do? Addiction. 2010 Aug;105(8):1326–1330. doi: 10.1111/j.1360-0443.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- 112.Hall W, Lynskey M. The challenges in developing a rational cannabis policy. Curr Opin Psychiatry. 2009 May;22(3):258–262. doi: 10.1097/YCO.0b013e3283298f36. [DOI] [PubMed] [Google Scholar]