Abstract
Progestogens have actions in the midbrain ventral tegmental area (VTA) to mediate motivated behaviours, such as those involved in reproductive processes, among female rodents. In the VTA, formation and actions of one progestogen, 5α-pregnan-3α-ol-20-one (3α,5α-THP; 3α,5α-THP), are necessary and sufficient to facilitate sexual responding (measured by lordosis) of female rodents. Although 3α,5α-THP can be produced following metabolism of ovarian progesterone, 3α,5α-THP is also a neurosteroid produced de novo in brain regions, such as the VTA. There can be dynamic changes in 3α,5α-THP production associated with behavioural experience, such as mating. Questions of interest are the sources and targets of 3α,5α-THP. Regarding sources, pregnane Xenobiotic Receptor (PXR) may be a novel factor involved in 3α,5α-THP metabolism in the VTA (as well as a direct target of 3α,5α-THP). We have identified PXR in the midbrain of female rats, and manipulating PXR in this region reduces 3α,5α-THP synthesis and alters lordosis as well as affective and social behaviours. Regarding targets, recent studies have focused on the role of membrane progestin receptors (mPRs). We have analyzed expression of two of the common forms of these receptors (mPRα/paqr7 and mPRβ/paqr8) in female rats. Expression of mPRα was observed in peripheral tissues and brain areas, including hypothalamus and midbrain. Expression of mPRβ was only observed in brain tissues and was abundant in the midbrain and hypothalamus. To our knowledge, studies of these receptors in mammalian models have been limited to expression and regulation, instead of function. A question that was addressed was the functional effects of progestogens via mPRα and mPRβ in the midbrain of hormone-primed rats for lordosis. Studies to date suggest that mPRβ may be an important target of progestogens in the VTA for lordosis. Together, these studies demonstrate that PXR is involved in production of 3α,5α-THP in the midbrain VTA. Moreover, mPRs may be a target for progestogens’ actions in the VTA for lordosis.
Keywords: progesterone, non-genomic, lordosis, neurosteroids, mating
Introduction
The role of progesterone, and other progestogens (referring herein to progesterone and its neuroactive products, including 5α-pregnan-3α-ol-20-one; 3α,5α-THP), beyond their pro-gestational effects are of interest. Progestogens are involved in facilitating successful mating before gestation, have organizing effects on the nervous system during gestation/perinatally and then can alter adult behaviours that ultimately are adaptive (reducing stress/anxiety, enhancing cognition and conferring protection to neural insults/aging; see reviews on these topics by: [1–5]). Of interest are the novel sources and targets of progestogens for such effects.
To be able to ask questions about the sources and targets of progestogens for effects on varied central nervous system functions, it is useful to focus on a behaviour that is reliant upon progestogens, and then extend this approach to other behaviours that are regulated by progestogens. As such, in our laboratory, we utilise mating behaviour of female rodents, with one measure being the lordosis response. Lordosis refers to the mating posture of female rodents to allow intromissions by the male. Lordosis is a quantifiable behaviour under hormonal control, and the brain circuitry underlying it is well-characterised [6–8]. Lordosis is observed under appropriate endocrine and environmental contexts, including progestogens having actions in midbrain and hypothalamic regions. By using such a behaviour that is dependent upon progestogens’ actions in the midbrain, for example, we can investigate the requisite factors for formation as well as actions of progestogens in this region. These studies then inform subsequent investigations on how these same mechanisms may be conserved across species, brain regions and other behaviours (e.g. affect, affiliation, cognition, and neuroprotection).
From studies using this approach, it is clear that progesterone has actions in the midbrain ventral tegmental area (VTA) to mediate motivated behaviours, such as those involved in reproductive processes, among female rodents. In the VTA, formation and actions of the progestogen, 3α,5α-THP (a.k.a. allopregnanolone), are necessary and sufficient to facilitate lordosis (reviewed recently in [9]). Although 3α,5α-THP can be produced following metabolism of ovarian progesterone, 3α,5α-THP is also a neurosteroid produced de novo in brain regions, such as the VTA. Because actions of 3α,5α-THP are necessary and sufficient in the VTA for lordosis, we have been utilizing this behaviour as a bioassay to ask questions about factors involved as sources and targets of progestogens, which will be discussed throughout this review. Indeed, these studies addressing mechanistic questions about progestogens in the VTA using lordosis as the in vivo assay are then extended to other areas of interest regarding progestogens’ effects across the lifespan.
Another intriguing finding related to 3α,5α-THP’s vital role in reproductive behaviours is that there can be dynamic changes in 3α,5α-THP production associated with behavioural experiences, such as mating. For example, we have demonstrated that midbrain 3α,5α-THP levels are highest following paced mating (where female control the timing of male contacts’ [10,11]). In addition to such a response from a social challenge like mating, previous work by Purdy and colleagues demonstrating rapid and robust changes in 3α,5α-THP synthesis following acute environmental and physical stressors (footshock, swim stress; [12]). Moreover, chronic stressors during gestation (e.g. restraint stress, immune challenges, environmental disruptions of dams) or adulthood (social isolation) reduce 3α,5α-THP levels. Additionally, reducing 3α,5α-THP synthesis in the brain is associated with greater stress responding [13–18] As such, one notion is that 3α,5α-THP is critical for homeostatic regulation.
This review will focus on the identifying novel sources and targets of 3α,5α-THP in the midbrain VTA. First, novel sources of 3α,5α-THP will be a focus. Previous work demonstrating that manipulating enzymes for 3α,5α-THP formation in the midbrain attenuates lordosis will be discussed. How these enzymes may be downstream of a novel factor, the pregnane xenobiotic receptor (PXR), and its role as a key regulatory factor for behavioural-induced 3α,5α-THP formation in the midbrain will be addressed. Second, novel targets of 3α,5α-THP will be a focus. Studies establishing that 3α,5α-THP has rapid effects that do not involve nuclear progestin receptors (PRs) in the VTA will be discussed. Then, the role of membrane progestin receptors (mPRs) as targets of progestogens in the midbrain for lordosis will be addressed. Studies are described that support PXR as a novel factor in production of, and mPRs as novel targets of, 3α,5α-THP, using the lordosis model, in its role as a regulator of homeostatic responses, related to stress, motivated behaviours and plasticity (Figure 1).
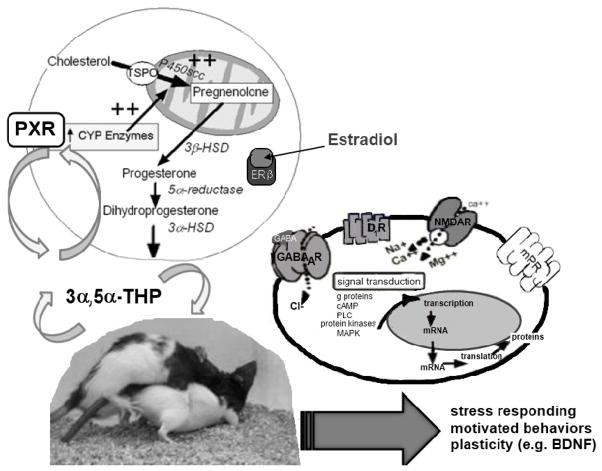
Figure 1.
Proposed relationship between pregnane xenobiotic receptor (PXR), 3α,5α-THP, and target substrates that may be involved in biosynthesis of 3α,5α-THP in the midbrain ventral tegmental area (VTA) with mating, including oestradiol role via oestrogen receptor beta (ERβ). Moreover, 3α,5α-THP is a ligand of PXR and, thus, may activate PXR to underlie behaviour (mating) induced neurosteroidogenesis. 3α,5α-THP has novel membrane targets including GABAA, dopamine type 1 (D1), NMDA and membrane progestin receptors (mPRs) that are important for actions of 3α,5α-THP in the VTA for processes underlying homeostasis, such as stress responding, motivated behaviors and plasticity (e.g. brain-derived neurotrophic factor; BDNF).
Sources of 3α,5α-THP
Formation of 3α,5α-THP can occur following metabolism from progesterone from peripheral glands (e.g. ovaries, adrenals, placenta) or its de novo synthesis. Regarding metabolism, progesterone, secreted from peripheral glands, travels in circulation to the brain (as well as many other target organs) and then can be metabolised by enzymes to other neuroactive metabolites. A pathway to form 3α,5α-THP from metabolism of progesterone involves sequential actions of 5α-reductase (an irreversible action that forms dihydroprogesterone) and then 3α-hydroxysteroid dehydrogenase (3α-HSD). Oestradiol can enhance progestogen metabolism, and studies in our laboratory suggest that the beta form of the estrogen receptor may be involved for such effects (reviewed in [19]). However, these progestogens can also be formed in the brain itself, independent of these peripheral sources [20–27]. The biosynthetic pathway for neurosteroid production involves many recognized factors, including the 18kDA translocator protein (TSPO, a.k.a. peripheral-type benzodiazepine receptor), the steroidogenic acute regulatory (StAR) protein, cytochrome P450-dependent C27 side chain cleavage enzymes (P450scc), 3β-hydroxysteroid dehydrogenase (3β-HSD), 5α-reductase, and 3α-HSD. TSPO and StAR have actions to transport cholesterol (a requisite precursor for all steroids) into the mitochondria. In the mitochondria, cholesterol is oxidised by P450scc to form, which is then metabolised by 3β-HSD to progesterone. Progesterone can then be metabolised to form 3α,5α-THP, via actions of 5α-reductase and 3α-HSD. Thus, 3α,5α-THP can be formed following conversion of peripheral and central sources of progesterone.
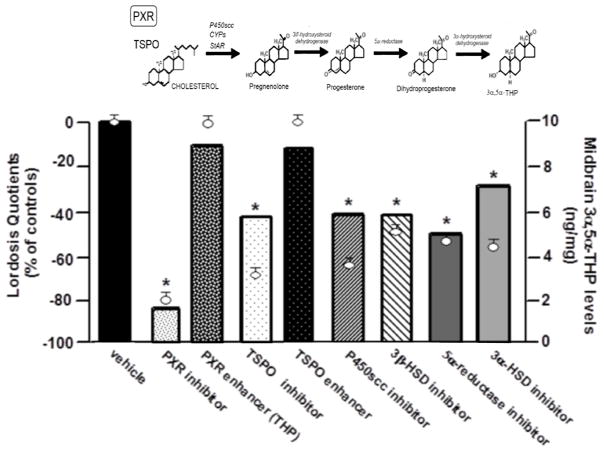
Reducing activity at any of these rate-limiting steps for biosynthesis and/or metabolism to 3α,5α-THP in the midbrain VTA attenuates lordosis of rodents. First, these enzymes are expressed in the brain, with high levels reported in the midbrain and corticolimbic regions of rats [28–32]. Using RT-PCR and western blotting, we have identified mRNA and protein expression of StAR, P450scc, 3β-HSD, 5α-reductase, and 3α-HSD in the midbrain of pro-oestrous rats (those that are naturally-receptive with high progestogen levels; [33]). Second, infusions of antagonists to these targets to the VTA of receptive rats reduce lordosis responding ([5,9,34–36], See Figure 2). Third, genetic manipulations support these effects of pharmacological manipulations of these factors for lordosis. For example, mice that are deficient in 5α-reductase have lower lordosis as well as midbrain 3α,5α-THP levels when primed with oestradiol and progesterone, compared to wildtype mice [37]. Yet, 5α-reductase knockout mice have increased lordosis when administered 3α,5α-THP [37], suggesting that the deficit in lordosis is related to formation of 3α,5α-THP, not responses to 3α,5α-THP. Fourth, studies investigating age-related changes in reproductive behaviours support the notion that alterations in formation of 3α,5α-THP in the brain modifies reproductive responses. Among middle-aged rats, there is variability in the timing of reproductive senescence (defined by acyclicity, low fertility, and low fecundity) and mating behaviours, which may be related to capacity to metabolise progesterone and dihydroprogesterone in the midbrain as well as reward pathway targets, such as the diencephalon [38]. Fifth, we have observed the same pattern of effects for such central manipulations of these factors required for 3α,5α-THP synthesis in gonadally-intact, ovariectomised, or ovariectomised and adrenalectomised rats, suggesting that peripheral sources of progesterone were not necessary for these effects [5]. Together, these studies demonstrate the importance of 3α,5α-THP in the midbrain for lordosis; however, another question is the role of mating for biosynthesis of 3α,5α-THP in the brain.
Figure 2.
Intra-ventral tegmental area (VTA) infusions of positive modulators enhanced lordosis (bars) and increased 3α,5α-THP(circles). Infusions of inhibitors blocked increases of 3α,5α-THP levels in the VTA and decreased lordosis quotient. * indicates significant difference from vehicle-infused receptive controls, p < 0.05.
Mating induces 3α,5α-THP formation in the brain. Levels of 3α,5α-THP are increased in the midbrain VTA following mating [5,9–11,30,31]. Other reproductively-relevant behaviours, such as exploration (in an open field), anxiolysis (in an elevated plus maze), or social interaction with another female do not produce the same increases in 3α,5α-THP in the midbrain as does mating among pro-oestrous rats [10,11]. In addition to being specific to mating, this behaviour-induced 3α,5α-THP synthesis is most robust following a semi-naturalistic mating paradigm utilized in the lab, called paced mating, compared to a standard mating paradigm. With paced mating compared to standard mating, the testing chamber is larger and divided so that females can control the timing (i.e. “pace”) of their sexual contacts with males. The pacing task is then more akin to a naturalistic mating scenario among rats, is spontaneously observed among naïve females, and enhances reproductive success (increases fertility and fecundity; [40]). Among females that do not spontaneously pace their contacts, despite being exposed to and mating with a male in a pacing chamber, there are lower levels of 3α,5α-THP in the midbrain following testing, compared to that observed among females that do spontaneously pace in the task [41]. As well, there are greater increases in 3α,5α-THP in the midbrain following paced compared to standard mating [5,9–11]. Notably, behaviour-induced biosynthesis of 3α,5α-THP does not only occur in the midbrain. Following paced mating, there are rapid enhancements in the levels of 3α,5α-THP in the hypothalamus, striatum/diencephalon, hippocampus and cortex [41], further support of a role of 3α,5α-THP for behaviours beyond reproduction. As well, behavioural-induced biosynthesis of 3α,5α-THP occurs rapidly, and in the absence of peripheral sources of progestogens, such as the ovaries, adrenals, and placenta, suggesting that the brain is the source for mating-induced 3α,5α-THP formation. A question of continued investigations is the upstream factors that may be driving this response.
Role of pregnane xenobiotic receptor
Current investigations are aimed at understanding the role of PXR as a novel factor for 3α,5α-THP’s synthesis in the midbrain VTA. A well-known function of PXR is to detect both exogenous and endogenous toxins and up-regulates gene transcription for proteins that metabolize and/or dispose of these. A focus heretofore has been on these actions of PXR in the liver and other secretory organs (e.g. intestines and kidneys) as a “master regulator” in these tissues [42–45], but more recent work supports the importance of PXR for clearance and metabolism in the nervous system (described below). Activated PXR regulates gene transcription in a ligand-dependent fashion, which promotes the production of a wide array of proteins, including CYP enzymes, which metabolize many drugs and hormones [45–46]. Indeed, 3α,5α-THP is a ligand of PXR (albeit, the specificity of this response is not entirely clear [45–46]), further suggesting interaction of PXR and 3α,5α-THP in regulating homeostatic processes. Current studies are investigating the role of PXR for cholesterol metabolism/clearance and induction of CYP enzymes important for neurosteroidogenesis.
The evidence in support of a potential role of PXR in central nervous system function, including neuroendocrinology, is growing. First, PXR is expressed in the brain. The traditional thinking was that PXR was predominantly a liver factor. However, there has been identification of PXR in the central nervous system of several species, such as rodents, rabbits, pigs, and [9,33,46–50]. Of particular importance to behavioural-induced 3α,5α-THP synthesis, PXR is expressed in the midbrain VTA. Studies using microarray, RT-PCR and qPCR, western blotting and enzyme-linked immunosorbance assays have confirmed the presence of PXR gene, RNA, and protein in the midbrain of mated female rats [9,10,33,51]. PXR expression in the midbrain may be sensitive to hormonal state as pro-oestrous rats have higher levels than do dioestrous rats or male rats [9,10,33,51]. Second, activation of PXR with ligands, such as progestogens 3α,5α-THP enhance lordosis responding of dioestrous or ovariectomised, oestradiol-primed rats [52–53]. Given that PXR is expressed in brain and body and can be activated by many environmental and pharmacological agents to alter many different CYP enzymes that are involved in steroid metabolism and clearance of many drugs, it was important to begin investigating the specificity of these effects of PXR manipulations. Third, studies using knock down of expression of PXR in the midbrain VTA with infusions of antisense oligodeoxynucleotides (AS-ODN) targeted to PXR have revealed some of its functional effects. Among cycling rats, PXR AS-ODNs reduced midbrain PXR expression and 3α,5α-THP levels and attenuated lordosis responding, proceptivity and aggression/rejection behaviours towards the males, of pro-oestrous rats [9,10,33,51]. There were no effects of AS-ODN infusions to dioestrous rats, which have low progestogens and reduced capacity for mating-induced increases in 3α,5α-THP levels. Over the estrous cycle, there are sequential increases in oestradiol followed by elevations in progestogen levels during pro-oestrus. A question is then PXR manipulations are oestradiol- and/or progestogen-dependent. Additionally, oestradiol enhances 5α-reductase activity, consequently increasing brain 3α,5α-THP, and is required for 3α,5α-THP to facilitate lordosis of ovariectomised female rats [34]. As such, whether there are distinct effects of oestradiol to involve PXR was investigated. Among ovariectomised rats primed with oestradiol (but not vehicle alone) and administered 3α,5α-THP and PXR AS-ODNs to the VTA, there was a reduced capacity to respond to 3α,5α-THP infusions or behavioural-induced 3α,5α-THP production in the midbrain [54]. These data show that replacement of 3α,5α-THP following knock down of PXR in the VTA does not reverse effects of PXR knock down for lordosis, suggesting binding of 3α,5α-THP to PXR may be important for these effects on lordosis and mating-induced 3α,5α-THP. Fourth, studies are ongoing to characterize the behaviour and (neuro)steroid responsiveness of commercially-available animal models that are genetic knockouts of PXR (mice from Taconic Inc, and rats from S.A.G.E. Labs). Moreover, how these PXR manipulations may alter expression and/or activity of downstream factors important for neurosteroidogenesis are of ongoing interest. Together, these data suggest that PXR is a homeostatic regulator for behavioural-induced 3α,5α-THP formation.
Potential role of liver X receptor
Another potential target of interest as a novel factor involved in production of 3α,5α-THP is the liver X receptor, a relative of PXR as a member of the nuclear receptor superfamily. 3α,5α-THP can be produced, and secreted, by both glia and neurones, independent of peripheral secretion; indeed, it has been suggested that LXR and PXR may have respective roles in glia and neurones [1]. Although both of these receptors have been traditionally considered in their roles as liver factors, evidence is mounting regarding their expression and functional effects in the central nervous system. The studies investigating expression and functions of PXR in the brain are described in the previous section. In the case of LXR, there have been recent reports of its role in neurogenesis in the developing mouse midbrain [55] as well as an anxiety phenotype in the female LXR knockout mouse [56]. Indeed, a consideration is the role of these receptors for cholesterol metabolism and clearance, which has clinical relevance for development, aging and neurodegenerative disorders (e.g. Alzheimer’s disease, multiple sclerosis, diabetes neuropathy; Niemann-Pick disease; 46, 57–62). Indeed, a role of 3α,5α-THP has likewise been implicated in these disorders [1,3,5,63,64]. Of interest to us is how PXR and LXR may act synergistically for efficient cholesterol metabolism and mating-induced 3α,5α-THP, and how these effects may, ultimately, be neuroprotective or facilitate plasticity.
Targets of 3α,5α-THP
Following its formation in the midbrain VTA, a question is by what receptor mechanism may 3a,5α-THP be having its functional effects. Traditional notions about how steroids have effects are those that involve binding to their intracellular cognate steroid receptors. Cognate, nuclear progestin receptors (nPRs) were first characterized ~40 years ago [65]. As with the traditional actions of other steroid receptors, nPRs bind ligands and act as transcription factors to evoke expression of mRNAs leading to changes in protein synthesis, and, thereby, biological actions. Both progesterone and dihydroprogesterone bind with a high affinity for intracellular progestin receptors, which are located in reproductive tissues and brain areas, such as the hypothalamus [66].
Studies investigating the mechanisms of action of 3α,5α-THP suggest that is does not require actions at nPRs for its functional effects on lordosis. This idea has been supported by several studies focused on investigating whether 3α,5α-THP in the midbrain VTA meets criteria for steroid receptor actions in this region: 1) receptors are expressed; 2) activation of receptors increase behavioural response; 3) blockade of receptors attenuate behavioural response; 4) genetic knockouts of receptors show an attenuated behavioural response; 5) timing suggests effects are too rapid for new protein synthesis; 6) effects are observed when relegated to the cell membrane. First, nPRs are low in the VTA, and there is little evidence of oestradiol-induced nPRs in this region [30,31,66]. Second, activating nPRs with nPR agonists directed at the VTA do not enhance lordosis of ovariectomised rodents [7,30,31]. Third, conversely, nPR antagonists do not alter progestogen-facilitated lordosis [67–69]. Fourth, there are similar effects of progestogens to increase lordosis of nPR knockout mice and their wildtype counterparts [70]. Fifth, progestogens to the VTA have rapid effects to increase neuronal firing here as well as lordosis (all in less than 5 minutes, which is considered outside of the timeframe for mRNA translation, transcription and new protein synthesis; [71]). Sixth, unbound progestogens permeate cell membranes with ease, but if they are conjugated to a large protein, like bovine serum albumin, passage into the cell is restricted; yet, conjugated progestogens to the VTA enhance lordosis of ovariectomised rodents [72,73]. Together, these studies demonstrated that 3α,5α-THP has rapid, membrane-relegated effects that do not require nPRs in the VTA for lordosis of female rodents.
3α,5α-THP, like other neurosteroids, has actions through ion channel-associated membrane receptors for rapid functional changes, and there are several of these targets in the VTA to consider in their role for progestogen-facilitated lordosis. There is a long history of investigating the role of 3α,5α-THP through γ-aminobutyric acid type A (GABAA) receptors. 3α,5α-THP, even compared to GABAergic anxiolytics (benzodiazepines and barbiturates), has higher efficacy at GABAA receptors, and directly increases chloride channel currents when in very low (nanomolar) concentrations [74–78]. The effects of 3α,5α-THP as a positive modulator GABAA receptors have been well-characterized in the VTA [7,30,31] and elsewhere in the brain [79–81]. Blocking GABA receptors in the VTA attenuates lordosis of female rodents [7,30,31,82]. The midbrain VTA is a well-described part of the dopaminergic reward pathway, and progestogens can alter density of dopamine type 1 receptors as well as dopamine release in the midbrain [30,31]. We have demonstrated that blocking dopamine type 1, but not type 2, receptors in this region reduces progestogen-facilitated lordosis responding of female rodents; and lordosis can be enhanced with dopamine type 1 agonists infused to the VTA [7,83,84]. Thus, actions via GABAA and dopamine type 1 receptors in the VTA may be required for 3α,5α-THP lordosis of female rodents.
Regarding the circuitry in the VTA, a suggestion is that there is an interaction between these effects of 3α,5α-THP via GABAA, dopamine and glutamate targets (as well as their downstream signal transduction pathways; [7]). In the VTA, there are dopamine type 1 receptor-expressing GABAergic terminals that synapse on dopamine cell bodies that express GABAA and NMDA receptors [7,85,86]. There are also GABAergic interneurones in the midbrain VTA that provide input to dopamine-containing cell bodies and therefore enhance dopamine release when activated by GABA [87]. As well, activation of dopamine type 1 receptors on GABAergic afferents in the VTA enhances GABA release [88], and we have demonstrated that blocking GABAA receptors in the VTA attenuates progestogen-facilitated lordosis and effects of dopamine type 1 agonists to enhance lordosis [89]. Furthermore, 3α,5α-THP is a negative modulator of glutamate, reducing its excitatory effects [90], and 3α,5α-THP increases levels of glutamate in the midbrain [30]. NMDAR agonists to the VTA enhance lordosis [91]. Thus, some of the membrane neurotransmitter targets in the VTA that are important for 3α,5α-THP-facilitated lordosis are GABA, dopamine and glutamate.
A series of experiments investigated the downstream targets of 3α,5α-THP via these neurotransmitter receptors in the midbrain VTA for lordosis by determining the requisite roles of G-proteins, adenylyl cyclase, phospholipase C, and protein kinases. These studies demonstrated in ovariectomised hormone-primed rats and hamsters that pharmacologically blocking G-proteins, adenylyl cyclase, phospholipase C, and protein kinases A or C in the midbrain VTA, attenuated progestogen-facilitated lordosis, and enhancements by GABAA and dopamine type 1 receptor agonists (reviewed in [7]). An additional question is whether 3α,5α-THP may be having actions via other G-protein coupled receptor targets in the VTA for these effects on lordosis. One such target of interest is membrane progestin receptors (mPRs).
Role of membrane progestin receptors
mPRs were characterized over a decade ago in aquatic species [92]. These receptors are members of the progestin and adiponectin (PAQR) family, which contains 4 adiponectin receptor like (class I receptors), 5 unique mPR members (α ~ε, class II receptors) and two hemolysin receptor like receptors [93–95]. The members of class II PAQR family, which consist of mPRα ~ε, exist only in vertebrates, and members of the other two classes are present both in vertebrates and non-vertebrates, including yeast and bacteria [93,95]. The mPRs are conserved across a broad range of many vertebrates, from fish to humans [92,93,95], and specifically bind progestogens in these species: e.g. fish (goldfish, seatrout, and zebrafish), frog, and mammals (cattle, rat, mouse, human) [93,96–98]. Thus, mPRs are conserved across species.
It can be argued that mPRs are most well-known for their roles in reproductive processes based upon their expression patterns. A focus herein will be on the most well-studied of the mPRs, mPRα and mPRβ (paqr7 and pagr8, respectively). mPRα was first characterized in spotted seatrout, and is expressed abundantly in reproductive (testes, ovary, uterus), kidneys, and brain and spinal cord among vertebrates, including fish, mice and human [92,99–101]. Functions of mPRα for reproductive processes have been described relating to rapid progestogen signaling in maturation of fish oocytes [92, 97, 99]; sperm motility in fish [98], progesterone-signaling in human myometrial cells, breast cancer cells and lymphocytes [95, 102–104], activity in rat and sheep reproductive tissues[105,106], and rapid gonadotropin releasing hormone secretion among mice [107]. mPRβ is also proposed to be involved in reproductive functions, including the controlled beating of cilia of the fallopian tubes of mice and humans [108]. Thus, this comparative literature supports a role of mPRα and mPRβ in reproduction across species. Of continued interest is the role of these receptors as targets of progestogens, such as 3α,5α-THP, for behavioural effects.
Beyond effects in peripheral reproductive organs, evidence is growing for the role of mPRs in the central nervous system. Two of the receptor subtypes that have received the most interest are mPRα and mPRβ. We have demonstrated with RT-PCR that there are differences in expression of mPRα and mPRβ in the body (spleen, heart, lung, kidney, liver, intestines) and brains (prefrontal cortex, hippocampus, amygdala, hypothalamus, midbrain) of pro-oestrous rats and mice. Among rats, mPRα is expressed in the body and brain (with high levels in the midbrain; [109]). That there was great abundance of mPRα found in both central and peripheral tissues of rats could indicate a coordinated up-regulation of the receptor in pro-oestrus. There was more specific expression of mPRβ in the brain versus the body, with high levels in the midbrain [109]. Other studies have confirmed such differences, such that mPRβ >mPRα protein expression in the rat brain, specifically the midbrain and hypothalamus [110,111]. In addition to this evidence of region-specificity, there are hormonal influences for expression of these mPRs. mPRα and mPRβ are increased during pro-oestrus, compared to diestrus, of rats in the mediobasal hypothalamus [112]. Expression of mPRβ is higher than mPRα in the midbrain, hippocampus, lateral and medial septum, and thalamus of ovariectomised, hormone-primed rats [110–112]. Indeed, there seems to be regulation of mPR expression in rats by oestradiol in these brain regions involved in reproductive behaviours [109]. Recent studies have begun characterizing the expression of mPRs among mice. Among pro-oestrous mice, there was abundant expression of both mPRα and mPRβ in brain (including the midbrain), but lower expression in peripheral tissues assessed compared to that observed in the brain [113]. Thus, these data suggest that the midbrain may be one brain regions containing mPRs as a target of progestogens among rats and mice.
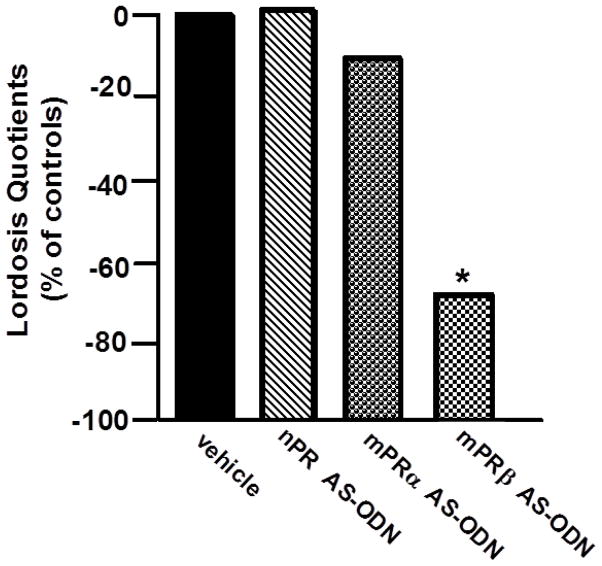
The functional effects of mPRα and mPRβ among rats and mice are of interest. Rapid change in lordosis of rodents is one of the best-studied in vivo models for novel, rapid actions of progestogens. As such, to investigate this question, infusions of AS-ODNs targeted against mPRα and/or mPRβ were infused intracerebroventricularly (ICV) or to the VTA of ovariectomised, hormone-primed rats and mice. Among rats, administration of mPRα AS-ODNs ICV reduced lordosis and expression of mPRα in the hypothalamus and VTA. Infusions to the VTA of AS-ODNs targeted against mPRα and/or mPRβ reduced respective expression of these receptor subtypes in the VTA. Moreover, mPRβ or mPRα +mPRβ, but not mPRα, AS-ODNs significantly reduced lordosis quotients, lordosis ratings, and proceptivity quotients, and significantly increased aggression/rejection behaviours towards males compared to control infusions. This pattern of reproductive behaviour was only observed among rats that were oestradiol and progesterone-primed, not those primed with oestradiol alone, suggesting the progestogen-dependency of these effects. These data support the role of mPRβ as a target of progestogens in the midbrain VTA for sexual receptivity of rats [109]. Among mice, comparisons were made for manipulations of mPRs α and β among those on a C57, C57×129 wildtypes, or nPR knockout background for reproductive responses following infusions of AS-ODNs ICV or to the VTA. Among mice, lordosis was reduced with mPRα, mPRβ, or mPRαβ AS-ODNs infused ICV [61]. When infused directly to the VTA, only mPRβ or mPRα+mPRβ AS-ODNs reduced lordosis. These effects were observed in C57 or C57×129 wildtypes, but not in nPRKO mice [113]. Together, these data support a functional role of mPRβ in the midbrain VTA as a likely target of progestogens for rapid facilitation of lordosis (See Figure 3).
Figure 3.
Intra-ventral tegmental area (VTA) infusions of nPR and mPRα AS-ODN do not influence lordosis, but lordosis is reduced by infusions of mPRβ AS-ODN to the VTA * indicates significant difference from control infusions
Conclusion
The midbrain VTA is a well-described part of the dopaminergic reward pathway as well as important for naturally-motivating behaviours, such as mating (Frye, 2009). Studies discussed herein describe the role of novel factor involved in production of (e.g. PXR), and targets for (e.g. mPRs), the neurosteroid, 3α,5α-THP, to have actions in the VTA for reproductive behaviours and other social challenges. Progestogens, such as 3α,5α-THP, are necessary for the full complement of behaviours important for successful mating (i.e. social contacts, pacing of such contacts, lordosis, suppression of anxiety and aggression in favor of pro-affiliative behaviours). Moreover, engaging in paced mating enhances 3α,5α-THP in the brain rapidly and without reliance on peripheral sources of prohormones, such as progesterone. If part of the progestogen metabolic or biosynthetic pathway is blocked in the midbrain, including PXR, we attenuate 3α,5α-THP facilitated lordosis and mating induced biosynthesis of 3α,5α-THP. We have recently observed that knocking down PXR in the VTA reduces expression of brain derived neurotrophic factor in the hippocampus [59], substantiating further studies on plasticity in this circuitry for social challenges, such as mating. Neurosteroids, such as 3α,5α-THP, are endogenous neuromodulators, synthesized de novo in brain, that have actions at membrane targets, such as neurotransmitters as well as G-protein coupled receptors, such as mPRβ in the midbrain VTA. Together, these studies demonstrate that PXR is a novel target involved in production of 3α,5α-THP in the midbrain VTA, acting as a homeostatic regulator. Moreover, mPRs may be a novel target for progestogens’ actions in the VTA for lordosis. How these targets involve behavioural and neural plasticity is of continued interest.
Acknowledgments
This research was supported by funding from the National Science Foundation (IOS-0957148), National Institute of Mental Health (MH-06769801, RMH067698B), and the National Institute of General Medical Sciences of the National Institutes of Health (P20GM103395). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Assistance provided by members of the laboratory, past and present, and many collaborators on the studies described herein is greatly appreciated.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9:241–50. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Nicola AF, Labombarda F, Deniselle MC, Gonzalez SL, Garay L, Meyer M, Gargiulo G, Guennoun R, Schumacher M. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front Neuroendocrinol. 2009;30:173–87. doi: 10.1016/j.yfrne.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Frye CA. Steroids, reproductive endocrine function, and cognition. A review. Minerva Ginecol. 2009;61:563–85. [PubMed] [Google Scholar]
- 4.Frye CA. Steroids, reproductive endocrine function, and affect. A review. Minerva Ginecol. 2009;61:541–62. [PubMed] [Google Scholar]
- 5.Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009;34:S143–61. doi: 10.1016/j.psyneuen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBold JF, Malsbury CW. Facilitation of sexual receptivity by hypothalamic and midbrain implants of progesterone in female hamsters. Physiol Behav. 1989;46:655–60. doi: 10.1016/0031-9384(89)90347-8. [DOI] [PubMed] [Google Scholar]
- 7.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–13. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm Behav. 2008;54:347–54. doi: 10.1016/j.yhbeh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Frye CA. Novel substrates for, and sources of, progestogens for reproduction. J Neuroendocrinol. 2011;23:961–73. doi: 10.1111/j.1365-2826.2011.02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frye CA, Koonce CJ, Walf AA. Knocking down pregnane xenobiotic receptor in the midbrain reduces formation of allopregnanolone in the midbrain and hippocampus, and brain-derived neurotrophic factor levels in the hippocampus, among paced mated female rats. Frontiers Cell Neurosci. 2013 (in revision) [Google Scholar]
- 11.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–74. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–72. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- 13.Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–41. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunton PJ, Russell JA. Neuroendocrine control of maternal stress responses and fetal programming by stress in pregnancy. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1178–91. doi: 10.1016/j.pnpbp.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Llidó A, Mòdol L, Darbra S, Pallarès M. Interaction between neonatal allopregnanolone administration and early maternal separation: Effects on adolescent and adult behaviors in male rat. Horm Behav. 2013;63:577–85. doi: 10.1016/j.yhbeh.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Paris JJ, Brunton PJ, Russell JA, Walf AA, Frye CA. Inhibition of 5α-reductase activity in late pregnancy decreases gestational length and fecundity and impairs object memory and central progestogen milieu of juvenile rat offspring. J Neuroendocrinol. 2011;23:1079–90. doi: 10.1111/j.1365-2826.2011.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerberg B, Blaskey LG. Prenatal stress effects are partially ameliorated by prenatal administration of the neurosteroid allopregnanolone. Pharmacol Biochem Behav. 1998;59:819–27. doi: 10.1016/s0091-3057(97)00540-6. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–40. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Jensen EV, Jacobson HI, Walf AA, Frye CA. Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol Behav. 2010;99:151–62. doi: 10.1016/j.physbeh.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- 21.Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol. 2010;327:1–12. doi: 10.1016/j.mce.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22:10613–20. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78:1003–8. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- 24.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–92. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–56. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 27.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- 28.Cheng YJ, Karavolas HJ. Subcellular distribution and properties of progesterone (delta4-steroid) 5α-reductase in rat medial basal hypothalamus. J Biol Chem. 1975;250:7997–8003. [PubMed] [Google Scholar]
- 29.Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3β-hydroxysteroid dehydrogenase in the rat brain. J Neurochem. 1998;71:2231–8. doi: 10.1046/j.1471-4159.1998.71062231.x. [DOI] [PubMed] [Google Scholar]
- 30.Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001;40:226–233. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- 31.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–22. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–8. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- 33.Frye CA, Paris JJ, Walf AA, Rusconi JC. Effects and mechanisms of 3α, 5α,-THP on emotion, motivation, and reward functions involving pregnane xenobiotic receptor. Front Neurosci. 2011;5:136. doi: 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frye CA, Paris JJ, Rhodes ME. Exploratory, anti-anxiety, social, and sexual behaviors of rats in behavioral estrus is attenuated with inhibition of 3α,5α-THP formation in the midbrain ventral tegmental area. Behav Brain Res. 2008;193:269–76. doi: 10.1016/j.bbr.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frye CA, Paris JJ. Progesterone turnover to its 5α-reduced metabolites in the ventral tegmental area of the midbrain is essential for initiating social and affective behavior and progesterone metabolism in female rats. J Endocrinol Invest. 2011;34:188–99. doi: 10.3275/7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petralia SM, Jahagirdar V, Frye CA. Inhibiting biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioral oestrus. J Neuroendocrinol. 2005;17:545–52. doi: 10.1111/j.1365-2826.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- 37.Koonce CJ, Frye CA. Progesterone facilitates reproductive behaviors among c57, but not 5α-reductase type 1 mutant, mice. Pharmacol Biochem Behav. 2013 doi: 10.1016/j.bbr.2013.07.025. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walf AA, Paris JJ, Llaneza DC, Frye CA. I. Levels of 5α-reduced progesterone metabolite in the midbrain account for variability in reproductive behavior of middle-aged female rats. Brain Res. 2011;1379:137–48. doi: 10.1016/j.brainres.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frye CA, Vongher JM. 3α,5α-THP in the midbrain ventral tegmental area of rats and hamsters is increased in exogenous hormonal states associated with estrous cyclicity and sexual receptivity. J Endocrinol Invest. 1999;22:455–64. doi: 10.1007/BF03343590. [DOI] [PubMed] [Google Scholar]
- 40.Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90:375–85. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83:336–47. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dussault I, Forman BM. The nuclear receptor PXR: a master regulator of “homeland” defense. Crit Rev Eukaryot Gene Expr. 2002;12:53–64. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.30. [DOI] [PubMed] [Google Scholar]
- 43.Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 44.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Idle JR, Gonzalez FJ. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Xie W, Krasowski MD. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics. 2008;9:1695–709. doi: 10.2217/14622416.9.11.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–9. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 48.Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine asPXR activators. Toxicol Appl Pharmacol. 2004;199:251–65. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Marini S, Nannelli A, Sodini D, Dragoni S, Valoti M, Longo V, Gervasi PG. Expression, microsomal and mitochondrial activities of cytochrome P450 enzymes in brain regions from control and phenobarbital-treated rabbits. Life Sci. 2007;80:910–7. doi: 10.1016/j.lfs.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Ott M, Fricker G, Bauer B. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther. 2009;329:141–9. doi: 10.1124/jpet.108.149690. [DOI] [PubMed] [Google Scholar]
- 51.Frye CA, Koonce CJ, Walf AA, Rusconi JC. Motivated behaviors and levels of 3α,5α-THP in the midbrain are attenuated by knocking down expression of pregnane xenobiotic receptor in the midbrain ventral tegmental area of proestrous rats. J Med Sex. 2013;10:1692–706. doi: 10.1111/jsm.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frye CA, Rhodes ME. Infusions of 5α-pregnan-3α-ol-20-one (3α,5α-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviors and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 53.Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Behav Brain Res. 2008;187:88–99. doi: 10.1016/j.bbr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frye CA, Koonce CJ, Walf AA. Pregnane xenobiotic receptor knock down with antisense oligonucleotides abrogates effects of 3α,5α-THP replacement to reinstate lordosis of ovariectomized, estradiol-primed female rats. Psychopharmacology. 2013 (in revision) [Google Scholar]
- 55.Theofilopoulos S, Wang Y, Kitambi SS, Sacchetti P, Sousa KM, Bodin K, Kirk J, Saltó C, Gustafsson M, Toledo EM, Karu K, Gustafsson JÅ, Steffensen KR, Ernfors P, Sjövall J, Griffiths WJ, Arenas E. Brain endogenous liver X receptor ligands selectively promote midbrain neurogenesis. Nat Chem Biol. 2013;9:126–33. doi: 10.1038/nchembio.1156. [DOI] [PubMed] [Google Scholar]
- 56.Tan XJ, Dai YB, Wu WF, Warner M, Gustafsson JÅ. Anxiety in liver X receptor β knockout female mice with loss of glutamic acid decarboxylase in ventromedial prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109:7493–8. doi: 10.1073/pnas.1205189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cermenati G, Giatti S, Cavaletti G, Bianchi R, Maschi O, Pesaresi M, Abbiati F, Volonterio A, Saez E, Caruso D, Melcangi RC, Mitro N. Activation of the liver X receptor increases neuroactive steroid levels and protects from diabetes-induced peripheral neuropathy. J Neurosci. 2010;30:11896–901. doi: 10.1523/JNEUROSCI.1898-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cermenati G, Abbiati F, Cermenati S, Brioschi E, Volonterio A, Cavaletti G, Saez E, De Fabiani E, Crestani M, Garcia-Segura LM, Melcangi RC, Caruso D, Mitro N. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J Lipid Res. 2012;53:300–10. doi: 10.1194/jlr.M021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci U S A. 2006;103:13807–12. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitro N, Cermenati G, Giatti S, Abbiati F, Pesaresi M, Calabrese D, Garcia-Segura LM, Caruso D, Melcangi RC. LXR and TSPO as new therapeutic targets to increase the levels of neuroactive steroids in the central nervous system of diabetic animals. Neurochem Int. 2012;60:616–21. doi: 10.1016/j.neuint.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 61.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–23. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 62.Repa JJ, Li H, Frank-Cannon TC, Valasek MA, Turley SD, Tansey MG, Dietschy JM. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J Neurosci. 2007;27:14470–80. doi: 10.1523/JNEUROSCI.4823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–20. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith CD, Wekstein DR, Markesbery WR, Frye CA. 3α,5α-THP: a potential plasma neurosteroid biomarker in Alzheimer’s disease and perhaps non-Alzheimer’s dementia. Psychopharmacology. 2006;186:481–5. doi: 10.1007/s00213-005-0186-1. [DOI] [PubMed] [Google Scholar]
- 65.O’Malley BW, Sherman MR, Toft DO. Progesterone “receptors” in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci U S A. 1970;67:501–8. doi: 10.1073/pnas.67.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann N Y Acad Sci. 2003;1007:238–50. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 67.Frye CA. Inhibition of 5α-reductase enzyme or GABA(A) receptors in the VMH and the VTA attenuates progesterone-induced sexual behavior in rats and hamsters. J Endocrinol Invest. 2001;24:399–407. [PubMed] [Google Scholar]
- 68.Frye CA, Murphy RE, Platek SM. Anti-sense oligonucleotides, for progestin receptors in the VMH and glutamic acid decarboxylase in the VTA, attenuate progesterone-induced lordosis in hamsters and rats. Behav Brain Res. 2000;115:55–64. doi: 10.1016/s0166-4328(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 69.Frye CA, Vongher JM. GABA(A), D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 70.Frye CA, Vongher JM. Progesterone and 3α,5α-THP enhance sexual receptivity in mice. Behav Neurosci. 2001;115:1118–28. [PubMed] [Google Scholar]
- 71.Frye CA, Vongher JM. Progestins’ rapid facilitation of lordosis when applied to the ventral tegmentum corresponds to efficacy at enhancing GABA(A)receptor activity. J Neuroendocrinol. 1999;11:829–37. doi: 10.1046/j.1365-2826.1999.00367.x. [DOI] [PubMed] [Google Scholar]
- 72.Frye CA, DeBold JF. P-3-BSA, but not P-11-BSA, implants in the VTA rapidly facilitate receptivity in hamsters after progesterone priming to the VMH. Behav Brain Res. 1993;53:167–75. doi: 10.1016/s0166-4328(05)80276-1. [DOI] [PubMed] [Google Scholar]
- 73.Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm Behav. 1996;30:682–91. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- 74.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 75.Gee KW, McCauley LD, Lan NC. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol. 1995;9:207–27. [PubMed] [Google Scholar]
- 76.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 77.McCauley LD, Liu V, Chen JS, Hawkinson JE, Lan NC, Gee KW. Selective actions of certain neuroactive pregnanediols at the gamma-aminobutyric acid type A receptor complex in rat brain. Mol Pharmacol. 1995;47:354–62. [PubMed] [Google Scholar]
- 78.Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–5. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- 79.Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- 80.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology. 2009;34:S48–58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Frye CA, Paris JJ. Infusions of bicuculline to the ventral tegmental area attenuates sexual, exploratory, and anti-anxiety behavior of proestrous rats. Pharmacol Biochem Behav. 2009;93:474–81. doi: 10.1016/j.pbb.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frye CA, Walf AA, Sumida K. Progestins’ actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors. Pharmacol Biochem Behav. 2004;78:405–18. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 84.Sumida K, Walf AA, Frye CA. Progestin-facilitated lordosis of hamsters may involve dopamine-like type 1 receptors in the ventral tegmental area. Behav Brain Res. 2005;161:1–7. doi: 10.1016/j.bbr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- 86.Churchill L, Dilts RP, Kalivas PW. Autoradiographic localization of gamma-aminobutyric acid A receptors within the ventral tegmental area. Neurochem Res. 1992;17:101–6. doi: 10.1007/BF00966870. [DOI] [PubMed] [Google Scholar]
- 87.Willick ML, Kokkinidis L. The effects of ventral tegmental administration of GABAA, GABAB and NMDA receptor agonists on medial forebrain bundle self-stimulation. Behav Brain Res. 1995;70:31–6. doi: 10.1016/0166-4328(94)00181-e. [DOI] [PubMed] [Google Scholar]
- 88.Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–88. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frye CA, Walf AA, Petralia SM. In the ventral tegmental area, progestins have actions at D1 receptors for lordosis of hamsters and rats that involve GABA A receptors. Horm Behav. 2006;50:332–7. doi: 10.1016/j.yhbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Park-Chung M, Wu FS, Farb DH. 3α-Hydroxy-5α-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons. Mol Pharmacol. 1994;46:146–50. [PubMed] [Google Scholar]
- 91.Petralia SM, DeBold JF, Frye CA. MK-801 infusions to the ventral tegmental area and ventromedial hypothalamus produce opposite effects on lordosis of hormone-primed rats. Pharmacol Biochem Behav. 2007;86:377–85. doi: 10.1016/j.pbb.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith JL, Kupchak BR, Garitaonandia I, Hoang LK, Maina AS, Regalla LM, Lyons TJ. Heterologous expression of human mPRa, mPRb and mPRg in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–1173. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 95.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 96.Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller JL. The role of Xenopus membrane progesterone receptor beta in mediating the effect of progesterone on oocyte maturation. Mol Endocrinol. 2007;21:664–73. doi: 10.1210/me.2006-0256. [DOI] [PubMed] [Google Scholar]
- 97.Tokumoto M, Nagahama Y, Thomas P, Tokumoto T. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen Comp Endocrinol. 2006;145:101–108. doi: 10.1016/j.ygcen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Tubbs C, Thomas P. Progestin signaling through an olfactory G protein and membrane progestin receptor-α in Atlantic croaker sperm: potential role in induction of sperm hypermotility. Endocrinology. 2009;150:473–484. doi: 10.1210/en.2008-0512. [DOI] [PubMed] [Google Scholar]
- 99.Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors α and β in transfected cells. J Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- 100.Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez SL, De Nicola AF, Schumacher M, Guennoun R. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience. 2010;166:94–106. doi: 10.1016/j.neuroscience.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas P, Giudice LC. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 103.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–116. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 104.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 105.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147:4151–4159. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 106.Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- 107.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on GnRH release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nutu M, Weijdegård B, Thomas P, Thurin-Kjellberg A, Billig H, Larsson DG. Distribution and hormonal regulation of membrane progesterone receptors beta and gamma in ciliated epithelial cells of mouse and human fallopian tubes. Reprod Biol Endocrinol. 2009;7:89–102. doi: 10.1186/1477-7827-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frye CA, Walf AA, Kohtz AS, Zhu Y. Membrane progestin receptors in the midbrain ventral tegmental area are required for progesterone-facilitated lordosis of rats. Horm Behav. 2013 doi: 10.1016/j.yhbeh.2013.05.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2011a;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zuloaga DG, Yahn SL, Pang Y, Quihuis AM, Oyola MG, Reyna A, Thomas P, Handa RJ, Mani SK. Distribution and oestrogen regulation of membrane progesterone receptor-β in the female rat brain. Endocrinology. 2012;153:4432–4443. doi: 10.1210/en.2012-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu B, Arbogast LA. Gene expression profiles of intracellular and membrane progesterone receptor isoforms in the mediobasal hypothalamus during pro-oestrus. J Neuroendocrinol. 2009;21:993–1000. doi: 10.1111/j.1365-2826.2009.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frye CA, Walf AA, Kohtz AS, Zhu Y. Membrane progestin receptors in the midbrain ventral tegmental area are required for progesterone-facilitated lordosis of mice. Physiol Behav. 2013 doi: 10.1016/j.yhbeh.2013.05.012. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]