Abstract
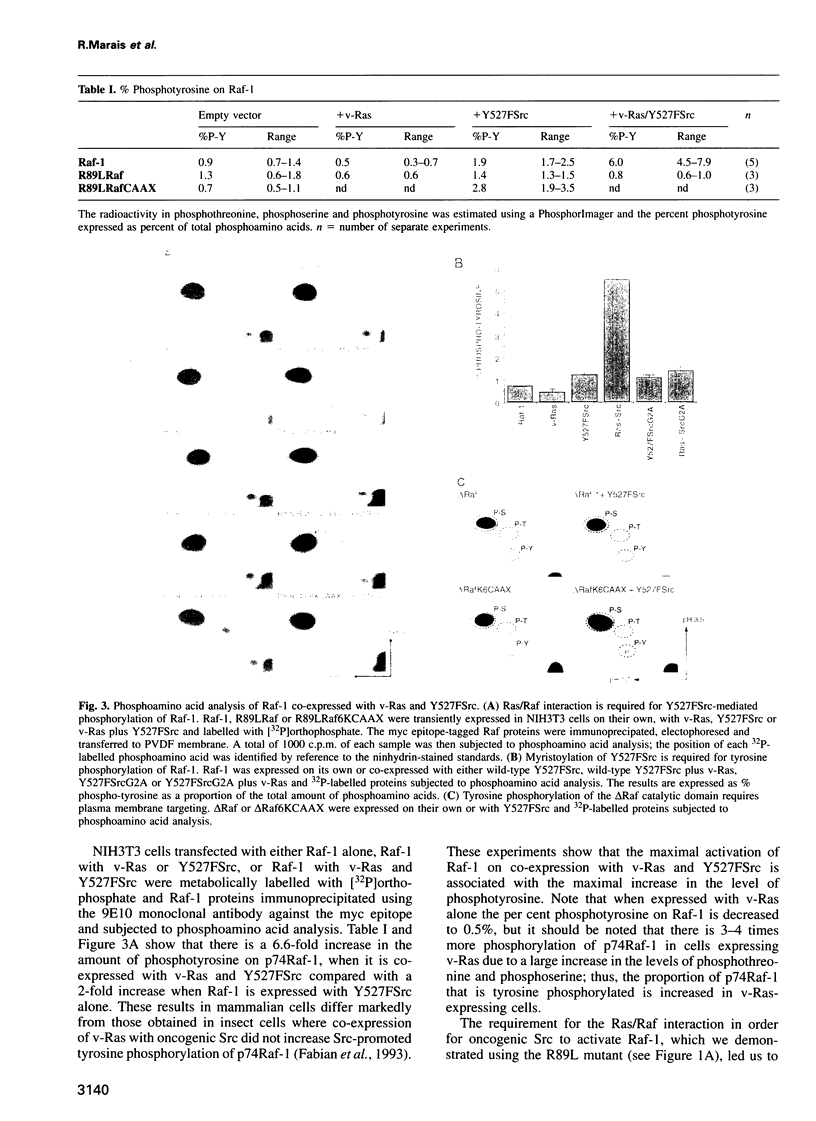
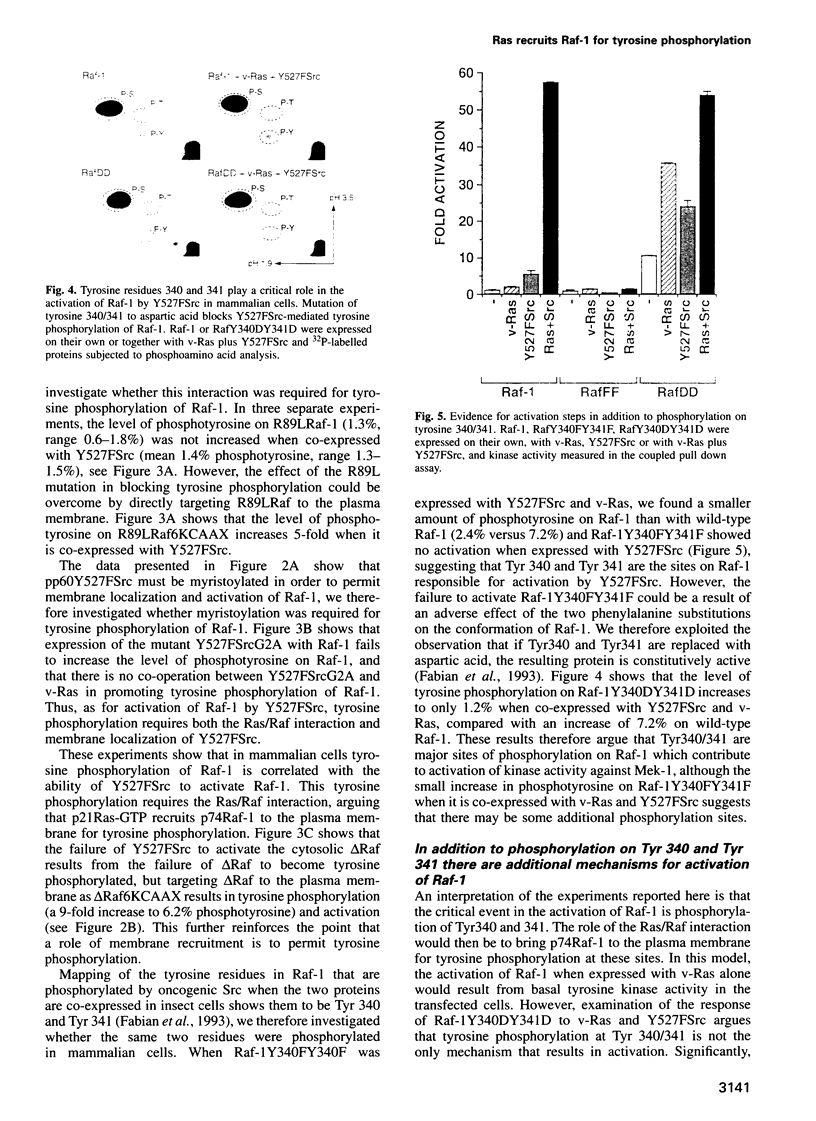
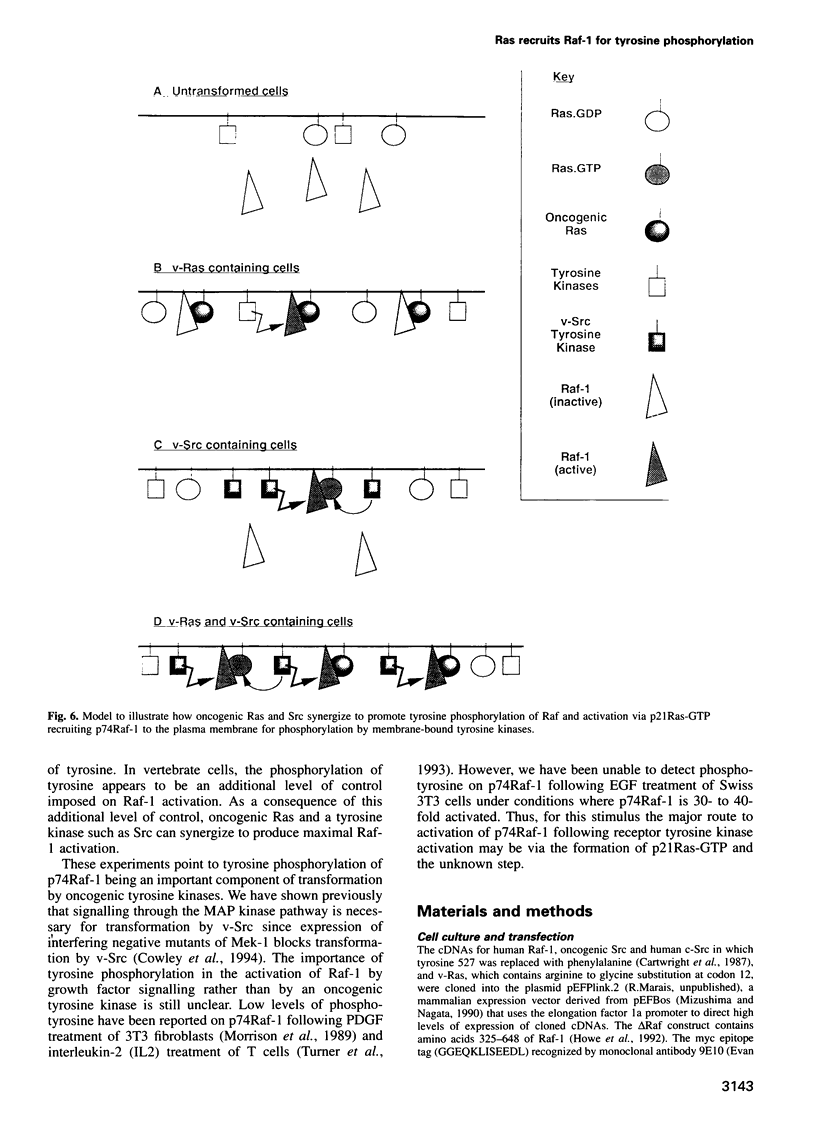
A central feature of signal transduction downstream of both receptor and oncogenic tyrosine kinases is the Ras-dependent activation of a protein kinase cascade consisting of Raf-1, Mek (MAP kinase kinase) and ERKs (MAP kinases). To study the role of tyrosine kinase activity in the activation of Raf-1, we have examined the properties of p74Raf-1 and oncogenic Src that are necessary for activation of p74Raf-1. We show that in mammalian cells activation of p74Raf-1 by oncogenic Src requires pp60Src to be myristoylated and the ability of p74Raf-1 to interact with p21Ras-GTP. The Ras/Raf interaction is required for p21Ras-GTP to bring p74Raf-1 to the plasma membrane for phosphorylation at tyrosine 340 or 341, probably by membrane-bound pp60Src. When oncogenic Src is expressed with Raf-1, p74Raf-1 is activated 5-fold; however, when co-expressed with oncogenic Ras and Src, Raf-1 is activated 25-fold and this is associated with a further 3-fold increase in tyrosine phosphorylation. Thus, p21Ras-GTP is the limiting component in bringing p74Raf-1 to the plasma membrane for tyrosine phosphorylation. Using mutants of Raf-1 at Tyr340/341, we show that in addition to tyrosine phosphorylation at these sites, there is an additional activation step resulting from p21Ras-GTP recruiting p74Raf-1 to the plasma membrane. Thus, the role of Ras in Raf-1 activation is to bring p74Raf-1 to the plasma membrane for at least two different activation steps.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D. R., Saito Y., Campbell D. G., Cohen P., Sithanandam G., Rapp U., Ashworth A., Marshall C. J., Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994 Apr 1;13(7):1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987 Apr 10;49(1):83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P., Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994 Jun 17;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian J. R., Daar I. O., Morrison D. K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993 Nov;13(11):7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian J. R., Vojtek A. B., Cooper J. A., Morrison D. K. A single amino acid change in Raf-1 inhibits Ras binding and alters Raf-1 function. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5982–5986. doi: 10.1073/pnas.91.13.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl W. J., Muslin A. J., Kikuchi A., Martin J. A., MacNicol A. M., Gross R. W., Williams L. T. Activation of Raf-1 by 14-3-3 proteins. Nature. 1994 Oct 13;371(6498):612–614. doi: 10.1038/371612a0. [DOI] [PubMed] [Google Scholar]
- Freed E., Symons M., Macdonald S. G., McCormick F., Ruggieri R. Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science. 1994 Sep 16;265(5179):1713–1716. doi: 10.1126/science.8085158. [DOI] [PubMed] [Google Scholar]
- Fu H., Xia K., Pallas D. C., Cui C., Conroy K., Narsimhan R. P., Mamon H., Collier R. J., Roberts T. M. Interaction of the protein kinase Raf-1 with 14-3-3 proteins. Science. 1994 Oct 7;266(5182):126–129. doi: 10.1126/science.7939632. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S., Scolnick E. M., Dixon R. A., Vogel U. S. Modulation of guanine nucleotides bound to Ras in NIH3T3 cells by oncogenes, growth factors, and the GTPase activating protein (GAP). J Biol Chem. 1990 Nov 25;265(33):20437–20442. [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Han M., Golden A., Han Y., Sternberg P. W. C. elegans lin-45 raf gene participates in let-60 ras-stimulated vulval differentiation. Nature. 1993 May 13;363(6425):133–140. doi: 10.1038/363133a0. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Haystead C. M., Hu C., Lin T. A., Lawrence J. C., Jr Phosphorylation of PHAS-I by mitogen-activated protein (MAP) kinase. Identification of a site phosphorylated by MAP kinase in vitro and in response to insulin in rat adipocytes. J Biol Chem. 1994 Sep 16;269(37):23185–23191. [PubMed] [Google Scholar]
- Heidecker G., Kölch W., Morrison D. K., Rapp U. R. The role of Raf-1 phosphorylation in signal transduction. Adv Cancer Res. 1992;58:53–73. doi: 10.1016/s0065-230x(08)60290-0. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Irie K., Gotoh Y., Yashar B. M., Errede B., Nishida E., Matsumoto K. Stimulatory effects of yeast and mammalian 14-3-3 proteins on the Raf protein kinase. Science. 1994 Sep 16;265(5179):1716–1719. doi: 10.1126/science.8085159. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Ernst W. H., Pingoud V., Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993 Dec 15;12(13):5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P. Determination of phosphoamino acid composition by acid hydrolysis of protein blotted to Immobilon. Methods Enzymol. 1991;201:21–27. doi: 10.1016/0076-6879(91)01005-m. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Williams L. T. The post-translational modification of ras p21 is important for Raf-1 activation. J Biol Chem. 1994 Aug 5;269(31):20054–20059. [PubMed] [Google Scholar]
- Leevers S. J., Paterson H. F., Marshall C. J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994 Jun 2;369(6479):411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Mark G. E., MacIntyre R. J., Digan M. E., Ambrosio L., Perrimon N. Drosophila melanogaster homologs of the raf oncogene. Mol Cell Biol. 1987 Jun;7(6):2134–2140. doi: 10.1128/mcb.7.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994 Oct 27;371(6500):762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M. L., Weber M. J., Bishop J. M., McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol. 1993 Oct;13(10):6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithanandam G., Kolch W., Duh F. M., Rapp U. R. Complete coding sequence of a human B-raf cDNA and detection of B-raf protein kinase with isozyme specific antibodies. Oncogene. 1990 Dec;5(12):1775–1780. [PubMed] [Google Scholar]
- Songyang Z., Carraway K. L., 3rd, Eck M. J., Harrison S. C., Feldman R. A., Mohammadi M., Schlessinger J., Hubbard S. R., Smith D. P., Eng C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995 Feb 9;373(6514):536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Campbell D. G., Nakielny S., Hidaka H., Leevers S. J., Marshall C., Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992 Nov;11(11):3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D., Macdonald S. G., Cadwallader K., Symons M., Hancock J. F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994 Jun 3;264(5164):1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G., Fumagalli S., Koegl M., Courtneidge S. A., Draetta G. Csk inhibition of c-Src activity requires both the SH2 and SH3 domains of Src. EMBO J. 1993 Jul;12(7):2625–2634. doi: 10.1002/j.1460-2075.1993.tb05923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S., Cohen P., Paterson H., Marshall C., Rapp U., Grand R. J. Specific association of activated MAP kinase kinase kinase (Raf) with the plasma membranes of ras-transformed retinal cells. Oncogene. 1993 Nov;8(11):3175–3181. [PubMed] [Google Scholar]
- Turner B. C., Tonks N. K., Rapp U. R., Reed J. C. Interleukin 2 regulates Raf-1 kinase activity through a tyrosine phosphorylation-dependent mechanism in a T-cell line. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5544–5548. doi: 10.1073/pnas.90.12.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Warne P. H., Viciana P. R., Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993 Jul 22;364(6435):352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- Wartmann M., Davis R. J. The native structure of the activated Raf protein kinase is a membrane-bound multi-subunit complex. J Biol Chem. 1994 Mar 4;269(9):6695–6701. [PubMed] [Google Scholar]
- Williams N. G., Roberts T. M., Li P. Both p21ras and pp60v-src are required, but neither alone is sufficient, to activate the Raf-1 kinase. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2922–2926. doi: 10.1073/pnas.89.7.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- Zheng C. F., Guan K. L. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994 Mar 1;13(5):1123–1131. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]