SUMMARY
The peripheral terminals of primary nociceptive neurons play an essential role in pain detection mediated by membrane receptors like TRPV1, a molecular sensor of heat and capsaicin. However, the contribution of central terminal TRPV1 in the dorsal horn to chronic pain has not been investigated directly. Combining primary sensory neuron-specific GCaMP3 imaging with a trigeminal neuropathic pain model, we detected robust neuronal hyperactivity in injured and uninjured nerves in the skin, soma in trigeminal ganglion, and central terminals in the spinal trigeminal nucleus. Extensive TRPV1 hyperactivity was observed in central terminals innervating all dorsal horn laminae. The central terminal TRPV1 sensitization was maintained by descending serotonergic (5-HT) input from the brainstem. Central blockade of TRPV1 or 5-HT/5-HT3A receptors attenuated central terminal sensitization, excitatory primary afferent inputs, and mechanical hyperalgesia in the territories of injured and uninjured nerves. Our results reveal new central mechanisms facilitating central terminal sensitization underlying chronic pain.
INTRODUCTION
Small-diameter nociceptive neurons whose cell bodies reside in dorsal root ganglia (DRG) and trigeminal ganglia (TG) play essential roles in pain signal detection, transmission, and modulation (Basbaum et al., 2009). The peripheral axons of these pseudo-unipolar neurons innervate tissues such as skin, muscle, and visceral organs to detect painful stimuli, while their central axons transmit these signals to secondary-order neurons in the spinal cord dorsal horn (in the case of DRG neurons) and the analogous trigeminal subnucleus caudalis (Vc, in the case of TG neurons). Peripheral tissue and nerve injury can lead to hyperalgesia, a state in which painful stimuli are perceived as more painful than normal. One major contributor to hyperalgesia is augmented sensitivity of primary nociceptive neurons, a phenomenon called peripheral sensitization (Treede et al., 1992). Although sensitization of peripheral terminals can be readily detected by sensory nerve recordings in the skin-nerve preparation, technical barriers have limited investigation of the role of the central terminals of primary sensory neurons in sensitization and hyperalgesia.
TRPV1, a non-selective cation channel, is a key molecular component of pain detection and modulation (Caterina et al., 2000; Caterina et al., 1997). Although TRPV1 protein is present throughout many nociceptive neurons (i.e. along peripheral and central axons) (Caterina et al., 2000; Guo et al., 1999), previous studies have been mainly focused on its function as a molecular sensor of noxious heat and capsaicin in the peripheral terminals. Hyperalgesia resulting from tissue injury or inflammation is often associated with sensitization of TRPV1 channel activity which can occur through several mechanisms such as phosphorylation, interaction with phospholipid PIP2, trafficking, and association with accessory proteins (Bhave et al., 2002; Lukacs et al., 2007; Zhang et al., 2005; Zhang et al., 2008). Recent studies have implicated TRPV1 in the modulation of sensory transmission from the central terminals of primary afferents to spinal dorsal horn neurons (Gregus et al., 2012; Sikand and Premkumar, 2007; Tognetto et al., 2001; Yang et al., 1998). However, an understanding of the mechanisms by which TRPV1 or other molecules contribute to central terminal sensitization events underlying persistent pain will require approaches that allow one to directly monitor presynaptic neuronal activity and its hyperactivity.
We combined primary sensory neuron-specific GCaMP3 imaging with a trigeminal neuropathic pain model and detected robust neuronal hypersensitivity in injured and uninjured nerves in the skin, soma in trigeminal ganglion, and central terminals in the spinal trigeminal nucleus. Strikingly, extensive TRPV1 hyperactivity was observed in central terminals innervating all dorsal horn laminae. The central terminal TRPV1 sensitization was maintained by upregulated descending serotonergic (5-HT) input from the rostral ventromedial medulla (RVM) in the brainstem. Central blockade of TRPV1 or 5-HT/5-HT3A receptor attenuated TRPV1-dependent central terminal sensitization, excitatory primary afferent inputs, and mechanical hyperalgesia in the territories of injured and uninjured nerves. Therefore, our results reveal a novel pain mechanism in which descending 5-HT from the RVM maintains central terminal sensitization by activation of presynaptic 5-HT3A receptors and subsequent facilitation of TRPV1 activity.
RESULTS
Generation and initial characterization of Pirt-GCaMP3 mice
To develop a better means of specifically monitoring primary sensory neuron activity, we generated a knock-in mouse line in which GCaMP3 expression is driven by the Pirt promoter. GCaMP3 is a genetically-encoded calcium indicator whose green fluorescent intensity is dramatically increased after binding to intracellular Ca2+ and which has been used to effectively visualize neuronal activity in several animal models (Tian et al., 2009). The Pirt promoter is a strong and selective pan-DRG and TG promoter which is expressed in almost all primary sensory neurons (Figure S1A), but which is not expressed in glia, in other peripheral tissues such as skin and muscle, or in the central nervous system (Kim et al., 2008). We compared Ca2+ imaging assays using conventional Fura2 dye loading of cultured dissociated wild-type DRG neurons and Pirt-GCaMP3 transgenic DRG neurons (Figure S2A). When treated with a high level of KCl (100 mM), both methods revealed activation of ~87% of total DRG neurons which is consistent with Pirt-GCaMP3 being present in almost all DRG neurons.
Developing a mouse trigeminal neuropathic pain model
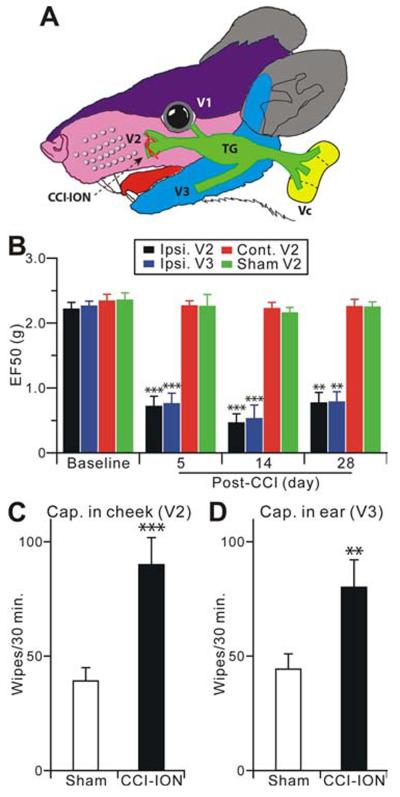
Due to its unique anatomical characteristics and clinical relevance, the trigeminal system has been used to study persistent or chronic pain. The trigeminal nerve originates from TG, which divides into three large branches: the ophthalmic (V1), maxillary (V2) and mandibular (V3), each of which innervate a distinct orofacial region (Figure 1A). To study peripheral and central mechanisms of neuropathic pain using Pirt-GCaMP3 mice, we adapted our rat model of chronic constriction injury of the infraorbital nerve (CCI-ION), the major branch of V2 (Klein et al., 1988), to mice (Figure 1A) (Okubo et al., 2013; Wei et al., 2008). For assessing mechanical hyperalgesia, von Frey filaments were applied to the whisker pad (injured V2 territory) or lower jaw (uninjured V3 territory) area. An active withdrawal of the head from the probing filament was defined as a nocifensive response (Supplemental Movie 1,2). The mechanical pain threshold was quantified as EF50, the mechanical force that produced a 50% response frequency. Compared with the sham group and baseline, CCI-treated animals exhibited significant decreases in EF50s (i.e. lower mechanical pain threshold) of both ipsilateral V2 and V3 regions measured at 5, 14, and 28 days after surgery (Figure 1B). There is no significant change in the mechanical pain threshold of the contralateral V2 after CCI-ION (Figure 1B). Therefore, CCI-ION mice showed long-lasting mechanical hyperalgesia in territories innervated by injured and uninjured nerves. In addition, capsaicin evoked nocifensive responses were also significantly increased in both ipsilateral V2 and V3 regions 14 days after CCI-ION compared to the sham group (Figure 1C,D). These results indicate that the mouse CCI-ION model exhibits primary hyperalgesia in the V2 skin, which is innervated by the injured ION, as well as a spread of hyperalgesia to the V3 area innervated by the uninjured V3 nerve. Extraterritorial hyperalgesia, i.e. the hyperalgesia found within the V3 skin, is a prominent component of many clinical chronic pain conditions and is thought to be related to CNS mechanisms (Ringkamp and Meyer, 2005).
Figure 1.
Trigeminal CCI-ION in Pirt-GCaMP3 mice shows long-lasting hyperalgesia from territories innervated by injured and uninjured nerves. (A) The CCI-ION and the anatomy in orofacial and trigeminal system are shown. (B) Mechanical hyperalgesia was tested at the ipsilateral V2 whisker pad (injured), the V3 lower jaw (uninjured), and contralateral V2 regions at 5, 14 and 28 days after CCI-ION (n=8) or sham (n=6) in mice. The data are expressed as an EF50 value and are presented as mean ± s.e.m. (C,D) Cheek injection of capsaicin evokes nocifensive facial wiping by the forepaw. The number of face wipes were measured within 30 minutes of capsaicin (0.5 μg) injection into the cheek (V2, injured) and the anterior part of ear skin (V3, uninjured; n=10 for each group). Data are presented as mean ± s.e.m. **p<0.01; ***p<0.001 vs. baseline (B), or sham (C,D).
Terminals of uninjured nerves in the skin became hypersensitized
We next used the CCI-ION model, in combination with Pirt-GCaMP3 imaging, to determine whether uninjured primary afferents are involved in extraterritorial hyperalgesia. We primarily used capsaicin to probe neuronal hypersensitivity since the dosage of capsaicin application can be quantitatively controlled. The complete absence of Ca2+ signals evoked by capsaicin in Pirt-GCaMP3;TRPV1−/− mice confirmed that capsaicin could be used to specifically monitor TRPV1 activity (Figure S2B). Importantly, we also determined that CCI-ION did not change GCaMP3 protein levels in the TG of Pirt-GCaMP3 mice (Figure S1B,C). Since GCaMP3 was expressed in nearly all neurons in the DRG and TG of Pirt-GCaMP3 mice and its expression levels remained constant after nerve injury, Pirt-GCaMP3 mice provided a suitable tool to monitor plasticity of primary sensory neurons under neuropathic pain conditions.
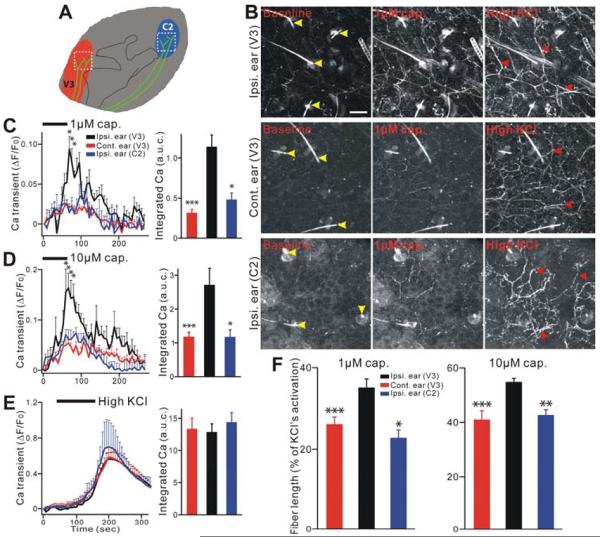
To examine whether neuronal activity of uninjured V3 nerves was affected by CCI-ION, we imaged peripheral nerve fiber and terminal activation in acutely isolated hairy ear skin explants from Pirt-GCaMP3 mice, 10–17 days after CCI-ION. Figure 2A shows a schematic diagram of a mouse ear and the location where images were taken in the V3 and C2 regions of ear skin. The anterior ear skin receptive field is innervated by the V3 nerve, similar to the lower jaw, but the former is better separated from the whisker pad. The posterior part of the ear skin is innervated by the cervical C2 DRG fibers (Figure 2A) (Neubert et al., 2005). To confirm the V3 innervation of the external ear, the retrograde nerve tracer DiI was injected into the skin over the anterior upper part of the external ear. DiI-labeled neurons were located in the V3 but not V1 or V2 division of the TG (Figure S3A). In contrast, DiI injection into the whisker pad (V2) only labeled neurons in V2 but not V3 division of the TG (Figure S3B).
Figure 2.
Ear skin nerve fibers in the secondary hyperalgesia region show peripheral hypersensitivity after CCI-ION in Pirt-GCaMP3 mice. (A) Schematic diagram of a mouse ear. Red or blue color indicates where V3 nerve fibers (green) or C2 nerve fibers are innervated into ear skin, respectively. White square dash lines indicate where the image was taken. (B) Representative GCaMP3 imaging of ear skin explants. Ear skin after CCI-ION in Pirt-GCaMP3 mice were activated by capsaicin (1 μM) or high KCl (100 mM). Upper rows show ipsilateral (on the CCI-ION side) V3 region. Middle rows show contralateral (uninjured side) V3 region. Lower rows show ipsilateral C2 region. Yellow arrow heads indicate hairs and hair follicles which are autofluoresecent. Red arrow heads indicate activated fibers and endings. Ipsi., ipsilateral; cont., contralateral. Scale bar: 50 μm. (C–E) In left panels time course of the amplitude of the Ca2+ transient that was evoked by capsaicin (C,1 μM; D, 10 μM) or KCl (E, 100 mM) application in ear skin. In right panels Ca2+ transient amounts with area under curve (a.u.c.) that was evoked by capsaicin (C,1 μM; D, 10 μM) or KCl (E, 100 mM) in ear skin. All population data for Ca2+ transient are expressed as the percentage of baseline Ca2+ transient (ΔF/F0) and are presented as mean ± s.e.m. Black bars indicate when stimuli were applied. (F) Length of ear skin fibers and endings after activation by capsaicin (1 μM and 10 μM), which was normalized to the length activated by KCl. Significant increases were found in ipsilateral ear (V3) as compared to contralateral ear (V3) or ipsilateral ear (C2). *p<0.05; **p<0.01; ***p<0.001.
The basal GCaMP3 fluorescence level is very low in nerve fibers in the ear skin without any stimulation. Ear explants were perfused with capsaicin and washed, and then treated again with a high level of KCl (100 mM). Interestingly, capsaicin application evoked robust and stronger Ca2+ responses in multiple nerve fibers of the anterior part of ipsilateral ear skin (V3) following CCI-ION when compared to Ca2+ responses in the contralateral ear skin (V3) and the posterior part of the ipsilateral ear skin (C2) (Figure 2B; Supplemental Movie 3,4). The capsaicin-induced Ca2+ response was dose-dependent and reversible (Figure 2B–D). At 1 μM capsaicin application, the peak amplitude of the absolute Ca2+ transient (ΔF/F0) in ipsilateral V3 (0.09±0.03) was significantly greater than that of contralateral V3 (0.02±0.00; n=10 explants per group) or that of ipsilateral C2 (0.01±0.01; n=3, P<0.05). We also performed area under the curve (AUC) measurements of the integrated Ca2+ elevation evoked by applied stimuli (Figure 2C). AUC (1 μM capsaicin) in ipsilateral V3 (1.14±0.15) was significantly larger than that of contralateral V3 (0.32±0.04; n=10 for each group; P<0.001) or ipsilateral C2 (0.48±0.08; n=3, P<0.05). Similar results were obtained with 10 μM capsaicin application (Figure 2D).
A high level of KCl activated a much larger population of afferent fibers than capsaicin did, but neither the peak amplitude nor integrated area of the KCl-evoked Ca2+ transient differ between the three groups (ipsilateral V3, contralateral V3, and ipsilateral C2) (Figure 2E). This is consistent with the finding that GCaMP3 levels are the same between ipsilateral and contralateral TG after CCI-ION (Figure S1B,C).
Besides comparing Ca2+ signal levels, we also examined changes in the cumulative length of nerve fibers activated by capsaicin after CCI-ION. These measurements were normalized to the total length of fibers activated by high KCl and expressed as a percentage of activated fiber length (Figure 2F). Following capsaicin application, the cumulative activated fiber length in ipsilateral V3 was significantly longer than that in contralateral V3 (n=10 per group, P<0.001) or ipsilateral C2 (n=3, P<0.05; Figure 2F). Together these data clearly indicate that hyperactivity occurs in the nerve fibers in the skin innervated by a neighboring uninjured nerve branche after CCI-ION.
Robust hyperactivity in multiple cell types in trigeminal ganglion after CCI-ION
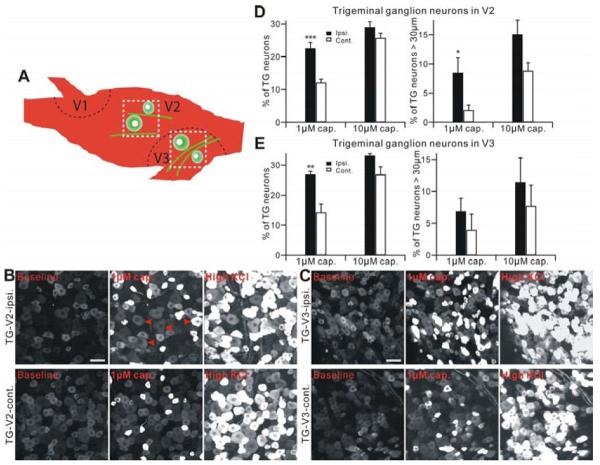
To evaluate neuronal activity in TG somata, we next imaged Ca2+ response in acutely isolated TG explants from Pirt-GCaMP3 mice. An advantage of visualizing TG explants is that neuronal cell bodies in TG retain their somatotopic organization and segregation (Figure 3A). The percentage of neurons that responded to 1 μM capsaicin was significantly increased in CCI-ION injured ipsilateral V2 (22.55±1.84%; normalized by 100 mM KCl activation) compared with the uninjured contralateral V2 TG (12.12±1.07%, P<0.001, n=12 explants) (Figure 3B,D). However, at 10 μM capsaicin there was no significant difference between the injured and uninjured V2 TG (Figure 3D; Supplemental Movie 5,6). Similar to the V2 region, the percentage of neurons in the CCI-ION ipsilateral V3 (26.91±1.05%) responding to 1 μM capsaicin was significantly increased compared to contralateral V3 TG (14.30±2.72%, n=4, P<0.01), but at 10 μM capsaicin no significant difference was observed between ipsilateral and contralateral V3 TG following CCI-ION (Figure 3C, E). Sham operated animals did not show any difference between ipsilalateral and contralateral V2 and V3 of TG (n=3, P>0.10, not shown). Interestingly, more of the capsaicin (1 μM) sensitive neurons in the V2 area of ipsilateral TG (8.46±2.66%, P<0.05) had large diameter cell bodies (>30 μm) when compared to the capsaicin sensitive neurons in the V2 of contralateral TG (2.1±0.83%, n=12) (Figure 3D). In contrast, no increased activation of large diameter cell bodies was found in V3 regions from ipsilateral compared with contralateral TG (Figure 3E).
Figure 3.
TG neurons show neuronal hyperactivity after CCI-ION in Pirt-GCaMP3 mice. (A) Schematic diagram of a mouse TG. Black dash lines indicate the border of three large divisions (V1, V2, V3) in TG (green cells). White square dash lines indicate where the image was taken. (B and C) Representative GCaMP3 imaging of TG explants. TG neurons of V2 (B) or V3 (C) division after CCI-ION in Pirt-GCaMP3 mice were activated by capsaicin (1 μM) or KCl (100 mM). Upper panels show ipsilateral V2 (B) or V3 (C) division of TG. Lower panels show contralateral V2 (B) or V3 (C) division of TG. Scale bar: 50 μm for both. Arrow heads in red indicate TG neurons which are over 30 μm diameter and activated by capsaicin. (D and E) Population data for V2 shown in (D) or for V3 shown in (E) are expressed as the percentage of KCl sensitive TG neurons and are presented as mean ± s.e.m. ipsi., ipsilateral; cont., contralateral. *p<0.05; **p<0.01; ***p<0.001.
TRPV1 and neuronal hyperactivity in central terminals innervating all dorsal horn lamina
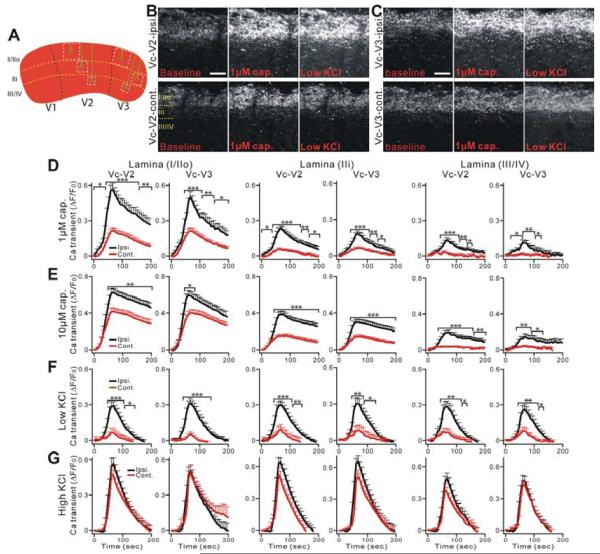
To examine the contribution of central fibers and terminals of primary sensory neurons to persistent pain, we next imaged the activation of central projecting axons and terminals of TG neurons in acute Vc slices from Pirt-GCaMP3 mice after CCI-ION. The segregation of trigeminal branches is also maintained in the central projection to Vc. V3, V2, and V1 axons terminate in the dorsomedial, middle, and ventrolateral regions of Vc, respectively (Nakajima et al., 2011; Rexed, 1952) (Figure 4A). This unique feature of the TG system allowed us to image specific Vc subdivisions in which sensory signals are received and transmitted from injured and uninjured peripheral axons. In addition, incoming C-fibers terminate in laminae I and II whereas A-fibers end predominately in lamina layers III and IV such that neuronal activities of different sensory fiber subtypes can be monitored accordingly (Mosconi et al., 2010). Figure 4A shows the laminar organization of V2 and V3 in Vc and where the images were analyzed. The Vc area was sliced using conventional vibratome slicing methods. The fluorescence intensity of a subset of sensory endings in superficial laminae was increased after capsaicin treatment (Figure 4B,C; Supplemental Movie 7,8). Besides capsaicin, we also used low KCl (20 mM) to examine overall neuronal excitability. Indeed, 20 mM KCl application evoked the activation of sensory endings in the superficial dorsal horn (C-fibers) as well as in deeper laminae (A-fibers) (Figure 4B, C). The GCaMP3-mediated Ca2+ signal intensity evoked by 1 μM capsaicin was significantly increased in the ipsilateral V2 and V3 (n=16, P<0.01; n=17, P<0.01, respectively) in the Vc slice from CCI-ION mice compared with that of the contralateral side (n=16, n=17 slices, respectively). The significant increase of activity in the ipsilateral V2 and V3 was found in all laminae (I/IIo, IIi, III/IV; Figure 4D). Similar results were obtained with 10 μM capsaicin (Figure 4E). In addition, more activated nerve terminals were seen in the ipsilateral deeper laminae (those innervated by larger diameter A-fibers) of the injured side.
Figure 4.
Central nerve fibers and terminals of Vc in TG system show strong central terminal hypersensitivity after CCI-ION in Pirt-GCaMP3 mice. (A) Schematic diagram of a mouse Vc. Black dash lines indicate the border of three large divisions (V1, V2, V3) in Vc. White square dash lines indicate where the region of the nerve fibers and terminals (green) was selected and analyzed to measure Ca2+ transient at different lamina (yellow dash lines). (B and C) Representative GCaMP3 imaging of Vc slices. Central fibers and terminals in V2 (B) or V3 (C) division of Vc after CCI-ION in Pirt-GCaMP3 mice were activated by capsaicin (1 μM) or 20 mM KCl. Upper panels show ipsilateral V2 (B) or V3 (C) division of Vc. Lower panels show contralateral V2 (B) or V3 (C) division of Vc. Scale bar: 50 μm. (D–F) Time course of the amplitude of the Ca2+ transient that was evoked by capsaicin (1 μM, 10 μM) or KCl (20 mM) application at different lamina (lamina I/IIo; IIi; III/IV) of V2 and V3 of Vc. Capsaicin was bath applied from 0 to 60 sec during the time course. (G) Ca2+ transients induced by KCl (100 mM) show no significant differences in ipsilateral and contralateral V2 and V3 of Vc after CCI-ION. The Ca2+ transient (ΔF/F0) was normalized to the value imaged in baseline. Ipsi., ipsilateral; cont., contralateral. *p<0.05; **p<0.01; ***p<0.001.
Low KCl showed dramatically significant increases in activity in the CCI-ION ipsilateral V2 and V3 (n=16, P<0.01 for both) of the Vc in all lamina (Figure 4F) compared with the contralateral side (n=16 for both). Responsiveness to high KCl (100 mM) was not significantly different between ipsilateral and contralateral V2 and V3 (n=17, P>0.10; n=18, P>0.10, respectively) (Figure 4G) suggesting that GCaMP3 protein levels at the central terminals were unchanged after CCI-ION. These data demonstrate the development of extensive hypersensitivity to capsaicin and low KCl in the central terminals of both injured and neighboring uninjured neurons following CCI-ION.
Descending 5-HT from RVM is upregulated after nerve injury and essential for neuropathic pain
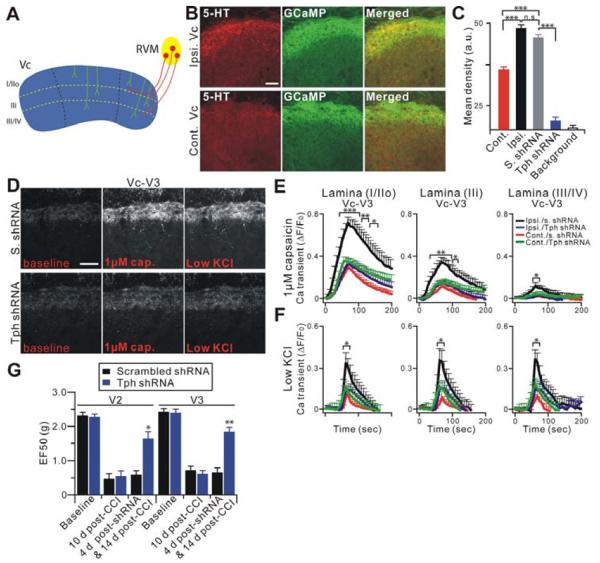
RVM in the brain stem is an important component of descending modulatory systems that project to the spinal dorsal horn and Vc, and is involved in facilitatory and inhibitory modulation of nociceptive inputs (Mason, 2001; Ren and Dubner, 2002). We and others have shown that 5-HT-dependent descending facilitation from the RVM is involved in persistent hyperalgesia after injury (Okubo et al., 2013; Suzuki et al., 2004; Wei et al., 2010). The robust hypersensitivity in central terminals of uninjured primary sensory neurons throughout multiple laminae of the V3 division of Vc after CCI-ION suggests a possible CNS mechanism underlying the sensitization of these central terminals. Here we investigated by immunostaining whether nerve injury causes any change in descending 5-HT positive fibers in the Vc sections (Figure 5A). Indeed, 14 days after CCI-ION, there was a dramatic increase in 5-HT immunoreactivity in the ipsilateral Vc compared with the Vc from sham animals or the contralateral side (Figure 5B). Importantly, the descending 5-HT positive fibers were in close vicinity to the central terminals of primary sensory neurons stained with GCaMP3 (Figure S4A). This is consistent with a previous study showing that descending 5-HT positive fibers form axo-axonic contacts onto the central terminals of primary sensory neurons (LaMotte and de Lanerolle, 1983).
Figure 5.
Descending 5-HT from RVM is upregulated after CCI-ION and nerve injury induced central terminal sensitization in Vc is blocked by depletion of descending 5-HT in the RVM. (A) Schematic diagram of descending RVM innervations into Vc. RVM (yellow) nerve fibers (red) innervate into dorsal lamina layers of Vc (blue). (B) Vc slices from mice 14 days after CCI-ION were doubly stained with anti-GFP (for GCaMP3; green) and anti-5-HT (red) antibodies. Scale bar: 50 μm. (C) Quantification of 5-HT levels in (B) and Figure S4B are expressed as the mean density. 5-HT level in ipsilateral Vc (Ipsi.; n=13, 48.40±1.35 arbitrary unit (a.u.)) after nerve injured is significantly higher than contralateral side (cont.; n=8, 34.33±1.55). Local gene transfer of Tph-2 shRNA into the RVM attenuated 5-HT in Vc (n=21, 17.18±1.59) compared with that by scrambled shRNA treatment (S.; n=10, 45.26±1.27) and background (n=21, 14.71±0.87). (D) Representative GCaMP3 imaging of Vc slices. Fourteen days after CCI-ION, central terminals in V3 of ipsilateral Vc slices, from mice, whose RVM was treated 3 days earlier with either Tph-2 (lower panels) or scrambled shRNA (upper panels), were activated by capsaicin (1 μM) or low KCl (20 mM). Scale bar: 50 μm. (E and F) Time course of the amplitude of the Ca2+ transient that was evoked by capsaicin (1 μM) or low KCl application at V3 in Vc. Capsaicin was bath applied from 0 to 60 sec during the time course. Ipsi./s. shRNA, ipsilateral scrambled shRNA (n=15); cont./s. shRNA, contralateral scrambled shRNA (n=15); ipsi./Tph shRNA, ipsilateral Tph-2 shRNA (n=17); cont./Tph shRNA, contralateral Tph-2 shRNA (n=17). (G) The effects of molecular depletion of descending 5-HT on mechanical hyperalgesia induced by CCI-ION at the V2 (injured) or V3 (uninjured) region. Behavioral measures were done before shRNAi (at 10 d after CCI-ION) and at 4 day after shRNAi (14 day after CCI-ION) with intra-RVM gene transfer of Tph-2 shRNA (n=8) or scrambled shRNA (n=7) *p<0.05; **p<0.01; ***p<0.001.
Local gene transfer of a plasmid encoding an shRNA targeting neuronal tryptophan hydroxylase-2 (Tph-2), a key enzyme in the synthesis of 5-HT (Invernizzi, 2007), into the RVM led to a significant reduction of 5-HT immunostaining in the Vc, confirming the specificity of the staining and the origin of the positive fibers (Figure 5C and Figure S4B).
To determine the contribution of descending 5-HT to the sensitization of primary afferent central terminals in Vc after nerve injury, we imaged Vc slices obtained from CCI-ION treated Pirt-GCaMP3 mice whose RVMs were treated with either Tph-2 shRNA or scrambled shRNA. Strikingly, TRPV1 hyperactivity in the central terminals in Vc was almost completely blocked by depletion of 5-HT from the RVM, but not by scrambled shRNA (Figure 5D,E and Figure S5A,B,D). This inhibitory effect of intra-RVM treatment of Tph-2 shRNA on TRPV1 hypersensitivity could be seen in laminae I to IV of both V2 (injured) and V3 (uninjured) subdivisions of the Vc. In addition, overall neuronal hyperactivity of central terminals to low KCl (20 mM) was also significantly suppressed by Tph-2 shRNA as compared to scrambled shRNA (Figure 5D,F and Figure S5A,C). In contrast, depletion of 5-HT in the RVM had no detectable effect on central terminal responsiveness to a stronger depolarizing stimulus, high KCl (100 mM) (Figure S5E). Similar descending 5-HT-dependent hypersensitivity of central terminals was also observed in neighboring uninjured V1 of the Vc and C1 spinal cord slices (Figure S6).
We next asked whether descending 5-HT is required for nerve injury-induced persistent pain states. We examined mechanical hyperalgesia 4 days after intra-RVM Tph-2 shRNA injection which was also 14 days after CCI-ION. Consistent with the imaging results, mechanical hyperalgesia in the facial territories of injured (V2) and uninjured (V3) nerve fibers was significantly attenuated by the depletion of descending 5-HT from RVM neurons (p<0.05 in V2, p<0.01 in V3 vs. before shRNA treatment) compared with the scrambled shRNA injected group (Figure 5G). Together these data suggest that nerve injury induced upregulation of descending 5-HT in Vc plays an important role in sensitizing central terminals likely through TRPV1 which contributes significantly to the neuropathic pain state.
Functional 5-HT3A receptors are present on central terminals of primary sensory neurons
The 5-HT3 receptor (5-HT3AR), the only ligand-gated cation channel with excitatory function in the 5-HT receptor family, has been found to mediate descending facilitation in the development of inflammatory and neuropathic pain (Lagraize et al., 2010; Okubo et al., 2013; Suzuki et al., 2004). 5-HT3AR is expressed in spinal and Vc dorsal horn neurons and primary afferent neurons as well as their central terminals (Doucet et al., 2007). In order to determine whether functional 5-HT3ARs are present on primary afferent central terminals, we examined activation of 5-HT3AR on primary afferent fibers in the Vc slices from Pirt-GCaMP3 mice. Application of the 5-HT3AR agonist SR57227 (10 μM) induced increases in fluorescence signals in some terminals in Vc slices from CCI-ION Pirt-GCaMP3 mice (Figure 6A,B). Interestingly, the number of SR57227-sensitive central terminals in the Vc from CCI-ION mice was significantly higher than found in the sham group (50% increase; n=4; p<0.05). The specificity of SR57227 activation was determined by pretreatment with the 5-HT3AR antagonist Y25130 (10 μM), which completely abolished the Ca2+ signals evoked by SR57227. Furthermore, SR57227 did not activate TRPV1 expressed in HEK293 cells (Figure S8C). Together these data suggest that functional 5-HT3ARs are present and upregulated in the central terminals after nerve injury.
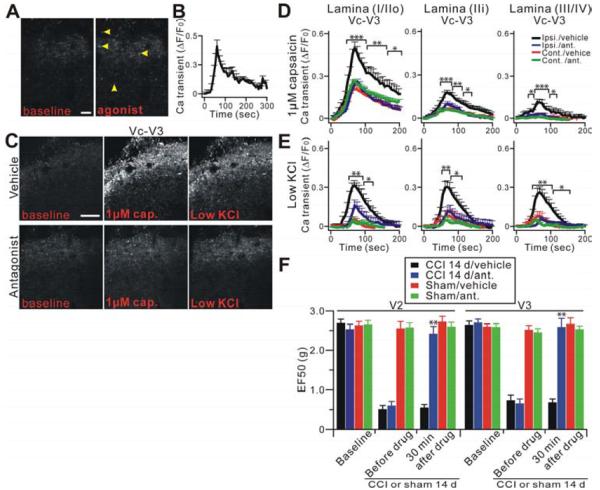
Figure 6.
Functional 5-HT3 receptors are present on central terminals of primary sensory neurons and mediate the sensitization of central terminals. (A) Representative Pirt-GCaMP3 imaging of Vc slices treated with 5-HT3AR agonist by bath application. Many central terminals in Vc after nerve injury were activated by the agonist (yellow arrowheads; n=9). Scale bar: 20 μm. (B) Time course of the amplitude of the Ca2+ transient that was evoked by 5-HT3AR agonist from (A). (C) Central terminals in V3 of ipsilateral Vc slices after nerve injury were bath pretreated with either the 5-HT3AR antagonist (lower panels) or vehicle (upper panels) for 30 min and then were activated by capsaicin (1 μM) or low KCl. Scale bar: 50 μm. (D and E) Strong hypersensitivity of central nerve fibers and terminals in Vc after nerve injury is significantly blocked by the antagonist. Time course of the amplitude of the Ca2+ transient that was evoked by capsaicin (1 μM) or low KCl application at V3 in Vc. Ipsi., ipsilateral (n=15); cont., contralateral (n=14); ipsi./ant., ipsilateral antagonist (n=12); cont./ant., contralateral antagonist (n=8). (F) Mechanical hyperalgesia was tested in V2 (injured) or V3 (uninjured) region at 14 days before and 30 min after intra-Vc 5-HT3AR antagonist (n=8) or vehicle (n=6) injection in nerve injury or sham mice (n=8, n=6 for antagonist or vehicle, respectively). *p<0.05; **p<0.01; ***p<0.001.
5-HT3AR mediates the sensitization of central terminals and TRPV1
It is possible that the sensitization of central terminals we detected after nerve injury is mediated by upregulated and spontaneously released 5-HT via activation of 5-HT3ARs on central terminals. To test this possibility, we perfused 5-HT3AR antagonist Y25130 (10 μM) onto Vc slices from CCI-ION Pirt-GCaMP3 mice for 30 minutes and then stimulated the tissues with capsaicin or low KCl. The functional blockage of 5-HT3AR significantly attenuated capsaicin-evoked GCaMP3 signals in the ipsilateral central terminals to a level comparable to that in the contralateral side of Vc slices from CCI-treated mice (Figure 6C,D and Figure S7A,B,D). The decrease in TRPV1 hyperactivity caused by Y25130 occurred in all laminae of the dorsal horn in both V2 (injured) and V3 (neighboring uninjured) subdivisions of the Vc (Figure 6C,D and Figure S7A,B,D). In addition, overall neuronal hypersensitivity of central terminals evaluated by low KCl (20 mM) was also significantly suppressed by the 5-HT3AR antagonist pretreatment compared with the contralateral side (Figure 6C,E and Figure S7A,C). In contrast, pretreatment of Y25130 had no effect on response to more robust depolarization of central terminals induced by 100 mM KCl (Figure S7E).
To determine whether blocking 5-HT3AR function can inhibit neuropathic pain, the 5-HT3AR antagonist Y25130 (130 pmol/0.5 μl) was microinjected in the ipsilateral Vc 14 days after CCI-ION. Indeed, mechanical hyperalgesia in facial territories innervated by injured (V2) and uninjured (V3) nerve fibers was significantly blocked by the 5-HT3AR antagonist (p<0.01, vs before drug; Figure 6F). These data suggest that 5-HT3AR activation in Vc may mediate medullary mechanisms of 5-HT-dependent descending facilitation underlying central terminal sensitization of uninjured nerves responsible for neuropathic pain.
5-HT3AR activation facilitates excitatory presynaptic input from primary afferents via TRPV1 sensitization
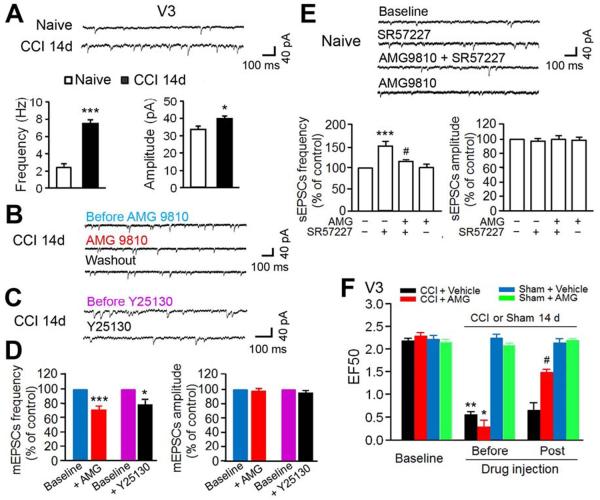
In order to determine the functional changes in Vc 5-HT3AR and presynaptic TRPV1 in the regulation of Vc glutamatergic transmission in trigeminal neuropathic pain after CCI-ION, we conducted whole-cell patch-clamp recordings of Vc dorsal horn neuronal activity in horizontal brainstem slices from adult mice. As shown in Figure 7A–C, spontaneous miniature excitatory postsynaptic currents (mEPSCs) were recorded from lamina II neurons identified by an evoked monosynaptic response to the attached trigeminal nerve root. Aδ/C fibers were electrically stimulated (Figure S8A) from attached dorsal roots of Vc slices from naïve mice and from the ipsilateral side of Vc slices from CCI-treated mice 14 days after injury. Application of the AMPA/kainite receptor antagonist CNQX (10 μM) completely blocked mEPSCs (3 neurons) confirming that the recorded EPSCs were induced by glutamatergic transmission. The frequency of mEPSCs recorded in V3 neurons was significantly increased 14 days after CCI-ION (7.5±0.44 Hz; p<0.001), compared to that in naïve mice (2.4±0.43 Hz). There was also a significant increase in mEPSC amplitudes after nerve injury (40.3±1.42 pA; p<0.05) compared to naïve mice (33.6±1.9 pA) (Figure 7A), suggesting both enhanced presynaptic excitatory input from primary nociceptive afferents and enhanced postsynaptic neuronal excitability. Moreover, in 9 out of 12 recorded V3 neurons from the Vc slices of mice 14 days after CCI-ION, bath application of the selective TRPV1 antagonist AMG9810 reversibly attenuated the CCI-induced increase in mEPSCs frequency but had no effect on the mEPSCs amplitudes (p<0.001, Figure 7B, D and Figure S8B). The same dose of AMG9810 had no effect on EPSCs in V3 neurons from naïve mice (Figure 7E). We tested AMG9810 in HEK293 cells to ensure its specificity to TRPV1 and it did not block 5-HT3AR activation (Figure S8D). These findings indicate that central terminal TRPV1 sensitization contributes to enhanced glutamatergic input from the uninjured trigeminal nerve after CCI-ION. In addition, application of the 5-HT antagonist Y25130 into Vc slices also significantly blocked increased frequency (p<0.05, n=7) but not amplitude of mEPSCs (Figure 7C, D), suggesting that presynaptic 5-HT3ARs in the central terminals are involved in the facilitation of primary afferent excitatory input.
Figure 7.
Central terminal TRPV1 and 5-HT3AR facilitate synaptic transmission to V3 lamina II neurons of the ipsilateral Vc after CCI-ION. (A) Upper: representative traces showed mEPSCs in one neuron of V3 division of the Vc from naïve or CCI-treated mice at 14d. Bottom: summarized mEPSCs frequency (left) and amplitude (right) from V3 neurons in the Vc of different groups (n=5–6 neurons per group). *p<0.05; ***p<0.001 versus naïve. (B) Original traces show mEPSCs during baseline control, application of selective TRPV1 antagonist AMG9810 (10 μM, 3 min) into Vc slices, and washout in one V3 neuron of the Vc from CCI 14 day-treated mice (right). (C) Original traces showed mEPSCs during baseline control and application of 5-HT3AR antagonist Y25130 (5 μM, 3 min) in one V3 lamina II neuron of Vc from CCI 14d-treated mice. (D) Normalized to the pre-drug treatment baseline mEPSCs, pooled data show significant reduction of frequencies (left) of mEPSCs in V3 neurons with blockade of Vc TRPV1 (n=9) or 5-HT3AR function (n=6) 14 days after CCI. However, there was no change of the amplitudes of mEPSCs (right). *p<0.05, ***p<0.001 vs. before drugs. (E) Upper: Typical traces showed sEPSCs (in the absence of TTX) during control, application of 5-HT3AR agonist SR57227 (10 μM, 3 min), pretreatment of AMG9810 plus application of SR57227, and application of AMG9810 alone in one V3 neuron of the Vc slices from naïve mice. Bottom: Normalized to the baseline sEPSCs, pooled data demonstrated that 10 μM SR57227-induced increase of the frequency (the left) but not the amplitude (the right) of sEPSCs in 7 neurons from the Vc slices of naïve mice. Pretreatment of AMG9810 attenuated SR57227-induced increase of the frequency of sEPSCs without effects on baseline control. ***p<0.001, vs. baseline control; #p<0.05 vs. SR57227 alone. (F) Effects of intra-Vc injection of AMG9810 (5 pmol/0.5μl) on CCI-induced mechanical hyperalgesia 14 days after nerve injury (n=6–8 mice per groups). **p<0.01, vs. baseline; #, p<0.05 vs. before drug treatment.
Next, we recorded spontaneous EPSCs (sEPSCs) in V3 lamina II neurons of the Vc slices from naïve mice in the absence of TTX. We observed that superfusion of 5-HT3AR agonist SR57227 (n=7) in Vc slices significantly increased the frequency but not amplitude of sEPSCs (p<0.001 vs baseline), which was partially reversed by pretreatment of AMG9810 (p<0.05 vs. SR57227 alone, n=5) (Figure 7E). These data suggest that presynaptic TRPV1 mediates 5-HT3AR activation-induced enhancement of primary afferent glutamatergic release in Vc.
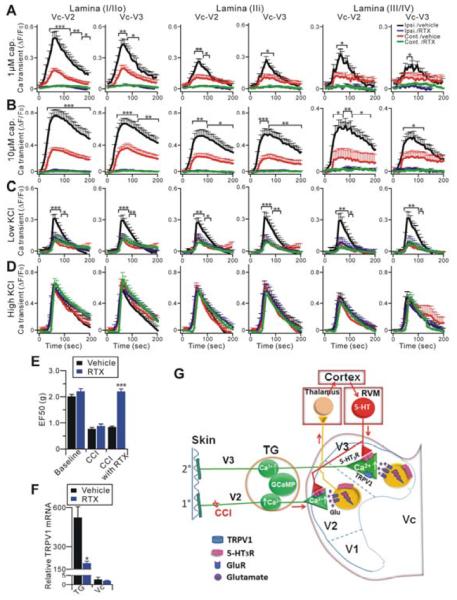
Finally, we examined what effect blockade of central terminal TRPV1 function would have on CCI-induced pain behavioral responses. Microinjection of AMG9810 into the ipsilateral Vc significantly attenuated mechanical hyperalgesia (p<0.05, n=6) 14 days after CCI and had no effect on mechanical thresholds in the sham group (n=6) (Figure 7F). A recent report has shown that TRPV1 is also expressed in interneurons in the spinal dorsal horn which contributes to the development of neuropathic pain (Kim et al., 2012). Kim et al. also demonstrated that 5 days after an intraperitoneal (i.p.) injection of the TRPV1 agonist resiniferatoxin (RTX), TRPV1+ neurons in the DRG but not in the spinal cord were selectively ablated. We i.p. injected RTX into CCI-ION mice in order to distinguish the action of TRPV1 at the central terminals of primary sensory neurons and dorsal horn neurons. However, we did both GCaMP imaging and pain behavioral testing 1 day after RTX treatment, instead of 5 days after treatment as done by Kim et al. (2012). The reason for this modification was that we wanted to use RTX to silence pre-synaptic TRPV1 channels but not ablate the TRPV1+ primary sensory neurons. Ablation of TRPV1+ neurons, as seen 5 days after RTX injection, would prevent us from analyzing the hyperactivity of pre-synaptic TRPV1 and their contribution to mechanical hyperalgesia. Capsaicin and low KCl-evoked neuronal hyperactivity of the Vc central terminals from RTX treated animals (i.e. 14 days after CCI-ION and 1 day after RTX injection) was significantly decreased when compared to vehicle treated CCI-ION mice (Figure 8A–C). However, high KCl-induced depolarization of the central terminals from RTX and vehicle treated Vc slices were comparable (Figure 8D), indicating the central terminals are still functional after RTX treatment. Strikingly, after RTX treatment (n=7), CCI-ION mice exhibited a significant reduction in mechanical hypersensitivity compared with CCI-ION mice treated with vehicle (n=8) (Figure 8E). The selective reduction of TRPV1 mRNA level in the TG was confirmed at 5 days after RTX injection by quantitative real time RT-PCR (Figure 8F). However, the expression of TRPV1 by intrinsic neurons in the Vc was not affected by the RTX treatment even after 5 days. This indicated that systemic RTX did not silence TRPV1 in the dorsal horn neruons. These data suggests that central terminal TRPV1 is essential for nerve injury-induced mechanical hypersensitivity.
Figure 8.
Selective silencing of TRPV1 in the central terminals of primary sensory neurons blocks neuronal hypersensitivity of the central terminals and neuropathic pain. (A–D) Vc slices from CCI-ION in Pirt-GCaMP3 mice which were i.p. injected with either RTX (n=17) or vehicle (n=14), were imaged in response to capsaicin and KCl. The time course of the amplitude of the Ca2+ transient that was evoked by capsaicin (1 μM, 10 μM) or KCl (20 mM) application at different lamina (lamina I/IIo; lamina IIi; lamina III/IV) of V2 and V3 of Vc is shown. Capsaicin was bath applied from 0 to 60 sec during the time course. Capsaicin-evoked responses were completely gone in the Vc from RTX treated mice (A, B). (D) Ca2+ transients induced by high KCl (100 mM) show no significant differences in ipsilateral and contralateral V2 and V3 of Vc after CCI-ION in Pirt-GCaMP3 mice. The Ca2+ transient (ΔF/F0) was normalized to the value imaged in baseline. Ipsi., ipsilateral; cont., contralateral. (E) Effects of i.p. injection of RTX on CCI-induced mechanical hyperalgesia 14 days after nerve injury. (F) Quantitative real time RT-PCR was performed on total RNAs isolated from TG and Vc from RTX or vehicle-injected CCI-ION mice using TRPV1 specific primers (n=4 per group). TRPV1 levels were normalized by β-actin in each sample. *p<0.05; **p<0.01; ***p<0.001. (G) Schematic illustration of the proposed mechanism. CCI-ION-induced hyperactivity of primary nociceptive fibers in TG V2 subdivision neurons and nociceptive signal input leads to dorsal horn V2 neuron activation in the Vc; neuronal activation then ascends to the thalamus and the cerebral cortex. Pain modulatory signals then descend to the RVM. Enhanced 5-HT release from the RVM leads to enhanced activation of 5-HT3ARs and subsenquent TRPV1 on the central terminals of primary afferents in the V2 and V3 subdivision of the ipsilateral Vc resulting in central terminal sensitization of injury and uninjured nerve fibers and a spread of behavioral hypersensitivity to uninjured nearby facial skin.
DISCUSSION
Imaging neuronal activity in primary sensory neurons from tissue explants and slices has been a challenge given that their nerve endings and cell bodies are densely surrounded by other cells and therefore are difficult to selectively load with Ca2+ sensitive chemical dyes. By specifically expressing a genetically-encoded Ca2+ sensitive indicator in almost all DRG and TG neurons in Pirt-GCaMP3 mice, we successfully detected activation of primary sensory neurons in the trigeminal system at peripheral and central terminals as well as in their cell bodies, in tissue explant and in slice preparations. The advantages of this method are manifold: simple tissue preparation and imaging procedures; excellent spatial resolution; sensitive, high-efficiency simultaneous imaging of multiple neurons; preservation of somatotopic organization; and stable expression of GCaMP3 after nerve injury.
Previous immunostaining data have shown that nerve injury causes large diameter neurons which normally do not express TRPV1 to become TRPV1 positive (Hudson et al., 2001). Consistent with these findings, our imaging data demonstrate that following nerve injury, the somata of many large diameter neurons in the injured V2 are activated by capsaicin. Although some of these may represent C or Aδ-nociceptors, others are likely to include large-diameter low-threshold Aβ mechanoreceptors; this corresponds with our observation of increased capsaicin sensitivity in projections to lamina III and IV of the Vc, which normally receive only large-diameter non-nociceptive input (Devor, 2009; Li et al., 2011). These findings support the idea of a phenotypic switch in injured neurons that formerly detected only innocuous stimuli to a state involving nociceptor-like characteristics (Costigan et al., 2009). Although a similar gain of capsaicin sensitivity was not evident from imaging of the uninjured V3 somata, examination of the uninjured afferents innervating lamina III and IV of the Vc showed acquired capsaicin sensitivity (comparing Figure 4D,E with Figure 3D,E). Furthermore, responsiveness to low KCl was also augmented in the central terminals of both injured and uninjured afferents indicating overall neuronal sensitization. These data suggest that sensitization occurs in both injured and uninjured afferents, and that sensitization in the central terminals of uninjured neurons is more evident than in their cell bodies.
Recent studies from our and other groups have shown that descending 5-HT from RVM facilitates persistent pain (Okubo et al., 2013; Wei et al., 2010). However, the underlying mechanisms are far from clear, since many 5-HT receptor subtypes are present on both central terminals of primary sensory neurons and intrinsic dorsal horn neurons. Therefore, it is difficult to determine onto which neuronal population and nerve fibers within the dorsal horn, 5-HT exerts a facilitating effect. In fact, most studies have focused on the effect of descending modulation on intrinsic dorsal horn neurons. We identified a mechanism in which supraspinal descending 5-HT sensitizes the central terminals of primary sensory neurons via a cascade that successively involves presynaptic 5-HT3AR and TRPV1 to promote chronic neuropathic pain (Figure 8G). After nerve injury, the upregulation of descending 5-HT and subsequent engagement of primary afferent central terminal 5HT3AR activation are likely drivers of central sensitization in addition to postsynaptic effects on dorsal horn neurons. Our study demonstrates that central terminal sensitization driven by supraspinal descending facilitation contributes to spinal sensitization and spreading pain hypersensitivity from injured (V2) to surrounding uninjured (V3) territories during some persistent or chronic pain states.
Clinical studies have demonstrated an analgesic effect of selective 5-HT3R antagonists in patients with neuropathic pain (McCleane et al., 2003). Our findings suggest novel approaches for treatments with 5-HT3R antagonists in chronic pain conditions in which active descending facilitation possibly maintains central terminal sensitization. The development of agents that suppress 5-HT/5-HT3R dependent and TRPV1-involved central terminal sensitization may be necessary to achieve favorable treatment effects.
Previous studies have shown that TRPV1 activity can be potentiated by 5-HT via both metabotropic and ionotropic 5-HT receptors such as 5-HT2, 3, 4, and 7 receptors (Loyd et al., 2011; Ohta et al., 2006; Sugiuar et al., 2004). Multiple signaling pathways, including protein kinase A and C (PKA and PKC), have been implicated in the potentiation. It is conceivable that other 5-HT receptors other than 5-HT3AR are involved in the central terminal TRPV1 sensitization. A candidate molecular link between 5-HT3AR and its downstream effector TRPV1 is PKC since it can be activated by 5-HT3AR and phosphorylation of TRPV1 by PKC leads to potentiation of TRPV1 activity (Khan and Hichami, 1999; Vay et al., 2012). Previous studies have shown that the sensitization of TRPV1 resulted in larger membrane depolarizations which led to a lower threshold of neuronal activation and increased overall excitability (Neelands et al., 2008; Zhang et al., 2011). Low KCl, which induced weak excitation of naïve central terminals in contralateral Vc, could strongly activate the central terminals in ipsilateral Vc as indicated by increase in Ca2+ signals. The stronger activation by low KCl is due to sensitization of TRPV1 in the ipsilateral central terminals. It has also been previously reported that TRPV1 activation in cranial visceral afferent terminals allows calcium entry into the terminals; this entry increases the rate of glutamate vesicle fusion and release (Shoudai et al., 2010). So while TRPV1 acts as a molecular sensor for noxious heat and capsaicin in peripheral nerve terminals, its hyperactivity in central terminals also contributes to overall central terminal sensitization during chronic pain states. Although TRPV1 is not a mechanotransducer per se, blocking its activity centrally inhibited mechanical hyperalgesia. Therefore, our current study directly demonstrates a new role for TRPV1 and its facilitation in the central terminals and a new strategy to treat chronic pain. It is possible that TRPV1-independent mechanisms also contribute to the neuronal hypersensitivity of the central terminals of primary sensory neurons since 5-HT3AR may modulate other downstream channels besides TRPV1.
Our current study has been focused on a CNS mechanism of central terminal sensitization by 5-HT-dependent descending facilitation after nerve injury. Several possible mechanisms might contribute to the sensitization of uninjured peripheral terminals in the skin. It appears unlikely that sensitization of terminals in the V3 ear skin is due to a direct interaction between the terminals of injured nerves in the V2 skin and those of uninjured nerves in the V3 ear skin, since the distance between the two receptive fields examined is large. Given that the most distal site of close contact between the V2 and V3 neurons is within the trigeminal ganglion, a more likely mechanism is that the somata of injured nerves, their central axons, and/or the intraganglionic domains of their peripheral axons secrete molecules such as nerve growth factor to sensitize neighboring uninjured nerves (Shinoda et al., 2011). By retrograde and anterograde trafficking of channels, receptors, and other molecules, nerve endings in the skin and in the spinal cord/Vc, respectively, would then become hypersensitive to painful stimuli. Future experiments combining GCaMP imaging with pharmacological, electrophysiological, and genetic approaches will be necessary to definitively address these mechanistic possibilities. Regardless, our current study directly demonstrates a new role for TRPV1 and its facilitation in primary afferent central terminals and a new strategy to treat chronic pain.
METHODS
Animals
Pirt-GCaMP3 mice were generated by targeted homologous recombination to replace the entire coding region of the Pirt gene with the GCaMP3 sequence fused with a neomycin resistance gene sequence and put in frame with the Pirt promoter. Pirt-GCaMP3 heterozygotes were used in all experiments. Details are available in Supplemental Data.
Animal model
All experiments were performed in accordance with protocols approved by the Animal Care and Use Committee at the Johns Hopkins University School of Medicine and University of Maryland Dental School. The mice used in the tests were backcrossed to C57Bl/6 mice for at least six generations and were 1 to 2 month-old males. To produce the CCI-ION model, chronic constriction injury was performed using loose ligatures of unilateral ION via an intraoral approach. Mice were anesthetized with pentobarbital sodium (50 mg/kg i.p.) and the ION was loosely tied with two-four chromic gut (4.0) ligatures through an incision in the palatal-buccal mucosa. The incision was closed using tissue glue. The sham-operated mice received only a unilateral nerve exposure without ligatures.
Behavioral test
Behavioral tests were conducted by experimenters blind to conditions. To reduce any effects of restraint, either by hand (Seino et al., 2009) or grasping the tail (Krzyzanowska et al., 2011), on behavioral assessment of the mice, we adopted a less stressful method (Aita et al., 2010) and developed a more precise probing technique to test mechanical hypersensitivity in V2 and V3 skin. Mice were placed in a box (3×3×4 inch) with the top, bottom and four walls made of black wire mesh and allowed to freely move. Von Frey filaments were applied to the skin near the center of the vibrissa pad within the infraorbital territory (V2) and the skin within the mandibular nerve territory (V3). A brisk or active withdrawal of the head from the probing filament was defined as a response. See Supplemental Data for details.
Brainstem microinjection and gene transfer
As previously described (Wei et al., 2010), Suresilencing™ shRNA plasmids for Tph-2 (TCAACATGCTCCATATTGAAT) and scrambled control (ggaatctcattcgatgcatac) (SuperArray, Frederick, MD, USA) (0.5 μg/0.5 μl) was injected into the RVM. Details are available in the Supplemental Data.
RTX treatment
Eleven days after ION-CCI, mice were intraperitoneally injected with RTX or vehicle (dissolved in a mixture of 10% Tween 80 and 10% ethanol in normal saline) as a single dose in two injections of 50 μg/kg and 150 μg/kg on days 1 and 2, consecutively. The mice were injected under 3% isoflurane inhalation anesthesia and maintained anesthesia for 2 – 3 hours after RTX or vehicle. Mechanical thresholds and GCaMP3 imaging were performed 1 day after the second RTX injection. The mRNA levels of TRPV1 in the TG and Vc from CCI-ION mice at 5 days after RTX or vehicle injection were deterimed by quantitative RT-PCR using TRPV1 specific primers: 5' ATCATCAACGAGGACCCAGG 3' and 5' TGCTATGCCTATCTCGAGTGC 3'.
Pirt-GCaMP3 Ca2+ imaging
Ten to 17 days after CCI-ION, two month old mice were sacrificed by CO2 asphyxiation. Ear skin, TG explants, and Vc slice was acutely isolated from Pirt-GCaMP3 mice and used to image and determine Ca2+ transients. All experiments were performed at room temperature (~25°C), in a chamber with stimuli applied by bath application. GFP signals from Pirt-GCaMP3 mice were measured to see Ca2+ transients using green emitted light. Single photon Ca2+ imaging was performed with a 700 Zeiss confocal microscope, using the 488 nm line of a solid state laser for excitation of Pirt-GCaMP3 and a 488 nm laser main dichroic beam splitter (MBS) and a 505–555 nm variable secondary dichroic mirror (VSD) to detect the emission of green fluorescence. Details are available in the Supplemental Data.
Whole cell patch clamp recording in Vc slices
The brain stem was dissected and horizontally sliced at a thickness of 400 μm. Slices were kept in an 50 ml glass beaker containing carbogenated protective aCSF at 33 °C. After at least 1 hour's recovery, the brainstem slice was transferred into a recording chamber and was continuously perfused (10–15 ml/min) with oxygenated Krebs' solution (composition in mM: NaCl 126, NaHCO3 26, glucose 10, KCl 2.5, CaCl2 2, MgCl2 1.2, and NaH2PO4 1.25) at room temperature. sEPSCs or mEPSCs were recorded continuously at a holding potential of −70 mV in the absence or presence of 0.5 μM tetrodotoxin, respectively. To block inhibitory transmission, 1 μM strychnine sulfate and 10 μM bicuculline methiodide were included in the superfusate in all the experiments. Blind whole-cell voltage-clamp recordings were performed with series resistance compensation using an EPC 10 amplifier (Heka, Germany). See Supplemental Data for details.
Analysis
Group data were expressed as mean ± standard error of the mean. Unless otherwise noted unpaired Student's t test was used to determine significance in statistical comparisons and differences were considered significant at p<0.05. Animal behavior data were analyzed with two-way ANOVA test. Patch clamp recording data were analyzed with Mini Analysis 6.0.3 (Synaptosoft, USA). The statistical significance was set at p<0.05 using Student's t-test or Kolmogorov–Smirnov (K-S) test.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Loren Looger at Howard Hughes Medical Institute Janelia Farm for providing us GCaMP3 cDNA; Yixun Geng for technical assistance; Matthias Ringkamp and David Ginty for helpful comments and discussion. We thank Chip Hawkins and Holly Wellington of Mouse ES cell Core (P30NS050274) and Transgenic Core at Johns Hopkins University School of Medicine for assistance with Pirt-GCaMP3 mouse generation. This work was supported by National Institutes of Health Grants (R01DE022750 and R01GM087369 to X.D.; R01DE018573 to F.W.; T32NS070201 to Y.S.K.) and Johns Hopkins University Brain Science Institute grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST These conflicts are being managed by the Johns Hopkins Office on Policy Coordination.
REFERENCES
- Aita M, Byers MR, Chavkin C, Xu M. Trigeminal injury causes kappa opioid-dependent allodynic, glial and immune cell responses in mice. Molecular pain. 2010;6:8. doi: 10.1186/1744-8069-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau R.W.t. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annual review of neuroscience. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2009;196:115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- Doucet E, Latremoliere A, Darmon M, Hamon M, Emerit MB. Immunolabelling of the 5-HT 3B receptor subunit in the central and peripheral nervous systems in rodents. The European journal of neuroscience. 2007;26:355–366. doi: 10.1111/j.1460-9568.2007.05659.x. [DOI] [PubMed] [Google Scholar]
- Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6721–6726. doi: 10.1073/pnas.1110460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. The European journal of neuroscience. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. The European journal of neuroscience. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- Invernizzi RW. Role of TPH-2 in brain function: news from behavioral and pharmacologic studies. Journal of neuroscience research. 2007;85:3030–3035. doi: 10.1002/jnr.21330. [DOI] [PubMed] [Google Scholar]
- Khan NA, Hichami A. Ionotrophic 5-hydroxytryptamine type 3 receptor activates the protein kinase C-dependent phospholipase D pathway in human T-cells. The Biochemical journal. 1999;44(Pt 1):199–204. [PMC free article] [PubMed] [Google Scholar]
- Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133:475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, Na HS, Bae YC, Kim SJ, Kim JS, et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74:640–647. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Klein BG, Renehan WE, Jacquin MF, Rhoades RW. Anatomical consequences of neonatal infraorbital nerve transection upon the trigeminal ganglion and vibrissa follicle nerves in the adult rat. The Journal of comparative neurology. 1988;268:469–488. doi: 10.1002/cne.902680402. [DOI] [PubMed] [Google Scholar]
- Krzyzanowska A, Pittolo S, Cabrerizo M, Sanchez-Lopez J, Krishnasamy S, Venero C, Avendano C. Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. Journal of neuroscience methods. 2011;201:46–54. doi: 10.1016/j.jneumeth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience. 2010;171:1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte CC, de Lanerolle NC. Ultrastructure of chemically defined neuron systems in the dorsal horn of the monkey. III. Serotonin immunoreactivity. Brain research. 1983;274:65–77. doi: 10.1016/0006-8993(83)90521-8. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Weiss G, Henry MA, Hargreaves KM. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain. 2011;152:2267–2276. doi: 10.1016/j.pain.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annual review of neuroscience. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- McCleane GJ, Suzuki R, Dickenson AH. Does a single intravenous injection of the 5HT3 receptor antagonist ondansetron have an analgesic effect in neuropathic pain? A double-blinded, placebo-controlled cross-over study. Anesthesia and Analgesia. 2003;97:1474–1478. doi: 10.1213/01.ANE.0000085640.69855.51. [DOI] [PubMed] [Google Scholar]
- Mosconi T, Woolsey TA, Jacquin MF. Passive vs. active touch-induced activity in the developing whisker pathway. The European journal of neuroscience. 2010;32:1354–1363. doi: 10.1111/j.1460-9568.2010.07396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Tsuboi Y, Suzuki I, Honda K, Shinoda M, Kondo M, Matsuura S, Shibuta K, Yasuda M, Shimizu N, et al. PKCgamma in Vc and C1/C2 is involved in trigeminal neuropathic pain. J Dent Res. 2011;90:777–781. doi: 10.1177/0022034511401406. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Jarvis MF, Faltynek CR, Surowy CS. Elevated temperatures alter TRPV1 agonist-evoked excitability of dorsal root ganglion neurons. Inflammation research : official journal of the European Histamine Research Society. 2008;57:404–409. doi: 10.1007/s00011-008-7223-6. [et al] [DOI] [PubMed] [Google Scholar]
- Neubert JK, Mannes AJ, Keller J, Wexel M, Iadarola MJ, Caudle RM. Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Res Brain Res Protoc. 2005;15:119–126. doi: 10.1016/j.brainresprot.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K, Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. The Journal of physiology. 2006;576:809–822. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo M, Castro A, Guo W, Zou S, Ren K, Wei F, Keller A, Dubner R. Transition to persistent orofacial pain after nerve injury involves supraspinal serotonin mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5152–5161. doi: 10.1523/JNEUROSCI.3390-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. The Journal of comparative neurology. 1952;96:414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Meyer RA. Injured versus uninjured afferents: Who is to blame for neuropathic pain? Anesthesiology. 2005;103:221–223. doi: 10.1097/00000542-200508000-00002. [DOI] [PubMed] [Google Scholar]
- Seino H, Seo K, Maeda T, Someya G. Behavioural and histological observations of sensory impairment caused by tight ligation of the trigeminal nerve in mice. Journal of neuroscience methods. 2009;181:67–72. doi: 10.1016/j.jneumeth.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Shinoda M, Asano M, Omagari D, Honda K, Hitomi S, Katagiri A, Iwata K. Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7145–7155. doi: 10.1523/JNEUROSCI.0481-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14470–14475. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. The Journal of physiology. 2007;581:631–647. doi: 10.1113/jphysiol.2006.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain research. 2004;1019:68–76. doi: 10.1016/j.brainres.2004.05.108. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, Matera M, Geppetti P, Bianchi A. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Progress in neurobiology. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. British journal of pharmacology. 2012;165:787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, Guo W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neuroscience letters. 1998;255:135–138. doi: 10.1016/s0304-3940(98)00730-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59:450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Han P, Neelands TR, McGaraughty S, Honore P, Surowy CS, Zhang D. Coexpression and activation of TRPV1 suppress the activity of the KCNQ2/3 channel. The Journal of general physiology. 2011;138:341–352. doi: 10.1085/jgp.201110618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.