Abstract
Genome-wide association studies have been successful in identifying common variants that influence the susceptibility to complex diseases. From these studies, it has emerged that there is substantial overlap in susceptibility loci between diseases. In line with those findings, we hypothesized that shared genetic pathways may exist between multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS). While both diseases may have inflammatory and neurodegenerative features, epidemiological studies have indicated an increased co-occurrence within individuals and families. To this purpose, we combined genome-wide data from 4088 MS patients, 3762 ALS patients and 12 030 healthy control individuals in whom 5 440 446 single-nucleotide polymorphisms (SNPs) were successfully genotyped or imputed. We tested these SNPs for the excess association shared between MS and ALS and also explored whether polygenic models of SNPs below genome-wide significance could explain some of the observed trait variance between diseases. Genome-wide association meta-analysis of SNPs as well as polygenic analyses fails to provide evidence in favor of an overlap in genetic susceptibility between MS and ALS. Hence, our findings do not support a shared genetic background of common risk variants in MS and ALS.
INTRODUCTION
Multiple sclerosis (MS, OMIM: 126200) is a common disease of the central nervous system characterized by inflammation, demyelination and axonal loss (1). Large extended families with the disease are extremely rare (2), but a genetic component in susceptibility to MS has been clearly demonstrated (1). Currently known risk variants include four classical human leukocyte antigen (HLA) alleles and >50 single-nucleotide polymorphisms (SNPs) outside the HLA region (3,4).
Amyotrophic lateral sclerosis (ALS, OMIM: 105400) is a neurodegenerative condition with devastating impact. Multiple cellular events contribute to the pathobiology, including mitochondrial dysfunction, excitotoxicity, protein aggregation in the cytosol, impaired axonal transport, neuroinflammation and dysregulated RNA signaling (5). About 10–20% of cases are familial, and up to 50% of these can be explained by known mutations in 18 genes including SOD1, FUS, TARDBP and C9orf72 (6). The majority of patients are isolated cases, however. Not all results from genome-wide association studies (GWAS) have been replicated, but two regions of association have been confirmed in independent studies: a locus on chromosome 9 and variation in the UNC13A region (7–11).
One of the lessons learned in the GWAS era is the substantial overlap in susceptibility loci between diseases. This has been demonstrated for immune-related (12,13), metabolic (14) and psychiatric (15) disorders and indicates, sometimes unexpectedly, commonalities and differences between diseases. MS indeed shares several susceptibility loci with other immune-related disorders, including type 1 diabetes and Crohn's disease (3). However, besides the immune component, key features of neurodegeneration, i.e. axonal transection, neuronal cell atrophy and neuronal death, are early pathological events in MS (1). Moreover, the irreversible disability seen in patients correlates stronger with neuronal damage than with inflammatory demyelination (16), although the cause of the neuronal damage remains elusive. On the other hand, for diseases classified as neurodegenerative such as ALS, an inflammatory or immune component has been implicated but is not yet conclusive (17,18). Case reports have described patients affected by both diseases (19–24) and an increased co-occurrence of MS and ALS compared with what is expected has been observed (25,26). Studies also report an increased risk of MS among relatives of patients suffering from ALS and vice versa (27–29), and some but not all studies report geographical correlation in mortality rates of both diseases (30,31).
In order to assess the shared genetic contribution between MS and ALS, possibly through common pathways of neurodegeneration or inflammation, we investigated the overlap of common susceptibility variants using available GWAS data.
RESULTS
We first investigated previously reported (3,4,7–11) susceptibility loci in one disease for evidence of association in the other. None of the reported ALS susceptibility loci show evidence for association with MS (Table 1). Out of 56 established, independent MS susceptibility loci (3,4), 4 (7.1%) show nominal significance for association with ALS, but none survived multiple testing for the number of SNPs investigated (Table 2). As expected because of the overlap between the datasets used here and those used in the original studies of each disease separately, all previously reported risk factors for either MS or ALS show the same direction of effect for the respective disease in this dataset as in the original studies. Regarding the other disease, 4/5 reported ALS risk SNPs show the same direction of effect in MS as in ALS (sign test P = 0.38), and among established MS-associated SNPs, 26/56 (46%) SNPs show the same direction of effect in ALS (sign test P = 0.69). Four SNPs were previously highlighted for reaching suggestive P-values of <10–5 for association with disease course (bout onset versus primary progressive MS) (3). Only one of these shows evidence for association with ALS but in the opposite direction (data not shown).
Table 1.
Association for reported ALS susceptibility loci with MS
| Chromosome | Rsid | Position (hg19) | Gene | Risk allele | P ALS | OR ALS | P MS | OR MS |
|---|---|---|---|---|---|---|---|---|
| 1 | rs6700125 | 59702797 | FGGY (9) | T | 0.087 | 1.06 | 0.085 | 1.06 |
| 7 | rs10260404 | 154210798 | DPP6 (10) | C | 0.0049 | 1.10 | 0.55 | 1.02 |
| 9 | rs3849942 | 27543281 | C9orf72 (7,8) | T | 5.8E-06 | 1.19 | 0.26 | 1.04 |
| 12 | rs2306677 | 26636386 | ITPR2 (11) | A | 0.080 | 1.10 | 0.60 | 1.03 |
| 19 | rs12608932 | 17752689 | UNC13A (7) | C | 8.3E-09 | 1.21 | 0.39 | 0.97 |
Table 2.
Association for independent, established MS susceptibility loci with ALS
| Chromosome | Rsid | Position (hg19) | Gene | Risk allele | P MS | OR MS | P ALS | OR ALS |
|---|---|---|---|---|---|---|---|---|
| 1 | rs4648356 | 2709164 | MMEL1 (TNFRSF14) | C | 0.012 | 1.09 | 0.97 | 1.00 |
| 1 | rs11810217 | 93148377 | EVI5 | T | 0.00032 | 1.14 | 0.12 | 0.94 |
| 1 | rs11581062 | 101407519 | SLC30A7 | G | 0.032 | 1.08 | 0.025 | 1.08 |
| 1 | rs1335532 | 117100957 | CD58 | A | 1.2E-08 | 1.35 | 0.97 | 1.00 |
| 1 | rs1323292 | 192541021 | RGS1 | A | 0.0098 | 1.11 | 0.53 | 1.03 |
| 1 | rs7522462 | 200881595 | C1orf106 | G | 0.00083 | 1.13 | 0.023 | 0.92 |
| 2 | Rs6718520a (4) | 43325570 | ZFP36L2 (THADA) | A | 1.2E-05 | 1.16 | 0.84 | 1.01 |
| 2 | rs12466022 | 43359061 | ZFP36L2 (THADA) | C | 4.2E-05 | 1.16 | 0.76 | 0.99 |
| 2 | rs7595037 | 68647095 | PLEK | T | 1.6E-05 | 1.15 | 0.32 | 0.97 |
| 2 | rs17174870 | 112665201 | MERTK | C | 0.00012 | 1.15 | 0.79 | 1.01 |
| 2 | rs10201872 | 231106724 | SP140 | T | 0.00056 | 1.15 | 0.13 | 1.07 |
| 3 | rs669607 | 28071444 | intergenic | C | 2.5E-05 | 1.15 | 0.57 | 0.98 |
| 3 | rs2028597 | 105558837 | CBLB | G | 0.56 | 1.03 | 0.52 | 1.04 |
| 3 | rs2293370 | 119219934 | C3orf1 | G | 0.056 | 1.08 | 0.29 | 0.96 |
| 3 | rs9282641 | 121796768 | CD86 | G | 0.0015 | 1.22 | 0.52 | 0.96 |
| 3 | rs2243123 | 159709651 | IL12A | C | 0.17 | 1.05 | 0.25 | 1.04 |
| 4 | rs228614 | 103578637 | MANBA | G | 0.0092 | 1.18 | 0.23 | 0.625 |
| 5 | rs6897932 | 35874575 | IL7R | C | 0.0014 | 1.12 | 0.20 | 0.96 |
| 5 | rs4613763 | 40392728 | PTGER4 | C | 0.00014 | 1.19 | 0.87 | 0.99 |
| 5 | rs2546890 | 158759900 | IL12B | A | 3.8E-06 | 1.16 | 0.78 | 1.01 |
| 6 | rs12212193 | 90996769 | BACH2 | G | 0.0055 | 1.09 | 0.14 | 1.05 |
| 6 | rs802734 | 128278798 | PTPRK | A | 0.0014 | 1.12 | 0.89 | 1.00 |
| 6 | rs11154801 | 135739355 | AHI1 | A | 0.014 | 1.08 | 0.49 | 0.98 |
| 6 | rs17066096 | 137452908 | IL22RA2 | G | 0.00096 | 1.13 | 0.29 | 0.96 |
| 6 | rs1738074 | 159465977 | TAGAP | C | 0.00075 | 1.12 | 0.45 | 0.98 |
| 7 | rs354033 | 149289464 | ZNF767 | G | 0.00079 | 1.13 | 0.26 | 1.04 |
| 8 | rs1520333 | 79401038 | PKIA | G | 0.11 | 1.06 | 0.41 | 1.03 |
| 8 | rs4410871 | 128815029 | MYC | C | 0.018 | 1.09 | 0.54 | 1.02 |
| 9 | rs2150702 | 5893861 | MLANA | G | 2.5E-05 | 1.14 | 0.015 | 1.08 |
| 10 | rs3118470 | 6101713 | IL2RA | C | 0.00078 | 1.12 | 0.76 | 1.01 |
| 10 | rs1250550 | 81060317 | ZMIZ1 | A | 0.0024 | 1.11 | 0.66 | 0.98 |
| 10 | rs7923837 | 94481917 | HHEX | G | 0.015 | 1.08 | 0.18 | 0.96 |
| 11 | rs650258 | 60832282 | CD5 | C | 0.00018 | 1.14 | 0.097 | 0.95 |
| 11 | rs630923 | 118754353 | CXCR5 | C | 0.033 | 1.11 | 0.066 | 1.08 |
| 12 | rs1800693 | 6440009 | TNFRSF1A | G | NAb | NA | 0.67 | 1.01 |
| 12 | rs10466829 | 9876091 | CLECL1 | A | 0.0009 | 1.11 | 0.49 | 0.98 |
| 12 | rs12368653 | 58133256 | AGAP2 | A | 0.0018 | 1.10 | 0.31 | 0.97 |
| 12 | rs949143 | 123595163 | ARL6IP4 | G | 0.015 | 1.08 | 0.57 | 0.98 |
| 14 | rs4902647 | 69254191 | ZFP36L1 | C | 0.00022 | 1.12 | 0.72 | 0.99 |
| 14 | rs2300603 | 76005557 | BATF | T | 0.014 | 1.10 | 0.10 | 0.94 |
| 14 | rs2119704 | 88487689 | GPR65 | C | 0.045 | 1.13 | 0.23 | 0.93 |
| 16 | rs2744148 | 1073552 | SOX8 | G | 0.023 | 1.10 | 0.30 | 0.95 |
| 16 | rs7200786 | 11177801 | CLEC16A | A | 8.8E-05 | 1.14 | 0.58 | 0.98 |
| 16 | rs13333054 | 86011033 | IRF8 | T | 0.063 | 1.09 | 0.98 | 1.00 |
| 17 | rs9891119 | 40507980 | STAT3 | C | 0.00016 | 1.13 | 0.86 | 0.99 |
| 17 | rs180515 | 58024275 | RPS6KB1 | G | 0.093 | 1.06 | 0.74 | 1.01 |
| 18 | rs7238078 | 56384192 | MALT1 | T | 0.00075 | 1.13 | 0.99 | 1.00 |
| 19 | rs1077667 | 6668972 | TNFSF14 | C | 0.033 | 1.10 | 0.10 | 0.94 |
| 19 | rs8112449 | 10520064 | CDC37 | G | 0.14 | 1.05 | 0.83 | 0.99 |
| 19 | rs874628 | 18304700 | MPV17L2 | A | 0.021 | 1.09 | 0.65 | 0.98 |
| 19 | rs2303759 | 49869051 | DKKL1 | G | 0.0075 | 1.11 | 0.034 | 1.08 |
| 20 | rs2425752 | 44702120 | NCOA5 | T | 0.0001 | 1.14 | 0.40 | 0.97 |
| 20 | rs2248359 | 52791518 | CYP24A1 | C | 0.00085 | 1.12 | 0.29 | 1.04 |
| 20 | rs6062314 | 62409713 | ZBTB46 | T | 0.047 | 1.14 | 0.52 | 1.04 |
| 22 | rs2283792 | 22131125 | MAPK1 | G | 0.00036 | 1.12 | 0.23 | 1.04 |
| 22 | rs140522 | 50971266 | ODF3B | T | 0.0022 | 1.12 | 0.72 | 0.99 |
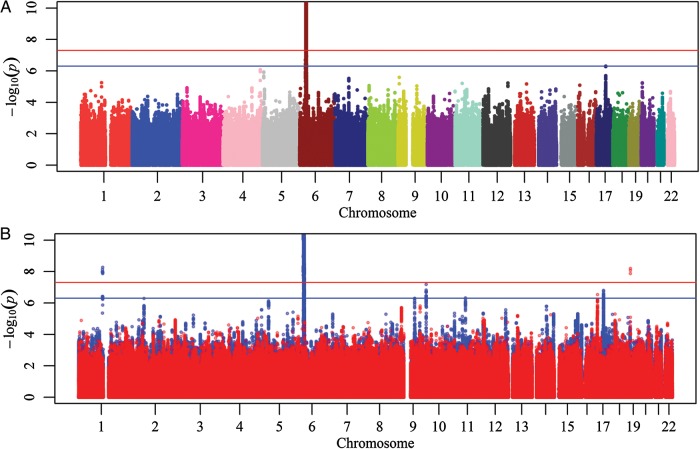
We next combined summary results from both MS and ALS datasets in a meta-analysis, looking for modest effects in each dataset that strengthen each other in the combined analysis. The combined analysis of both diseases included a total of 5 440 446 SNPs (Fig. 1). The genomic inflation factor (λs) was 1.033 for MS, 0.997 for ALS and 1.005 for the combined MS–ALS meta-analysis. In the meta-analysis, the HLA region reaches genome-wide significance, but this is driven by the MS component (P ALS with same direction of effect ≥0.01). One region, near NPEPPS on chromosome 17 (rs2935183), reaches suggestive association levels of P < 5 × 10−7 but is once again driven by MS [P (MS) = 6.5 × 10−7; P (ALS) = 0.41].
Figure 1.
Manhattan plot of (A) a combined MS–ALS analysis and (B) an overlay of the individual components consisting of both diseases (blue: MS, red: ALS). The y-axis has been cut off at -logP = 10. Red and blue horizontal lines indicate genome-wide (P < 5 × 10−8) and suggestive (P < 5 × 10−7) evidence.
Lastly, we investigated the possibility of an overlap of small susceptibility effects (polygenic score or ‘en masse’ effect). Therefore, we tested collectively SNPs that reached certain thresholds in the MS or ALS GWASs for association with ALS and MS, respectively. After correction for multiple testing, none of the models were significantly associated with disease (Tables 3 and 4), with the best model for each disease explaining only 0.05% of the phenotypic variance. To test whether the lack of association may have been affected by association results in the HLA region (which is known to be strongly associated with MS, but not with ALS), we repeated the polygenic analysis excluding SNPs in the HLA region (removing all SNPs on chromosome 6 between 29 and 33 Mb). This did not influence the results (Supplementary Material, Table S1).
Table 3.
Polygenic score based on MS data in ALS
| Model | P-value | Number of SNPs | Nagelkerke r2 corrected for baselinea |
|---|---|---|---|
| <5E−8 | 0.820 | 75 | 5.4E−06 |
| <5E−7 | 0.963 | 90 | 2.0E−07 |
| <5E−6 | 0.987 | 114 | 0.0E+00 |
| <5E−5 | 0.827 | 184 | 5.0E−06 |
| <5E−4 | 0.880 | 633 | 2.4E−06 |
| <5E−3 | 0.414 | 3454 | 6.9E−05 |
| <0.05 | 0.775 | 22284 | 8.5E−06 |
| <0.1 | 0.848 | 38861 | 3.8E−06 |
| <0.2 | 0.986 | 66276 | 1.0E−07 |
| <0.3 | 0.743 | 89109 | 1.1E−05 |
| <0.4 | 0.459 | 108626 | 5.7E−05 |
| <0.5 | 0.412 | 125558 | 7.0E−05 |
aBaseline: PC1-3, dummy-coded cohorts.
Table 4.
Polygenic score based on ALS in MS
| Model | P-value | Number of SNPs | Nagelkerke r2 corrected for baselinea |
|---|---|---|---|
| <5E−8 | 0.843 | 3 | 4.5E−06 |
| <5E−7 | 0.785 | 4 | 8.4E−06 |
| <5E−6 | 0.500 | 7 | 5.2E−05 |
| <5E−5 | 0.452 | 49 | 6.4E−05 |
| <5E−4 | 0.928 | 389 | 9.3E−07 |
| <5E−3 | 0.306 | 3075 | 1.2E−04 |
| <0.05 | 0.032 | 22315 | 5.2E−04 |
| <0.1 | 0.050 | 38922 | 4.4E−04 |
| <0.2 | 0.040 | 66738 | 4.8E−04 |
| <0.3 | 0.057 | 89592 | 4.1E−04 |
| <0.4 | 0.048 | 108839 | 4.4E−04 |
| <0.5 | 0.074 | 125337 | 3.6E−04 |
aBaseline: PC1-5, dummy-coded cohorts.
DISCUSSION
In this study, we have applied several statistical approaches to the investigation of shared susceptibility loci between the neurological diseases MS and ALS, which are both thought to involve inflammatory and neurodegenerative components (1,17,18) and for which case reports and epidemiological studies have reported co-occurrence within individuals or families (19–29). The strength of the study is that different statistical approaches are consistent in demonstrating that the number of regions in the genome with evidence for an overlap in susceptibility between the two diseases is not more than expected by chance. Among 65 loci having previously been implicated in one disease or disease subgroup, only 5 show nominally significant association with the other disease and none survive correction for multiple testing. There was no significant enrichment for the same direction of effect in both diseases. In a combined analysis of both diseases, no region outside of the HLA reaches genome-wide significance and only one reaches suggestive association levels of P < 5 × 10−7. Moreover, for both these regions with evidence for association in both diseases, results appear driven by strong evidence in MS, despite sample sizes of similar magnitude for both diseases. Furthermore, the polygenic analysis demonstrates that it is unlikely that many common variants with effect sizes that are beyond the detection threshold for association are shared between the two diseases. This contrasts with other diseases where a polygenic risk score calculated for one disease is associated with related diseases, as in the example of schizophrenia and bipolar disorder (15).
MS is a clinically heterogeneous disease, with the majority of patients (∼80%) suffering from a bout onset form of the disease with relapses and remissions, possibly followed by secondary progression, and the remaining 20% being characterized by progression from onset (1). It has been speculated that both forms represent a continuous spectrum of disease phenotypes with risk factors driving the balance between inflammation and neurodegeneration (32). Genetic association studies have so far not provided evidence for a different pathogenesis of the two forms (3). On the contrary, HLA-DRB1*1501, the strongest risk factor in MS and especially immunological in nature, is shared between both bout onset and primary progressive MS. In this study, there was no evidence for shared loci with the same direction of effect between ALS and primary progressive MS.
A total of >50 common risk variants for MS have now been identified (3,4). There is a highly significant enrichment for immune system genes in this list, with only few variants having a potential neurological function (3). GWAS studies in ALS have seen limited success (8). This discrepancy in the number of common risk variants identified between immunological and other diseases has been suggested to reflect a history of selection and adaptation of variants influencing the immune system (33,34). Mutations in several genes cause familial forms of ALS, and it has been thought that less common (1–5%) or rare (<1%) variants play a role in sporadic forms of the disease as well (35). Similarly, first reports of less common and rare variants in MS are emerging (36,37). This category of variants, which are not well captured by current genome-wide association studies, may explain part of the heritability in MS and ALS that remains unaccounted for by common variants (‘missing heritability’), and potentially the shared neurodegenerative component. Next-generation sequencing offers a technology suited to address this hypothesis.
It has recently been demonstrated that a large proportion of ALS is related to a GGGGCC hexanucleotide repeat expansion in intron 1 of C9orf72 (38,39), located in a region on chromosome 9p previously highlighted in GWAS studies of ALS (7,8). We did not observe any association of the C9orf72 region with MS. This is in line with the fact that no repeat expansions were observed in a cohort of 215 MS patients (25). Hence, C9orf72 variation does not appear to be a risk factor for MS. It has been suggested that MS can act as a modifier that increases the likelihood of C9orf72 expansions becoming penetrant and causing concurrent ALS (25), although further investigation is required (40).
In summary, the overlap of common variants between MS and other autoimmune disorders is not matched by a similar overlap between MS and other neurological disorders, such as ALS in this study. Whether less common or rare variants explain some of the shared neurodegenerative or neuroinflammatory aspects of both diseases cannot be addressed with the currently available datasets and remains to be examined with emerging technologies.
MATERIALS AND METHODS
We used data from 6 datasets totaling 4088 MS patients and 7144 controls from a recent meta-analysis of MS genome-wide association studies (4). Imputation was performed using Beagle v3.1 and the 1000 Genomes Project (1000G) Phase I (a) reference panel (2010/11 data freeze, 2011/6 haplotypes), and analysis was performed as described previously using the post-imputation probabilities and the first five principal components (PC) as covariates (4), leading to association results for a total of 6 948 682 SNPs with INFO of >0.10 and a minor allele frequency of >0.01 in all 6 datasets.
The ALS study population consists of 3 762 patients and 4 886 controls over 11 cohorts, for which details have been described previously (7,41). Imputation was performed using Beagle v.3.1.1. software with the 1000G CEU Aug 2010 reference panel. Analysis on dosage data including 3 PC led to association results for 12 249 385 SNPs.
A/T and C/G SNPs were removed, and results from both datasets on 5 440 446 overlapping SNPs were combined using an inverse variance fixed-effects model as implemented in the PLINK software package (42). Power was >99% for OR of ≥1.2 and >80% for OR of ≥1.15 at a typical risk allele frequency of 30% and genome-wide significance (P < 5 × 10−8).
Polygenic risk scores were calculated per individual to test the collective impact of SNPs that are associated with ALS on MS and vice versa. For each trait (MS and ALS), we first pruned the association results of the GWAS by linkage disequilibrium (r2 = 0.1), preferentially keeping SNPs with lower P-values. We selected twelve sets of SNPs (models) based on their GWAS P-values (<5 × 10−8, <5 × 10−7, <5 × 10−6, <5 × 10−5, <5 × 10−4, <5 × 10−3, 0.05, <0.1, <0.2, <0.3, <0.4 and <0.5). The smallest model contains three SNPs, whereas the models of P < 0.5 contain >125 000 SNPs (Table 3). Next, we calculated a polygenic risk score in all individuals of the other GWAS by summing up the dosages of the risk alleles in each model, multiplied by the log-odds. We then tested the association between the risk score and the phenotype using logistic regression with the same number of PCs as used in the original analysis of each trait (ALS: PC1-3, MS: PC1-5) and dummy-coded cohorts as covariates. Nagelkerke r2 was calculated to test the variance explained by each model (43).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
FUNDING
A.G. is supported by the Belgian Charcot Foundation. A.G. and B.D. are supported by Wetenschappelijk Onderzoek Multiple Sclerose (WOMS), the Belgian Neurological Society (BNS) and the Research Fund KU Leuven (OT/11/087). Computational resources used in this work were provided by the Hercules Foundation and the Flemish Government – department EWI. This investigation was supported in part by a postdoctoral fellowship from the National Multiple Sclerosis Society to N.A.P. (FG 1938-A-1) and by National Institutes of Health funding to the International Multiple Sclerosis Genetics Consortium (R01 NS049477). J.E.L. received funding from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (R01 NS073873). A.A.C. receives salary support from the National Institute for Health Research (NIHR) Dementia Biomedical Research Unit at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health. P.V.D. and B.D. are clinical investigators of the Research Foundation Flanders (FWO-Vlaanderen). B.D. is supported by TEVA PHARMA NEDERLAND BV. W.R. is supported by Interuniversity Attraction Poles (IUAP) program P6/43 of the Belgian Federal Science Policy Office, the University of Leuven (GOA 11/014 and Methusalem) and the E von Behring Chair for Neuromuscular and Neurodegenerative Disorders. J.V. is supported by the Thierry Latran Foundation. P.I.W.d.B. is the recipient of a VIDI Award from the Netherlands Organization for Scientific Research (NWO project 016.126.354). For the ALS GWA study, we thank all patients and healthy volunteers who participated in the project; general practitioners and pharmacists. In France, this study was funded by the Association pour la Recherche sur la SLA; and the Association Réseau SLA Ile de France. Support was also provided by the ALS Therapy Alliance; Project ALS; the Angel Fund; the Pierre L. de Bourgknecht ALS Research Foundation; the Al-Athel ALS Research Foundation; the ALS Family Charitable Foundation and the National Institute of Neurological Disorders and Stroke (NS050557). The Irish ALS study was funded by The Muscular Dystrophy Association (USA); The Health Research Board of Ireland; The Irish Neurological Association Travel Award and The Irish Motor Neuron Disease Research Foundation. The ALS Study in Italy was funded by the Italian Ministry of Health (RF-PIE-2007-63565 and RF-2010-2309849); Regione Piemonte (2004-A317); the Fondazione Vialli e Mauro per la SLA Onlus (obiettivo 7) and the Federazione Italiana Giuoco Calcio (FIGC). The ALS study in the Netherlands was supported by the Prinses Beatrix Fonds (Kersten Foundation); VSB fonds; the Netherlands ALS Foundation and J.R. van Dijk; the Adessium Foundation to L.v.d.B.; the Netherlands Organization of Scientific Research NWO Investments (grant numbers 175.010.2005.011, 911-03-012); the Research Institute for Diseases in the Elderly (014-93-015; RIDE) and the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO) (project number 050-060-810). In addition, the research leading to these results has received funding from the European Community's Health Seventh Framework Programme (FP7/2007-2013) (grant agreement number 259867). In Sweden, the ALS study was supported by the Swedish Brain Research Foundation; the Hållstens Research Foundation; the Swedish Medical Society; the Björklund Foundation for ALS Research and the Swedish Association for the Neurologically Disabled to P.A. For the ALS UK study, we thank the Motor Neurone Disease Association of Great Britain and Ireland, the Medical Research Council (UK), the Wellcome Trust and the Psychiatry Research Trust (Tim Perkins Fund and Charcot Fund). This work was supported in part by the Intramural Research Programs of the US National Institutes of Health (NIH), National Institute on Aging (Z01-AG000949-02) and National Institute of Neurological Disorders and Stroke (NINDS). Members of the International Multiple Sclerosis Genetics Consortium (IMSGC) and the Australia and New Zealand MS Genetics Consortium (ANZGene) are listed in the supplementary file.
Supplementary Material
REFERENCES
- 1.Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Willer C.J., Dyment D.A., Cherny S., Ramagopalan S.V., Herrera B.M., Morrison K.M., Sadovnick A.D., Risch N.J., Ebers G.C. A genome-wide scan in forty large pedigrees with multiple sclerosis. J. Hum. Genet. 2007;52:955–962. doi: 10.1007/s10038-007-0194-6. [DOI] [PubMed] [Google Scholar]
- 3.The International Multiple Sclerosis Genetics Consortium and The Welcome Trust Case Control Consortium 2. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patsopoulos N.A., De Bakker P.I.W. Bayer Pharma MS Genetics Working Group, Steering Committees of Studies Evaluating IFNβ-1b and a CCR1-Antagonist, ANZgene Consortium, GeneMSA, The International Multiple Sclerosis Genetics Consortium. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann. Neurol. 2011;70:897–912. doi: 10.1002/ana.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferraiuolo L., Kirby J., Grierson A.J., Sendtner M., Shaw P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 6.Andersen P.M., Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat. Rev. Neurol. 2011;7:603–615. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 7.van Es M.A., Veldink J.H., Saris C.G., Blauw H.M., van Vught P.W., Birve A., Lemmens R., Schelhaas H.J., Groen E.J., Huisman M.H., et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat. Genet. 2009;41:1083–1087. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- 8.Shatunov A., Mok K., Newhouse S., Weale M.E., Smith B., Vance C., Johnson L., Veldink J.H., van Es M.A., van den Berg L.H., et al. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 2010;9:986–994. doi: 10.1016/S1474-4422(10)70197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunckley T., Huentelman M.J., Craig D.W., Pearson J.V., Szelinger S., Joshipura K., Halperin R.F., Stamper C., Jensen K.R., Letizia D., et al. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N. Engl. J. Med. 2007;357:775–788. doi: 10.1056/NEJMoa070174. [DOI] [PubMed] [Google Scholar]
- 10.van Es M.A., van Vught P.W., Blauw H.M., Franke L., Saris C.G., Van den Bosch L., de Jong S.W., de Jong V., Baas F., van't Slot R., et al. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 2008;40:29–31. doi: 10.1038/ng.2007.52. [DOI] [PubMed] [Google Scholar]
- 11.van Es M.A., Van Vught P.W., Blauw H.M., Franke L., Saris C.G., Andersen P.M., Van Den Bosch L., de Jong S.W., van‘t Slot R., Birve A., et al. ITPR2 as a susceptibility gene in sporadic amyotrophic lateral sclerosis: a genome-wide association study. Lancet Neurol. 2007;6:869–877. doi: 10.1016/S1474-4422(07)70222-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhernakova A., van Diemen C.C., Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat. Rev. Genet. 2009;10:43–55. doi: 10.1038/nrg2489. [DOI] [PubMed] [Google Scholar]
- 13.Cotsapas C., Voight B.F., Rossin E., Lage K., Neale B.M., Wallace C., Abecasis G.R., Barrett J.C., Behrens T., Cho J., et al. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet. 2011;7:e1002254. doi: 10.1371/journal.pgen.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohlke K.L., Boehnke M., Abecasis G.R. Metabolic and cardiovascular traits: an abundance of recently identified common genetic variants. Hum. Mol. Genet. 2008;17:R102–R108. doi: 10.1093/hmg/ddn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herz J., Zipp F., Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp. Neurol. 2010;225:9–17. doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Philips T., Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- 18.Saresella M., Piancone F., Tortorella P., Marventano I., Gatti A., Caputo D., Lunetta C., Corbo M., Rovaris M., Clerici M. T helper-17 activation dominates the immunologic milieu of both amyotrophic lateral sclerosis and progressive multiple sclerosis. Clin. Immunol. 2013;148:79–88. doi: 10.1016/j.clim.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Borisow N., Meyer T., Paul F. Concomitant amyotrophic lateral sclerosis and paraclinical laboratory features of multiple sclerosis: coincidence or causal relationship? BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-007975. doi:10.1136/bcr-2012–007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trojsi F., Sagnelli A., Cirillo G., Piccirillo G., Femiano C., Izzo F., Monsurro M.R., Tedeschi G. Amyotrophic lateral sclerosis and multiple sclerosis overlap: a case report. Case Rep. Med. 2012;2012:324685. doi: 10.1155/2012/324685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G., Esiri M.M., Ansorge O., DeLuca G.C. Concurrent multiple sclerosis and amyotrophic lateral sclerosis: where inflammation and neurodegeneration meet? J. Neuroinflammation. 2012;9:20. doi: 10.1186/1742-2094-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen J.A., Stein R., Baker R.A., Royden Jones H. Muscle atrophy associated with multiple sclerosis: a benign condition or the onset of amyotrophic lateral sclerosis? J. Clin. Neurosci. 2008;15:706–708. doi: 10.1016/j.jocn.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Dynes G.J., Schwimer C.J., Staugaitis S.M., Doyle J.J., Hays A.P., Mitsumoto H. Amyotrophic lateral sclerosis with multiple sclerosis: a clinical and pathological report. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:349–353. doi: 10.1080/146608200750139837. [DOI] [PubMed] [Google Scholar]
- 24.Hader W.J., Rpzdilsky B., Nair C.P. The concurrence of multiple sclerosis and amyotrophic lateral sclerosis. Can. J. Neurol. Sci. 1986;13:66–69. doi: 10.1017/s0317167100035824. [DOI] [PubMed] [Google Scholar]
- 25.Ismail A., Cooper-Knock J., Highley J.R., Milano A., Kirby J., Goodall E., Lowe J., Scott I., Constantinescu C.S., Walters S.J., et al. Concurrence of multiple sclerosis and amyotrophic lateral sclerosis in patients with hexanucleotide repeat expansions of C9ORF72. J. Neurol. Neurosurg. Psychiatry. 2012;84:79–87. doi: 10.1136/jnnp-2012-303326. [DOI] [PubMed] [Google Scholar]
- 26.Turner M.R., Goldacre R., Ramagopalan S., Talbot K., Goldacre M.J. Autoimmune disease preceding amyotrophic lateral sclerosis: an epidemiologic study. Neurology. 2013;81:1222–1225. doi: 10.1212/WNL.0b013e3182a6cc13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemminki K., Li X., Sundquist J., Hillert J., Sundquist K. Risk for multiple sclerosis in relatives and spouses of patients diagnosed with autoimmune and related conditions. Neurogenetics. 2009;10:5–11. doi: 10.1007/s10048-008-0156-y. [DOI] [PubMed] [Google Scholar]
- 28.Etemadifar M., Abtahi S.H., Akbari M., Maghzi A.H. Multiple sclerosis and amyotrophic lateral sclerosis: is there a link? Mult. Scler. 2012;18:902–904. doi: 10.1177/1352458511427719. [DOI] [PubMed] [Google Scholar]
- 29.Hemminki K., Li X., Sundquist J., Sundquist K. Familial risks for amyotrophic lateral sclerosis and autoimmune diseases. Neurogenetics. 2009;10:111–116. doi: 10.1007/s10048-008-0164-y. [DOI] [PubMed] [Google Scholar]
- 30.Landtblom A.M., Riise T., Boiko A., Söderfeldt B. Distribution of multiple sclerosis in Sweden based on mortality and disability compensation statistics. Neuroepidemiology. 2002;21:167–179. doi: 10.1159/000059518. [DOI] [PubMed] [Google Scholar]
- 31.Bostrom I., Riise T., Landtblom A.M. Mortality statistics for multiple sclerosis and amyotrophic lateral sclerosis in Sweden. Neuroepidemiology. 2012;38:245–249. doi: 10.1159/000338031. [DOI] [PubMed] [Google Scholar]
- 32.Hensiek A.E., Seaman S.R., Barcellos L.F., Oturai A., Eraksoi M., Cocco E., Vecsei L., Stewart G., Dubois B., Bellman-Strobl J., et al. Familial effects on the clinical course of multiple sclerosis. Neurology. 2007;68:376–383. doi: 10.1212/01.wnl.0000252822.53506.46. [DOI] [PubMed] [Google Scholar]
- 33.Corona E., Dudley J.T., Butte A.J. Extreme evolutionary disparities seen in positive selection across seven complex diseases. PLoS One. 2010;5:e12236. doi: 10.1371/journal.pone.0012236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casto A.M., Feldman M.W. Genome-wide association study SNPs in the human genome diversity project populations: does selection affect unlinked SNPs with shared trait associations? PLoS Genet. 2011;7:e1001266. doi: 10.1371/journal.pgen.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dion P.A., Daoud H., Rouleau G.A. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat. Rev. Genet. 2009;10:769–782. doi: 10.1038/nrg2680. [DOI] [PubMed] [Google Scholar]
- 36.De Jager P.L., Jia X., Wang J., de Bakker P.I., Ottoboni L., Aggarwal N.T., Piccio L., Raychaudhuri S., Tran D., Aubin C., et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat. Genet. 2009;41:776–782. doi: 10.1038/ng.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goris A., Fockaert N., Cosemans L., Clysters K., Nagels G., Boonen S., Thijs V., Robberecht W., Dubois B. TNFRSF1A coding variants in multiple sclerosis. J. Neuroimmunol. 2011;235:110–112. doi: 10.1016/j.jneuroim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 38.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renton A.E., Majounie E., Waite A., Simon-Sanchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Doormaal P.T.C., Gallo A., van Rheenen W., Veldink J.H., van Es M.A., Van den Berg L.H. Amyotrophic lateral sclerosis is not linked to multiple sclerosis in a population based study. J. Neurol. Neurosurg. Psychiatry. 2013;84:940–941. doi: 10.1136/jnnp-2012-304864. [DOI] [PubMed] [Google Scholar]
- 41.Chio A., Schymick J.C., Restagno G., Scholz S.W., Lombardo F., Lai S.L., Mora G., Fung H.C., Britton A., Arepalli S., et al. A two-stage genome-wide association study of sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 2009;18:1524–1532. doi: 10.1093/hmg/ddp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagelkerke N.J.D. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.