Abstract
The involvement of purinergic signalling in kidney physiology and pathophysiology is rapidly gaining recognition and this is a comprehensive review of early and recent publications in the field. Purinergic signalling involvement is described in several important intrarenal regulatory mechanisms, including tuboglomerular feedback, the autoregulatory response of the glomerular and extraglomerular microcirculation and the control of renin release. Furthermore, purinergic signalling influences water and electrolyte transport in all segments of the renal tubule. Reports about purine- and pyrimidine-mediated actions in diseases of the kidney, including polycystic kidney disease, nephritis, diabetes, hypertension and nephrotoxicant injury are covered and possible purinergic therapeutic strategies discussed.
Keywords: Glomerulus, Tubules, Sodium transport, ATP release, Kidney failure, Diabetes
Synopsis
Glomerulus and the renal vasculature
Mesangial cells
Podocytes
Kidney blood vessels
Physiological responses in the glomerulus and renal vasculature
Renal autoregulation
Myogenic responses to altered perfusion pressure
Tubuloglomerular feedback and the juxtaglomerular apparatus
Glomerular and medullary microcirculation
Renin release
Renal tubules
Proximal convoluted tubules
Loop of Henle
Distal tubules
Collecting duct
Water transport
Sodium transport
Release and metabolism of nucleotides
Flow-induced nucleotide release
Mechanism of nucleotide release
Metabolism by ectonucleotidases
Renal pathophysiology
Renal injury and failure
Polycystic kidney disease
Ischaemia
Nephritis
Hypertension
Diabetic nephropathy
Inflammation
Hyper- and hypothyroidism
Nephrotoxicant injury
Renal transplants
Summary
Studies of the involvement of purinergic signalling in kidney physiology and pathophysiology are growing rapidly, so we believe it is timely to prepare a comprehensive review of the history and current views about the wide variety of events mediated by receptors for purines and pyrimidines. Reviews are available on various aspects of the field, including:
Adenosine and regulation of renin secretion [159,273];
Adenosine and kidney function [69,248,259,349,384];
Glomerulus and tuboglomerular feedback [148,200,255,320];
Nucleotide signalling along the renal tubules (sodium and water transport) [12,28,77,103,192,215,216,233,288,297,308,328,372,381,383,409,414];
Cilia in renal epithelium and paracrine purinergic signalling [298];
Nervous control (ATP as a cotransmitter) [306];
Ectonucleotidases in the kidney [333];
Purinergic regulation of renal blood flow [45,146,147,150,153,165,256,261,321];
P2X receptors and kidney function [17].
Pathophysiology [30,113,402,403];
Physiological and pathophysiological renal actions of purines [113,118,160,161,217,379,402,403];
Polycystic kidney disease (PKD) [133,139,267];
Role of dinucleotide polyphosphates in chronic kidney disease and uremia [171].
Renal microvascular function and hypertension [117].
Sympathetic hyperactivity in renal disease [6];
Mechanotransduction in the renal tubule [400].
Human embryonic kidney (HEK-293) cells are often used as a recombinant expression system for the study of a variety of receptors, including P2 receptors (see e.g. [7,68,334,374]), even though they express native P2Y1, P2Y2 and P2Y4 receptors [88,89].
Glomerulus and the renal vasculature
The mammalian glomerulus is structurally complex, consisting of central glomerular tuft of endothelial (~20 % of total cells) and mesangial (~25 %) cells, encapsulated by a double layer of visceral (podocytes) and parietal epithelial (~55 %) cells ([266]; see Fig. 1). Most information concerning P2 receptor expression in the glomerulus comes from cell culture with very few studies on receptor distribution in the native mammalian glomerulus. Nevertheless, mRNA for P2Y1 and P2Y2 was identified in extracts from whole rat glomeruli [16]. P2Y2 immunoreactivity colocalised with podocytes and cells of the parietal sheets; P2Y1 immunoreactivity was limited to the mesangial cells. ATP evoked calcium transients in the intact glomerulus and in the isolated parietal sheet. These studies suggest that extracellular ATP may regulated glomerular ultrafiltration directly, independent of actions of the renal microvasculature. ATP can also relax glomeruli via P2Y receptors on endothelial cells resulting in release of nitric oxide (NO), supporting the notion that P2 receptors influence glomerular filtration rate (GFR) [166]. Uridine adenosine tetraphosphate may also act as an autocrine hormone affecting glomerular filtration rate [170]. Tubular sodium transport systems are more sensitive to diadenosine tetraphosphate (Ap4A) than systems involved in glomerular filtration rates [167]. β-Blockers induce relaxation of the glomerular microvasculature by releasing ATP, which acts via P2Y receptors on endothelial cells to produce NO, resulting in vasodilation [180]. Connexin (Cx) 40 hemichannels and extracellular ATP are the key molecular elements of the glomerular endothelial calcium wave [371]. A review that discusses the roles of ATP and adenosine in tubuloglomerular feedback (TGF) regulation of glomerular filtration is available [46]. In addition to the effects of GFR, P2 receptors in the extraglomerular mesangium play an important role in TGF, as discussed below.
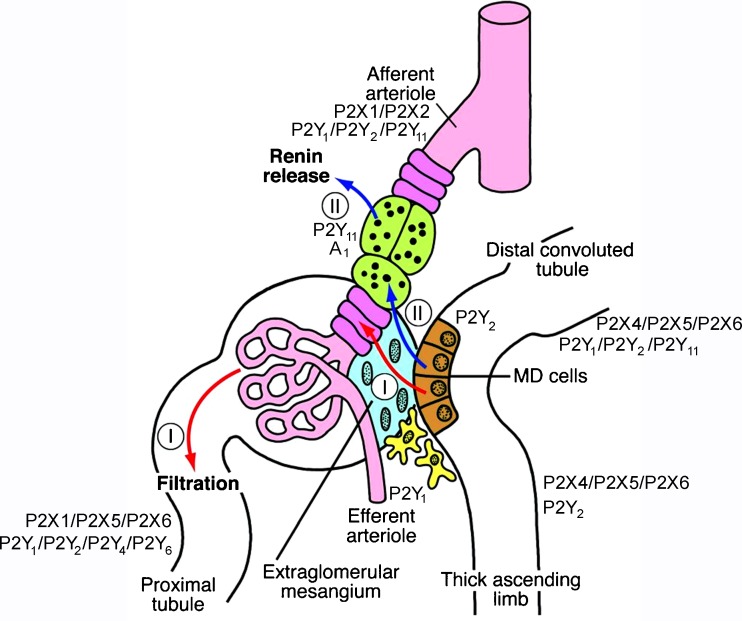
Fig. 1.
Schematic showing the structural and functional relationships within the JGA. The macula densa (MD) cells are shown in brown, the extraglomerular mesangium in blue, the vascular smooth muscle cells (VSMCs) in magenta, the renin-producing cells in green, the fibroblasts of the adjacent interstitium in yellow and the blood vessels in red. Note that both signalling pathways from the MD pass the extraglomerular mesangium, either to reach the VSMCs (I) to regulate filtration or the renin-producing cells (II) to mediate renin secretion (reproduced from [200], with permission from the American Society for Clinical Investigation)
Mesangial cells
Functionally, ATP and uridine 5′-triphosphate (UTP), probably acting via P2Y2 and/or P2Y4 receptors, increased inositol 1,4,5-trisphosphate formation and activated the p38-stress-activated protein kinase cascade in rat renal mesangial cells [144,286,361]. Extracellular ATP increases [Ca2+]i by release from intracellular stores, indicating mediation via P2Y receptors [121,282], although P2X receptor-mediated increase in [Ca2+]i has also been claimed [311]. Cultured mouse mesangial cells expressed P2X2, P2X4, P2X7, P2Y2 and P2Y4 receptors; mRNA (but not protein) for P2X1 and P2X3 receptors was also found [311]. Using RT-PCR, P2X1 receptor mRNA was shown to be expressed by an immortalised mouse mesangial cell line (G3) [131]. ATP and UTP stimulate the mitogen-activated protein kinase (MAPK) cascade and the MAPK pathway to promote proliferation of rat renal mesangial cells [143,145,157,323,392]. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2X7 receptors [326], although P2X4 receptors may also be involved in the apoptotic actions [347]. The generation of reactive oxygen species in rat mesangial cells may contribute to P2X7 receptor-induced apoptotic cell death [125]. ATP potentiates mesangial cell proliferation induced by growth factors [324] and growth hormones reverse desensitization of P2Y2 receptors in rat mesangial cells [122].
Diadenosine polyphosphates, which influence renal perfusion pressure, activate Cl− and non-selective cation conductance in rat mesangial cells as do ATP and angiotensin II (Ang II); Ap4A was the most effective of the dinucleotides [194,318,325]. Diadenosine pentaphosphate (Ap5A) and diadenosine hexaphosphate appear to play a regulatory role in mesangial cell proliferation [129]. Ap4A and Ap5A decrease, while diadenosine triphosphate appears to increase glomerular filtration rate [359].
The effect of sphingosine-1-phosphate, a potent mitogen of mesangial cells, is rapidly desensitized by activation of P2Y and P1 receptors [420]. ATP and UTP induce migration of mesangial cells by upregulating sphingosine kinase-1 expression and activity [193].
Adenosine inhibits platelet-derived growth factor in human glomerular mesangial cells via A2B receptors [73]. A1 and A2 receptors appear to mediate opposite actions on intracellular levels of cyclic adenosine monophosphate (cAMP) in mesangial cells [265]. A2 receptor-mediated hyperpolarisation of cultured rat mesangial cells has been described [283]. A2 receptors have been identified on human glomerular mesangial cells [350]. Adenosine activates mesangial cell proliferation [235], but can also induce apoptosis of mesangial cells [437]. High concentrations of glucose increase extracellular levels of ATP in mesangial cells, which in turn activates ERK1/2. This effect is partially dependent on the generation of reactive oxygen species and subsequent upregulation of transforming growth factor-1β [300].
Podocytes
Podocytes maintain the permselectivity of the glomerular filtration barrier. Podocyte function is implicated in human health: proteinuria is a significant independent risk factor for cardiovascular mortality and an indicator of underlying chronic kidney disease. Cultured human podocytes produce superoxide in response to extracellular ATP [115]. Increase in [Ca2+]i by purines and pyrimidines is mediated mainly by P2Y2 and P2Y6 receptors, but P2Y1, P2Y11 and P2X7 receptors are also expressed by podocytes [40,87,118,375]. ATP acting via P2 receptors stimulated AMP-activated protein kinase and suppressed superoxide generation in cultured mouse podocytes [287]. Functionally, activation of A2A receptors reduces glomerular proteinuria at least in part by preserving the structure and function of podocytes [9].
Kidney blood vessels
P2X1 receptors have been identified in the vascular smooth muscle of the rat renal, arcuate and interlobular arteries and in the afferent arteriole: P2X1 is not expressed in the efferent arteriole [51,375]. A P2X1-like receptor has been confirmed functionally in the afferent arteriole [149]. In the smooth muscle of the larger renal arteries, P2X2 receptor subunits have been immunolocalised [146,375], and at a molecular level, P2X4 receptor subunits are found, at least in arcuate and interlobular arteries [127]. At a protein level, however, P2X4 expression is limited to the vascular endothelium [242]. P2X7 receptors are also expressed in the healthy kidney: expression in the vascular smooth muscle and outer-adventitium is very low [218], whereas functionally significant expression is observed in the endothelium [242]. Of the P2Y receptors, P2Y1 is expressed in the endothelium of the large arteries and both afferent and efferent arterioles [375]. ATP released from renal tubular epithelial cells acts on pericytes to regulate the diameter of vasa recta capillaries that are in close proximity to renal tubules and are key to regulating renal medullary blood flow [61]. P1 receptors are expressed on mouse afferent arterioles and it was concluded that activation of A3 receptors blunted the vasoconstrictor effects mediated by A1 receptors [231].
Physiological responses in the glomerulus and renal vasculature
Infusion of ATP into the renal artery alters renal vascular resistance, although the vasoactive response is dependent upon species and basal vascular tone and can be influenced by the experimental approach [147]. Functionally, the larger renal arteries serve principally as conductance vessels [428], and renal vascular resistance, which determines renal blood flow, is regulated primarily through pressure-dependent vasoactivity of the preglomerular arterioles [351]. The interlobular arteries also contribute, but to a lesser extent [130]. The responsiveness to ATP of the arcuate and interlobular arteries and the glomerular arterioles of the rat has been assessed in the isolated perfused kidney preparation [149]. The preglomerular arteries were relatively insensitive to ATP, with micromolar concentrations required to cause a short-lived vasoconstriction. In contrast, sub-micromolar concentrations of ATP caused sustained contraction of the afferent arteriole. The efferent arteriole was unresponsive to extracellular ATP, consistent with the reported absence of P2 receptors in this section. In the isolated perfused rat kidney, intrarenal administration of ATP is normally vasoconstrictive, an effect potentiated by inhibition of NO synthesis (NOS) [79]. In contrast, ATP causes vasodilatation when baseline renal vascular resistance is high. This reflects P2Y-mediated production of NO [86]. More recent data have shown vasoactive actions of P2X4 and/or P2X7 in the rat renal artery [242]. It would appear that P2 receptor ‘tone’ influences renal vascular resistance, with P2Y-mediated vasodilatation opposing P2X-mediated vasoconstriction.
Renal autoregulation
Autoregulation of blood flow is an intrinsic property of most vascular beds. In the kidney, autoregulation is highly efficient so that renal blood flow is effectively independent of blood pressure over the physiological range [64]. Whole kidney autoregulation is governed through the combined influence of TGF and the intrinsic myogenic response of the vascular smooth muscle. These regulatory systems have overlapping operational frequencies and may interact to a degree [394] so that afferent arteriolar constriction through TGF enhances the myogenic response in the upstream vasculature [135].
Myogenic responses to altered perfusion pressure
The intrinsic myogenic response to altered perfusion pressure is both necessary and sufficient for full whole kidney autoregulation [64]. The myogenic response operates along the preglomerular vascular tree, with increased transmural pressure causing channel-mediated calcium influx and promoting reflex vasoconstriction of the vascular smooth muscle. Mechanistically, the underlying signalling processes are not fully defined, but local release of ATP is implicated. In the afferent arteriole, for example, pressure-mediated vasoconstriction is markedly blunted by pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) or suramin or by the saturation and subsequent desensitization of the P2 receptor system [151]. The central role of the P2 system is further suggested by the fact that pressure-induced reductions in afferent arteriole diameter are abolished in P2X1-deficient mice [152]. Pharmacological [272] or pathological [119] manoeuvres that impair P2X1 receptor signalling will also blunt whole kidney autoregulation of blood flow, both in vivo and in vitro. Finally, mice with a targeted deletion of the ectonucleotidase NTPDase1 exhibit enhanced pressure-induced vasoconstriction in the mesenteric artery [183]. This probably reflects the prolonged half-life of extracellular ATP and is consistent with a key role for local nucleotide signalling in the general myogenic response.
Tubuloglomerular feedback and the juxtaglomerular apparatus
TGF is a dynamic process whereby changes in the concentration of NaCl in the fluid emerging from the loop of Henle elicit inverse changes in the GFR of the nephron of origin. TGF is mediated by the juxtaglomerular apparatus (JGA), which includes a sensor, the macula densa and an effector (granulated cells in the afferent arteriole); other components of the JGA (e.g. mesangial cells) also play a role.
Changes in luminal NaCl concentration within the physiological range promote a directly correlated release of ATP from the basolateral membrane of macula densa cells [21,196]. Furthermore, the concentration of ATP in the cortical interstitium changes to reflect inhibition or activation of TGF [260]. These data suggest that ATP is the primary signalling molecule for TGF [22,258]. Gene targeting experiments, however, indicate that ATP is not the ultimate signal through which activation of TGF causes constriction of the afferent arteriole: hydrolysis of ATP to adenosine appears to be critical. A1 receptors mediate TGF in both rats [91] and mice [34]. In vivo TGF responses are blunted in mice lacking either the adenosine A1 receptor [222,356] or ecto-5′-nucleotidase, the enzyme catalysing the final stage of the degradation of ATP to adenosine [47]. This proposition is supported by a recent in vivo study in which the TGF response in mice (as assessed by changes in stop-flow pressure in the proximal tubule) was unaffected during intravenous infusion of PPADS or suramin [319]. Nevertheless, an anatomical consideration argues for involvement of the P2 receptor system in the TGF response: the ATP released from macula densa cells cannot activate directly P2 receptors in the afferent arteriole, being physically separated in most species by the extraglomerular mesangium. An intact mesangium is required for TGF responses [307]. Intracellular Ca2+ wave propagation occurs between rat juxtaglomerular cells; this is mediated by ATP and is involved in the synchronisation of renin release [424]. It was later shown that both ATP and gap junctions were integral components of the TGF calcium wave and that TGF activation causes a wave of increased cytosolic calcium to pass through the mesangium, to the granulated cells of the afferent arteriole and into the glomerular podocytes [285]. Propagation of this calcium wave was abolished by suramin but not by adenosine receptor antagonism. The P2 receptor response requires gap junctional coupling and is inhibited by antagonists against Cx37 and Cx40 [364]. It has been claimed recently that TGF adapts and stabilizes early distal delivery at a new set-point, via an A1 receptor-dependent mechanism [27].
Finally, the basolateral membrane of macula densa cells expresses a P2Y2-like receptor. The function of this receptor is unknown but it may provide a mechanism through which ATP release can be coupled to production [22]. A schematic illustrating the underlying mechanism of TGF is shown in Fig. 2.
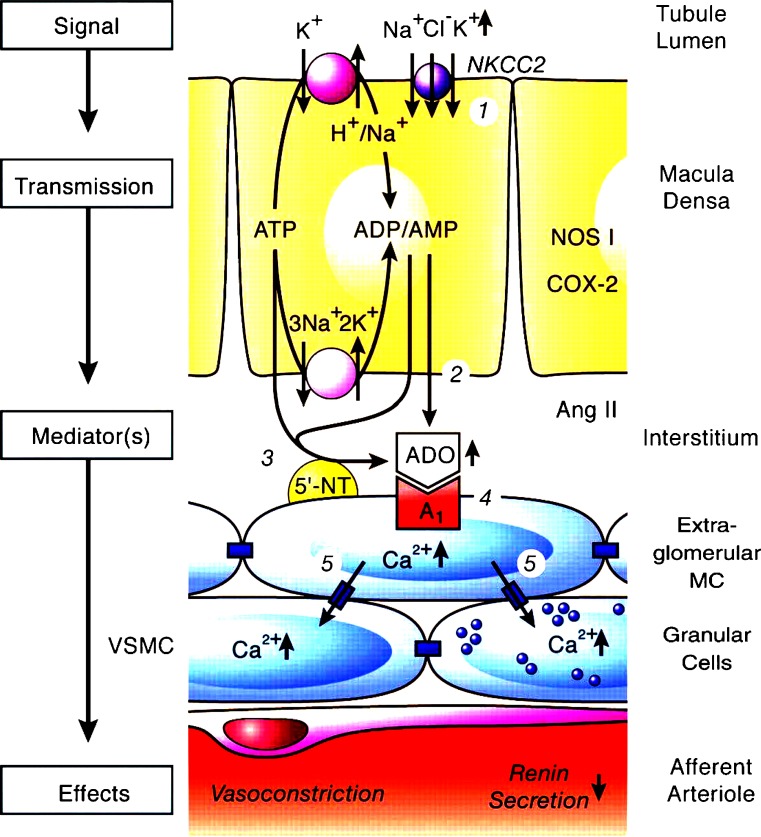
Fig. 2.
Proposed mechanism of adenosine acting as a mediator of the tubuloglomerular feedback. Numbers in circles refer to the following sequence of events. 1, Increase in concentration-dependent uptake of Na+, K+ and Cl− via the furosemide-sensitive Na+–K+–2Cl− co-transporter (NKCC2); 2 and 3, transport-dependent, intra- and/or extracellular generation of adenosine (ADO) and the extracellular generation involves ecto-5′-nucleotidase (5′-NT); 4, extracellular ADO activates adenosine A1receptors triggering an increase in cytosolic Ca2+ in extraglomerular mesangium cells (MC); 5, the intensive coupling between extraglomerular MC, granular cells containing renin and smooth muscle cells of the afferent arteriole (VSMC) by gap junctions allows propagation of the increased Ca2+ signal resulting in afferent arteriolar vasoconstriction and inhibition of renin release. Factors such as nitric oxide, arachidonic acid breakdown products or angiotensin (ANG) II modulate the described cascade. NOS I neuronal nitric oxide synthase, COX-2 cyclooxygenase-2 (reproduced from [384], with permission of the American Physiological Society)
Glomerular and medullary microcirculation
Infusions of nucleotide analogues into the renal artery exert powerful effects on regional blood flow, and these can be measured by laser Doppler flow probes inserted into specific regions of the kidney. In the rabbit, ATP evokes a biphasic response, with vasoconstriction of the medullary blood flow being followed by hyperaemia [82]. On the basis of relative agonist potency, the vasoconstriction was attributed to P2X1 receptors; the secondary vasodilatation, which was independent of NO, to adenosine receptors. In the rat, the net effect of ATP is influenced by sodium status. In sodium-restricted rats, ATP increased medullary blood flow in a NO-dependent manner [71]. In salt-loaded rats, ATP caused vasoconstriction in the outer medulla without affecting inner medullary flow. The authors speculated that the inner medullary vasodilatation reflected an effect of nucleotides on vasa recta pericytes. Consistent with this, purinergic cross-talk between the thick ascending limb and abutting vasa recta exerts a countervailing influence on Ang II vasoconstriction, an action lost during salt-sensitivity [263]. The integrated picture is far from clear, however, since data obtained in slices of rat kidney suggest that P2 receptor activation promotes vasoconstriction of the vasa recta due to contraction of pericytes [61].
Responses to adenosine, mediated by A2 receptors, modulated TGF by counteracting the effects of A1 receptor-mediated actions [44]. Both A2A and A2B receptors are functionally expressed in juxtamedullary afferent arterioles, and the dilator effects of adenosine are predominantly mediated by A2B receptors, which counteract A1 receptor-mediated vasoconstriction [84].
Renin release
The renin–angiotensin system is influenced by many factors, the final pathways of which converge at the level of altered intracellular calcium signalling in the granular cell; renin secretion is inversely related to [Ca2+]i [424]. The renin-containing epithelioid juxtaglomerular cells are modified vascular smooth muscle cells and are localised in the media of the afferent arteriole close to its entry into the glomerulus and are innervated by sympathetic nerves. ATP was shown to increase renin release from juxtaglomerular cells in an early paper [97]. Both renal juxtaglomerular and microvascular endothelial cells express P2 purinoceptors and ATP inhibited cAMP-stimulated renin release from juxtaglomerular cells only in the absence of endothelial cells [203]. P2Y receptors mediate stimulation of renin secretion in rat renal cortical slices [59]. ATP can stimulate the renin gene promoter via P2Y11 receptors [385]. Stimulation of sympathetic nerves released ATP as a cotransmitter with noradrenaline (NA) to elicit excitatory junction potentials in both smooth muscle and juxtaglomerular cells, to produce vasoconstriction and release of renin, respectively [38]. A recent paper reports that adenosine, formed during renal sympathetic nerve stimulation, enhances via A1 receptors the postjunctional effects of released NA, thereby contributing to renal sympathetic neurotransmission [162]. Juxtaglomerular A1 receptors are also involved in the control of the glomerular microcirculation [184]. Adenosine was shown to depress renin secretion in sodium-restricted rats, accompanied by a marked fall in GFR, while this effect was nearly abolished in sodium-loaded rats [274]. A2 receptors mediate arteriolar dilation and stimulation of renin secretion, whereas activation of A1 receptors mediates arteriolar constriction and inhibition of renin secretion [58,252]. Adenosine inhibits renin release by a mechanism that involves the juxtaglomerular cells located in the afferent arteriole at some distance from the glomerulus [343]. From other studies, it was concluded that adenosine decreases renin release via the activation of juxtaglomerular A1 receptors and that adenosine may be an inhibitory signal from the macula densa to juxtaglomerular cells [158,271]. In A1 receptor-deficient mice, TGF is abolished (see above) and there is increased plasma renin [34]. A1 receptors are required for the inhibition of renin secretion produced by an increase in blood pressure, suggesting that adenosine is responsible for baroreceptor-mediated inhibition of renin release; in contrast, stimulation of the renin system by low blood pressure appears to follow a different pathway [327].
Renal tubules
The pattern and distribution of P1 [345,390] and P2 receptors [14,49,375] along the rat renal tubule have been reported (see Fig. 3), and the major transporters for sodium and water are illustrated in Fig. 4. In recent years, our understanding of the functional consequences of receptor activation has advanced considerably, in part due to physiological studies in gene-targeted mice (see Table 1).
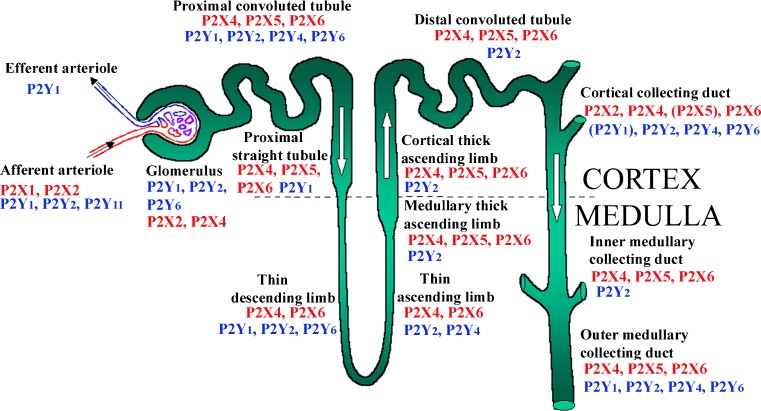
Fig. 3.
The distribution of P2 receptors along the nephron: an amalgam of available functional, mRNA and protein (immuno-) detection studies showing how widespread and overlapping it is. To date, there has been no full report of their distribution in native renal tissue of any species. P2Y is shown in blue and P2X in red; presence of those in parentheses is still uncertain. (updated from [381], with permission from the American Physiological Society)
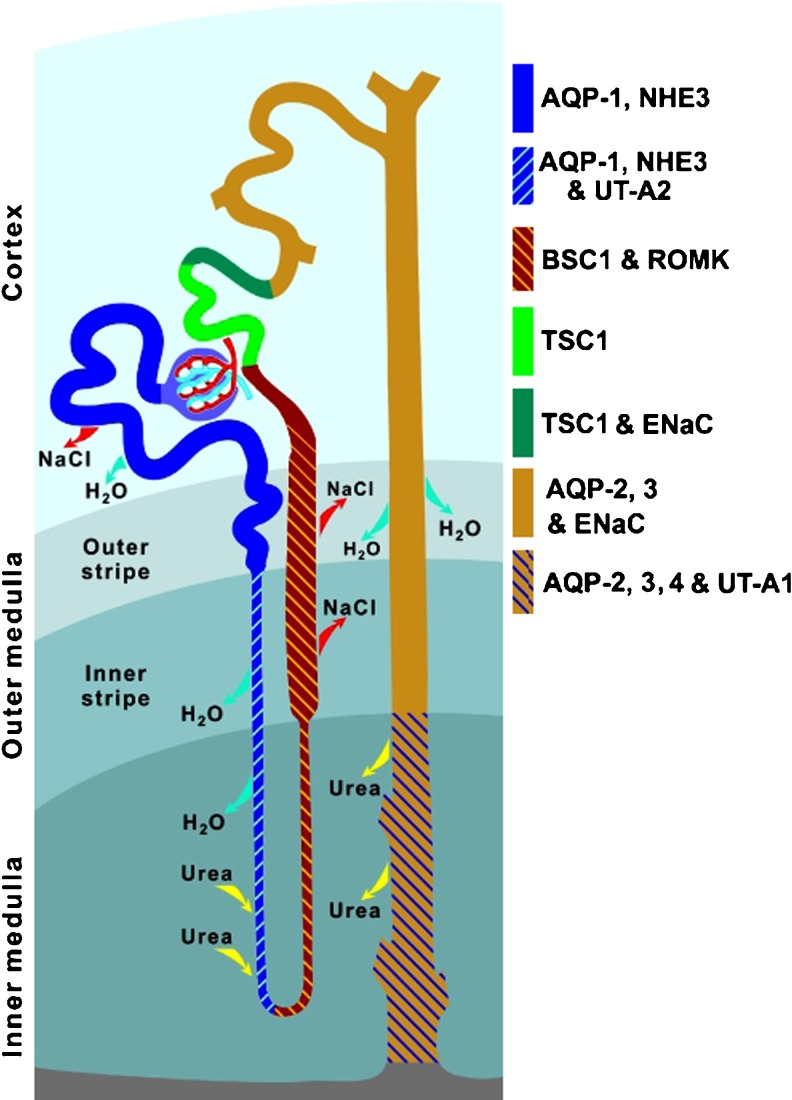
Fig. 4.
Architecture of rat nephron and collecting duct showing segmental localisation of major transporters and channels that play critical roles in water and solute reabsorption. AQP1, AQP2, AQP3, AQP4 Aquaporin water channel isoforms (reproduced from [189] with permission from Springer)
Table 1.
Effects of P2 receptor activation in kidney segments
| Kidney segment | P2 receptor | Action |
|---|---|---|
| Aldosterone-sensitive distal nephron | P2 | Decrease Na+ absorption |
| P2Y2 | Decrease aldosterone sensitivity and K+ excretion (with high K+ diet) | |
| Proximal tubule | P2Y1 | Increase [Ca2+]i, decrease bicarbonate reabsorption |
| P2Y2 | Increase [Ca2+]i and gluconeogenesis | |
| P2Y6 | Increase [Ca2+]i and inositol phosphates | |
| Loop of Henle | ||
| Thin descending limb | P2Y2 | Increase [Ca2+]i |
| Thin ascending limb | P2Y2 | Increase [Ca2+]i |
| Thick ascending limb | P2Y2 | Increase [Ca2+]i, decrease NKCC2 activity |
| Collecting ducts | P2 | Decrease phosphatidylinositol bisphosphate |
| P2X4 | Decrease ENaC activity in high [Na+] | |
| P2Y2 | Increase phospholipase C, decrease ENaC activity and H2O reabsorption | |
| Cortical collecting duct | P2Y2 | Increase [Ca2+]i and phospholipase C, decrease ENaC activity and small conductance K+ channel |
| Inner medullary collecting duct | P2Y2 | Increase [Ca2+]i, prostaglandin E2 protein kinase C and phospholipase C, decrease endothelin-1 release, cAMP and vasopressin-stimulated osmotic water permeability |
Proximal convoluted tubules
P2Y receptors were identified in the renal cortex over 20 years ago [254], and since then, a variety of approaches has begun to catalogue the distribution of specific receptor subtypes. P2Y1 and P2X5 receptors have been immunolocalised to the apical membrane in the S3 segment of the rat pars recta and P2Y4 and P2X6 receptor protein is found basolaterally in the proximal convoluted tubule (PCT). Low level expression of P2X4 protein is also seen in the PCT but not ascribed to a specific membrane domain [375]. Western analysis has shown the P2Y1 receptors in brush-border membrane vesicles from the S2 segment of rat PCT [12].
mRNA has been identified for P2Y1, 2, 4 and 6 receptors in the rat proximal tubule [14,15]. Measurements of Ca2+ transients following application of P2 receptor agonists of varying selectivity support expression of apical P2Y1-like receptors in an immortalised cell line with a proximal phenotype [178] and for basolateral P2Y1 receptors in native rat PCT [14,49]. Bailey and colleagues [15] also reported that basolateral uridine 5′-diphosphate (UDP) was effective in increasing [Ca2+]i, corroborating the presence of P2Y6 receptors. Finally, ATP and UTP were equipotent when applied to rat or rabbit basolateral membranes [14,422], implying P2Y2 or P2Y4 receptors. Notably, the immunohistochemical evidence in rats favours P2Y4 receptors [375].
In vivo, microperfusion studies show that adenosine nucleotides, applied from the luminal side, inhibited NHE3 activity in the rat PCT [11]. Adenosine diphosphate (ADP) was more effective than ATP, suggesting a P2Y1 receptor effect. This was supported by the observation that the P2Y1-selective agonist 2-methylthio ADP also had a potent inhibitory effect, which was blocked by the P2Y1-selective antagonist MRS2179. Addition of ATP to peritubular capillaries in vivo caused an increase in transepithelial bicarbonate reabsorption in rat PCT [70]. Increasing the viscosity of the peritubular perfusate also stimulated bicarbonate reabsorption, and this effect was blocked by peritubular suramin, suggesting P2 receptor mediation. (Shear stress was proposed as the activating factor.) Interestingly, the increase in bicarbonate reabsorption induced by ATP or by raised viscosity could be blocked by a NOS inhibitor. Thus, P2 receptors exert in distinct membrane domains opposing effects on sodium bicarbonate flux. This complexity of paracrine regulation is also observed for Ang II.
In the proximal tubules of P2Y2 receptor knockout mice, the expression of NaPT2 protein is increased but NHE3 abundance is normal [225]. Consistent with this, ATP inhibits phosphate uptake (and mRNA for NaPT2) in primary cultures of rabbit PCTs [211]. In the same preparation, ATP stimulates sodium–glucose co-transport by increasing both SGLT1 and SGLT2 protein expression [210].
A study in rats, using lithium clearance as an index of end proximal tubular fluid delivery, reported that intravenous infusion of the diadenosine polyphosphate, Ap4A, increased lithium clearance almost twofold. This occurred despite a fall in GFR and suggested that proximal tubular reabsorption was markedly reduced [353]. Ap4A can stimulate a number of P2 receptor subtypes, including P2Y1 and P2Y4 receptors [331,410], which are both expressed in the rat proximal tubule (P2Y1 apically, P2Y4 basolaterally); intravenous delivery of the agonist does not allow differentiation between these possibilities.
Effects on transport have also been observed in the amphibian kidney. Cells of the frog proximal tubule contain at least two different K+-selective conductances, both of which are regulated by extracellular ATP [312]. Extracellular ATP raises cytosolic Ca2+ and activates basolateral chloride conductance in Necturus proximal tubules [32]. A more recent paper presented evidence that P2X1 receptors played a role in the regulation of cell volume and K+ channels in frog renal proximal tubule cells [66].
Adenine-based and uracil-based nucleotides can also regulate metabolic functions in the proximal tubule. Early papers showed that ATP inhibited citrate synthase activity in the kidney cortex [33] and stimulated tetraethylammonium transport by rabbit renal brush border membrane vessels [240]. Gluconeogenesis is also stimulated by P2 receptor activation [48,247] and by diadenosine polyphosphates [75]. ATP and UTP were equipotent in stimulating gluconeogenesis, implicating P2Y2 or P2Y4 receptors [247]. Although these authors suggested P2Y2 mediation, these receptors have not been found in rat proximal tubules. P2Y4 receptors are expressed here, making a basolateral P2Y4-mediated effect more likely.
A long-term trophic role for purinergic signalling in the kidney has also been described, where ATP stimulates proliferation of proximal tubule cells via increase in [Ca2+]i and activation of p38, p44/42, MAPKs and cyclin-dependent kinase [212]. It has been claimed that there is paracrine stimulation of vascular smooth muscle proliferation by diadenosine polyphosphates released from proximal tubule cells [169], probably via P2Y receptors [39]. Tubular remodelling is complex, and in cultured mouse, proximal cells subjected to ATP depletion below ~15 % of control died uniformly of necrosis, while cells subjected to ATP depletion by 25 % or more of controls all died by apoptosis [224]. Prior heat stress or Zn2+ inhibits apoptosis in ATP-depleted kidney proximal tubule cells [396,399]. Extracellular ATP protects cultured proximal tubule cells against oxidative stress [213].
Adenosine, acting via P1 receptors, also plays roles in the regulation of proximal tubule activity. Adenosine A1 receptor mRNA was identified in rat nephron segments [423] and also A2A receptor mRNA [398]. Regulation of Na+-3HCO3− co-transport in rabbit proximal tubules via A1 receptors was claimed [360]. A1 receptors were characterised in human proximal tubule epithelial (HK-2) cells [367]. In the pig proximal tubule, Na+-ATPase activity is stimulated via adenosine A2A receptors, thus regulating sodium reabsorption [406]. Transepithelial fluxes of adenosine across human proximal tubule cells involve specific nucleoside transporters [80]. Adenosine is deaminated to inosine in isolated basolateral membranes from proximal tubules, leading to modulation of protein kinase (PK) A activity [8]. Low salt intake increases A1 receptor expression and function in rat proximal tubules [202]. Inhibition of A1 receptors increases fluid uptake in the proximal tubule [278]. Adenosine-induced PKA activation reduced Ang II-induced stimulation of phosphoinositide specific phospholipase (PL) Cβ [109].
The loop of Henle
P2X4 and P2X6 receptors have been immunolocalised to the rat thin descending limb of Henle [375]. Measurements of agonist-induced [Ca2+]i transients indicate a functional pyrimidine receptor in the basolateral membrane of this segment [14], but neither P2Y2 nor P2Y4 protein has been demonstrated. mRNA for P2Y1 and P2Y6 is expressed here [14,15], but again evidence of receptor protein is lacking. In the rat thin ascending limb, the functional evidence for a pyrimidine receptor in the basolateral membrane [14] is, in this case, supported by immunohistochemical confirmation of P2Y2 receptor protein; P2X4 and P2X6 protein expression has also been reported [375]. The physiological role of P2 receptors in the thin limbs is unknown, but this reflects the general absence of information regarding transport in these segments.
The expression and function of P2 receptors in the thick ascending limb (TAL) is increasingly well-defined but there appear to be species differences between rat and mouse. In the rat TAL, basolateral binding sites for adenosine-5′-(γ-thio)-triphosphate (ATPγS) are found [13], but this agonist does not discriminate well between different receptor (both P2Y and P2X) subtypes. P2Y2, P2X4 and P2X6 receptor proteins have been immunolocalised to the TAL [375] and mRNA is expressed here for P2Y1, 2, 4 and 6 subtypes [14,15,188].
Rat TAL segments appear poorly responsive to basolateral application of nucleotides, at least in terms of Ca2+ signalling [14,15]. In the mouse, however, basolateral ATP and UTP each causes large Ca2+ transients, consistent with P2Y2 receptor activation [13,280]. This work is extended with luminal application of ATP or UTP causing calcium transients in mouse medullary TAL (mTAL) perfused in vitro, an effect absent in P2Y2 knockout mice [174]. P2Y2 knockout mice have proven a useful tool for defining the role of these receptors in the kidney. These animals have increased expression of the Na+–K+–2Cl− co-transporter and an augmented natriuretic response to furosemide [309]. These data imply that P2Y2 receptors exert a tonic inhibitory effect on NaCl transport in mouse TAL. A recent study has further indicated that activation of basolateral P2X receptors triggers a marked reduction in NaCl absorption in mouse mTAL [237].
Further evidence directly supports a regulatory role for extracellular nucleotides on TAL function. In suspensions of rat mTAL, ATP increased intracellular NO production in a concentration-dependent manner, a response inhibited by suramin [340]. Flow-induced NO production is dependent on ATP release in TAL [41]. On the basis of agonist profiling, it was argued that the response was mediated primarily by P2X receptors. Further studies from the same group have defined NOS3 (eNOS) as the target for P2 receptor activation [338], in a process requiring activation of Akt1 (serine threonine kinase; aka PKB).
The physiological process coupling P2 receptor activation and NO production may be the flow rate of fluid thought the lumen of the TAL, which regulates nucleotide [174]. Increased flow within the physiological range has previously been shown to stimulate NO production [270] and promote translocation of NOS3 towards the apical membrane in TAL cells. Mechanistically, therefore, ATP is likely to reduce sodium flux in this segment via NO-mediated inhibition of apical transport processes and this has indeed been shown for both Na+–K+–2Cl− co-transporter [269] and Na+/H+ exchange activity [102]. Nevertheless, the primary effect of ATP may be reduction in basolateral Na+–K+–ATPase activity, since ATP reduces oxygen consumption in suspensions of TAL cells [339]. Pharmacological characterisation of this effect found it to be P2X-mediated and NOS-dependent. However, as with nucleotide-stimulated NO production, a weak inhibitory effect on oxygen consumption of UTP was found and there may be some additional P2Y involvement.
Distal tubules
The distal tubule is that segment of the nephron between the macula densa and the first confluence with another tubule in the collecting duct. It is a heterogeneous segment, incorporating the distal convoluted tubule, the connecting duct and the initial portion of the cortical collecting duct (CCD). The transport properties also vary: the distal convoluted tubule is the site of thiazide-sensitive sodium reabsorption and the epithelial sodium channel is expressed in the CCD. The connecting tubule, which is not well-defined in humans, has hybrid transport processes. P2 receptors have been identified in the native distal convoluted tubule and CCD ([13]; see [77]) and the collecting duct is discussed in detail below. However, many of the studies of distal tubule cells have been carried out on cell lines such as A6 (derived from Xenopus kidney) and Madin–Darby canine kidney (MDCK) cell. ATP activates both Cl− and K+ channels in distal nephron epithelial cells from the cell line A6 by a Ca2+-dependent mechanism [243,257,315], probably via P2Y2 receptors [19,250,251] and also in a rabbit distal convoluted tubule cell line [26]. The high-affinity Ca2+ channel of the distal tubule luminal membrane is regulated by ATP, and ATP plays a crucial role in the integrity of the cytoskeleton, which is also involved in the control of Ca2+ channels in this membrane [35,284]. Stretch-released ATP, acting through an autocrine PLC-dependent pathway, masks stretch activation of epithelial sodium channels (ENaC) in A6 distal tubule cells [232].
Connexin hemichannels have been shown to be localised in the luminal membrane of the distal nephron and may be the mechanism underlying ATP release from these cells and play a role in the regulation of salt reabsorption [239]. Multiple P2X receptors (P2X4, P2X5 and P2X6) have been immunolocalised in the distal tubule cells [411]. In particular, P2X4 and P2X6 receptors are present on the basolateral membranes of the rat distal tubule epithelium [375], and it has been claimed that P2X4-like receptors regulate ATP-stimulated epithelial Na2+ channel activity in distal tubule A6 epithelium [432].
The intercalated cells of the distal nephron protrude 1–3 μm further into the lumen than the principal cells; they release ATP in response to mechanical stress [136]. Extracellular nucleotides regulate Na+/H+ exchanger isoform 3 activity in the A6-NHE3-transfected cell line; A6-NHE3 cells are A6 cells selected on the basis of high transepithelial responsiveness to aldosterone [10]. The P2Y1 receptor in A6 cells can increase both cAMP/PKA and Ca2+/PKC intracellular levels, and it is claimed that the PKC pathway is involved in cystic fibrosis transmembrane conductance regulator activation [120]. A6 cells were used to show that aldosterone stimulates ATP release from the basolateral side of distal tubular cells; ATP then acts via purinoceptors to produce contraction of small groups of adjacent epithelial cells, which results in apical swelling that disrupts the ENaC interaction with the F-actin cytoskeleton, opening the channel and hence increasing sodium transport [112]. ‘Aldosterone escape’ refers to the excretion of sodium during high sodium intake; local purinergic tone in the aldosterone-sensitive distal nephron downregulates ENaC activity. P2Y2−/− mice had significantly less increased sodium excretion than wild-type mice [354]. It was concluded that control of ENaC by purinergic signalling is necessary for aldosterone escape and this was supported by another laboratory in a later paper, and in addition, it identified a potential role for NO and prostaglandins in response to aldosterone [434]. Mechanical stimulation of purinergic signalling leads to activation of transient receptor potential vanilloid (TRPV) 4 channels, an important component of the mechano-sensitive response of the aldosterone-sensitive distal nephron [236].
The MDCK cell line has been widely used for studies of distal tubule activities. An early paper showed that exogenous ATP stimulated ion transport in cultured MDCK cells [341]. Electrophysiological studies showed that ATP and UTP hyperpolarise MDCK cells by increasing the K+ conductance [95,206]. A later paper from this group showed that ATP increased [Ca2+]i; calcium then activates K+ channels and thus leads to hyperpolarisation of the cell membrane [281]. Regulation of transepithelial ion transport by two different receptors on the apical membrane of MDCK cells was claimed [430], the data implicating P2Y1 and P2Y2 (or P2Y4) receptors. Lanthanum inhibits UTP-induced Ca2+ mobilization in MDCK cells [164]. P2Y1, P2Y2 and P2Y11 receptor mRNA was shown to be expressed by MDCK cells, but the P2Y2 receptor was dominant in mediating ATP activation of cAMP formation [155,295,373,427]. A later paper showed expression of P2Y6 (as well as P2Y1, P2Y2 and P2Y11) receptors in MDCK cells [142]. Functional P2X7 receptors are also expressed by MDCK cells [163].
Mechanical stimulation of ATP release from MDCK cells (as well as COS-7 and HEK-293 cells) occurs on changing the medium and other experimental protocols with cultured cells, leading to P2Y1 and P2Y2 receptor activation [275]. Activation of P2Y receptors caused strong and persistent shrinkage of MDCK renal epithelial cells [195]. The cloning and tissue expression of MDCK P2Y2 receptors has been reported [426]. Cyclooxygenase (COX)-2 is constitutively expressed by MDCK cells, which participates in P2Y2 receptor-mediated signalling [276]. Transcellular ion currents in ATP-treated MDCK cells are mainly caused by the coupled function of apical and basolateral anion transporters providing transient Cl− secretion [31]. Adenosine induces ATP release via A1 receptors in MDCK cells [244]. Cl− secretion in MDCK monolayers treated with basolateral ATP is triggered by P2Y1 receptors and is mediated by subsequent [Ca2+]i-independent activation of PLA and PKA [3]. Mitochondria play an important role in adenosine-induced ATP release from MDCK cells [245]. Activation of the Na+–K+–Cl− co-transporter in MDCK cells is via Ca2+-independent signalling triggered by apical P2Y2 and basolateral P2Y1 receptors [4]. Rapid pressure changes induce both apical and basolateral ATP release [299]. In a later paper, it was shown that subtle flow changes sensed by the primary cilium induced nucleotide release, which amplified the epithelial [Ca2+]i response [296].
As reported earlier for proximal tubule cells, there is reversible tight junction disassembly during ATP depletion and repletion in MDCK cells [111]. MDCK epithelia spontaneously release ATP resulting in [Ca2+]i oscillations leading to modifications of steady-state renal function [105]. In a later paper, it was shown that the frequency of oscillations was increased by extracellular nucleotides and was decreased if the nucleotides were removed by apyrase [380]. Betaine serves as an osmolyte that is accumulated by tubular cells to maintain osmotic balance. Acute inhibition of the betaine transport by ATP and adenosine in MDCK cells has been reported [185]. Endogenous ATP release inhibits electrogenic Na+ absorption and stimulates Cl− secretion in MDCK cells [419]. Activation of c-Jun and/or p38 contributes to Na+–K+–Cl− co-transport suppression in MDCK cells by exposure to P2Y1 agonists [5]. The proto-oncoprotein SYT(SS18) controls ATP release and regulates cyst formation by polarised MDCK cells, suggesting that SYT plays a vital role in controlling epithelial morphogenesis and might explain the lethality of its loss in the developing embryo [56]. P2 receptor-mediated inhibition of vasopressin (AVP)-stimulated fluid transport and cAMP response in AQP2-transfected MDCK cell has been reported [186].
Adenosine modulates Mg2+ uptake in distal convoluted tubule cells via A1 and A2 receptors [181] and a volume-sensitive-like chloride conductance in a rabbit distal convoluted tubule cell line (DC1) [316]. Both basolateral and apical A1 receptors were shown to mediate sodium transport in cultured A6 cells [234].
Collecting ducts
A large number of P2X and P2Y receptor subtypes have been localised to the rat collecting duct. Immunohistochemistry has identified the expression of P2X1 (intercalated cells only, sodium-restricted only) P2X2, 4, 5 and 6 subunits and P2Y2, 4, 6, 11, 12and13 subtypes [188,375,409]. These immunohistochemical data have largely been validated by mRNA expression profiles in the rat tubule. P2Y1, 2, 4 and 6 metabotropic and P2X4 ionotropic receptor mRNAs were identified in the cortical and outer medullary collecting duct [13,15,409], with P2X1 and 6 receptor mRNAs also reported following dietary sodium restriction [409]. mRNA for P2Y1, 2, 4 and 6 subtypes was identified in the inner medullary collecting duct (IMCD) [188,433]. P2X1, 4, 5, 6 and 7 mRNAs have been localised to the murine cortical and outer medullary collecting duct, indicative of species differences in expression profiles [219]. In humans, only P2X4 has been detected in significant amounts in the collecting duct [50]. Activation of P2X receptors increased both sodium and water excretion [168]. Hypotonic treatment evokes biphasic ATP release across the basolateral membrane of cultured A6 cells [106,172].
Although the functional role of P2 receptors in the collecting duct is complex, several studies, using a combination of approaches, have demonstrated that extracellular nucleotides modulate water and electrolyte handling in this region of the nephron. These effects are important for sodium and water homeostasis since it is in this section of the nephron that urinary excretion is fine-tuned to meet the body’s overall requirements.
Water transport
The regulation of urine concentration and water homeostasis occurs in the collecting duct, under the control of AVP released from the brain. AVP increases the water permeability of the collecting duct by evoking the translocation to the apical membrane of the aquaporin 2 water channel (AQP2): this requires phosphorylation of AQP2 by PKA. In addition to these rapid, non-genomic effects, chronic AVP stimulation increases the water permeability of the collecting duct through the increased transcription of the AQP2 gene. Several studies have demonstrated that nucleotides have an inhibitory affect on the action of AVP in the collecting duct.
The ability of ATP to modulate AVP-induced water permeability in the collecting duct was first demonstrated in the mid-1990s. ATP was shown to reversibly inhibit AVPs’ actions in isolated perfused rabbit CCD and rat IMCD [187,188,314]. Since UTP and ATP were equipotent, the inhibitory effects were attributed to the activation of P2Y2 [188]. P2Y2 couples to the G-protein Gq, and as such, its stimulation causes the activation of PLC, increased inositol trisphosphate production and consequently the mobilization of [Ca2+]i. Both ATP and UTP stimulated intracellular calcium release in rat IMCD [74], and inhibition of calcium mobilization attenuated ATP’s effects in isolated rabbit CCD [314]. Notably, adenylate cyclase, stimulated by AVP and PLC, stimulated by ATP, are mutually inhibitory pathways. PLC activates PKC, which inhibits adenylate cyclase [369]. Indeed the inhibitory effects of P2Y2 receptors have been shown to be associated with PKC-dependent reductions in cAMP [187]. More recently, COX-1-mediated prostaglandin (PG) E2 synthesis has also been implicated in ATPs’ inhibitory effects. ATPγS stimulation of IMCD fractions from hydrated rats resulted in increased PGE2 synthesis [357,401], an effect that was blunted in IMCD fractions from dehydrated rats [357]. Furthermore, enhanced P2Y2 abundance (mRNA and protein) was documented in hydrated rats and was associated with increased PGE2 [191]. Since PGE2 decreases the water permeability of the collecting duct, these data add support to the concept that cross-talk between AVP and ATP provides an additional level of hydrosmotic regulation. Indeed, chronic stimulation of V2R with ddAVP, as would occur during dehydration, reduced P2Y2 abundance in rats [357]. However, the interaction between AVP and ATP may vary in the short and long term. In isolated perfused CCD, acute AVP exposure stimulated nucleotide secretion [264]. A recent paper has shown that ATP counteracts AVP-induced water permeability by increasing AQP2 degradation in lysosomes, preceded by ubiquitin internalization and by decreasing AQP2 gene transcription by reducing AVP-induced cAMP levels [29].
P2Y2 is located on both the apical and basolateral membranes of the collecting duct [188]; however, the demonstration that luminal ATP did not alter AVP-stimulated water permeability in isolated rat IMCD suggested that its effects are mediated by activation of P2Y2 receptors on the basolateral membrane [76]. Recent studies in immortalised mouse collecting duct cells (mpkCCDc14) have provided a possible mechanism for ATP’s inhibitory effect on water transport. Whereas ddAVP application resulted in increased AQP2 immunofluorescence at the apical membrane, ATP and ATPγS resulted in AQP2 internalisation. In addition to basolateral P2Y2 receptors, luminal P2X2 and P2Y4 stimulation may also be involved in ATP’s inhibitory effects. Co-expression of these receptors with AQP2 in Xenopus oocytes resulted in decreased membrane expression of AQP2 and, consequently, attenuated water permeability [415].
Gene deletion studies have substantiated the findings from the pharmacological manipulation. P2Y2−/− mice have increased medullary AQP2 expression and greater basal collecting duct fluid reabsorption than wild-type controls. These data suggest that P2Y2 stimulation provides a tonic inhibition of AVP actions at V2 receptors [309,382,433].
Sodium transport
The effects of extracellular nucleotides on sodium reabsorption in the collecting duct have largely been discerned through the evaluation of amiloride or benzamil-sensitive sodium transport, taken to reflect the activity of the epithelial sodium channel. Koster et al. [197] made the first demonstration that activation of P2 receptors inhibited benzamil-sensitive sodium transport. Using cultured rabbit collecting duct cells, they showed that both apically and basolaterally applied ATP inhibited benzamil-sensitive short circuit currents (an indicator of sodium transport). The inhibition of Na+ transport was dependent on PKC but not Ca2+ signalling. Since ATP and UTP were equipotent, and ADP had no effect, inhibition of ENaC-mediated sodium transport was attributed to activation of P2Y2 [197]. Subsequently, studies in the M-1 mouse collecting duct cell line demonstrated that amiloride-sensitive sodium reabsorption was reduced by both the apical and basolateral application of ATP and UTP. Since ADP and UDP had no effect, these results were consistent with the activation of P2Y2. Indeed, the presence of P2Y2 receptors in the cell line was confirmed by RT-PCR [63]. However, in contrast to the data from cultured rabbit collecting duct cells, the effects of ATP were not dependent on PKC activation [370]. In a different model, mIMCD-K2 mouse collecting duct cells, apical (but not basolateral) nucleotides inhibited sodium reabsorption, an effect, which based on mRNA expression and pharmacological profiling, was attributed to activation of P2Y1, P2Y2, P2X3 and P2X4 receptors [238]. More recently, studies in the mouse IMCD cell line (mIMCD-3) provided a possible mechanism for nucleotide-mediated ENaC inhibition. Extracellular nucleotides caused a reduction in serum- and glucocorticoid-inducible kinase-1 (SGK1) expression and activity. Since SGK promotes the insertion of ENaC into the apical membrane, it was postulated that nucleotides modulate sodium reabsorption through the regulation of SGK1 and, consequently, ENaC activity [220]. In addition, local phosphoinositide levels may modulate basal and acute ENaC activity. In immortalised mouse CCD cells, (mpkCCDe14), phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) concentrations correlated with ENaC activity. Given that the inhibition of both purinergic signalling and PLC rescued ENaC activity, it was postulated that purinergic regulation of PI(4,5)P2 concentrations, through the activation of P2Y receptors, modulates ENaC activity [288]. ENaC in the aldosterone-sensitive distal nephron is under tonic inhibition by local purinergic signalling responding to changes in dietary sodium intake [37]. Table 2 summarises P2 receptor actions on ECaC activity.
Table 2.
A summary of the effects of P2 receptor activation on ENaC activity (reproduced from [414], with permission from Springer)
| Epithelia | Species | P2 receptor involved (localisation) | Effect on I am-s | Mechanism | Reference |
|---|---|---|---|---|---|
| CCD | Mouse | P2Y2 (ap., baso.) | Inhibits | – | [214] |
| CD | Rat | P2X4-like (ap.) | Inhibits | – | [332] |
| CCD/OMCD | Rat | P2Y2/P2Y4 (ap.) | Inhibits | PKC | [413] |
| P2X4/P2X4/6 (ap.) | Inhibits or potentiates | ? or PI3K | [413] | ||
| M1 cell line | Mouse | P2Y2 (ap., baso.) | Inhibits | ↑ [H+]int | [63,370] |
| A6 cell line | Xenopus | P2Y2 (ap.) | Inhibits | PLC, ↓ PIP2, ↓ open prob. | [232] |
| P2X4-like (baso.) | Potentiates | PI3K, ↑ open prob. | [432] | ||
| CCD 1° cultures | Rabbit | P2Y2-like (ap., baso.) | Inhibits | PLC, PKC | [197] |
| mIMCD-K2 cell line | Mouse | P2X3, P2X4, P2Y1, P2Y2 (ap.) | Inhibits | – | [238] |
I am-s amiloride-sensitive current (i.e. ENaC-mediated current), ap. apical membrane, baso. basolateral membrane, ↑ increase, ↓ decrease, PKC protein kinase C, PLC phospholipase C, PI3K phosphoinositide 3-kinase, PIP2 phosphatidylinositol bisphosphate, MAPK mitogen-activated protein kinase, 1° primary cell cultures, open prob. single channel opening probability, CCD cortical collecting duct, OMCD outer medullary collecting duct
In addition to the inhibition of sodium reabsorption, ATP stimulation also increases Cl− secretion. In mIMCD-3 cells, ATP stimulates Cl− conductance by a calcium-dependent mechanism [352]. Extracellular ATP-induced calcium signalling requires both P2X and P2Y receptors in mIMCD-3 cells [417]. The TRPC3 is exclusively expressed in the apical membrane of principal cells of the collecting duct, both in vivo and in the mIMCD cell line. It has been shown that mIMCD-3 cells have two distinct calcium influx pathways: a store-operated channel activated by thapsigargin and basolateral ATP and TRPC3 channels activated by apical ATP [108]. Adenosine, acting on A2B receptors, also enhances Cl− secretion through cystic fibrosis transmembrane conductance regulator (CFTR) in IMCD-K2 cells [302]. The authors proposed that the adenosine receptor pathways might provide one mechanism for enhancing urine NaCl excretion in the setting of high dietary NaCl intake. It was later proposed that P2Y1 and P2Y2 receptors operate in tandem in IMCD cells to enhance urinary NaCl excretion in these conditions [303]. A recent whole kidney study showed that in rats on high sodium intake, adenosine had the potential to enhance renal excretion [201].
Further to the results from cell culture, nucleotide-induced inhibition of sodium transport has also been demonstrated in native collecting duct tissue. In isolated perfused mouse collecting ducts, the application of luminal ATP and UTP caused increased calcium release [67] and inhibition of amiloride-sensitive sodium transport [214], effects which were attributed to the stimulation of P2Y2 receptors. Consistent with P2Y2 receptors being the primary mediator of nucleotide-induced inhibition of ENaC, ATP’s effects on sodium reabsorption were significantly reduced in collecting duct cells isolated from P2Y2−/− receptor mice. However, residual ATP effects implicated the involvement of other P2 receptors [289]. Recently, P2Y2 receptor activation has been shown to increase renal Na+ excretion and decrease blood pressure [310].
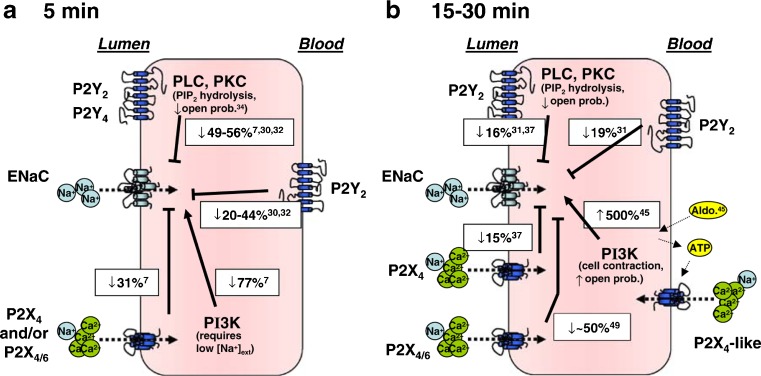
Shirley et al. [332] provided the first in vivo evidence that P2 receptors on the apical membrane of the collecting duct inhibit sodium reabsorption. Rats were maintained on a low sodium diet to induce ENaC expression and urinary recovery of 22Na during microperfusion of the late distal nephron used to assess sodium reabsorption. Notably, despite the firm evidence from in vitro studies in mice showing P2Y2-mediated inhibition of sodium reabsorption in the collecting duct, P2Y2/P2Y4 ‘selective’ agonists had no effect in the rat preparation, and the involvement of a P2X heteromer was hypothesised [332]. More recently, patch-clamp studies in split open rat CCDs have demonstrated the involvement of both apical P2X and P2Y receptors in the modulation of ENaC activity. Activation of P2Y2 and P2Y4 receptors resulted in PLC-dependent inhibition of ENaC activity. Interestingly, activation of P2X4 and P2X4/6 receptors caused an inhibition of ENaC activity when luminal concentrations of sodium were high (145 mM); however, when sodium concentrations were reduced to more physiological levels (50 mM), activation of the receptors potentiated ENaC activity [412,413]. In accordance with a P2X4 component to purinergic regulation of sodium transport is the demonstration that in the ‘distal-like’ cell line, Xenopus A6 cells, activation of basolateral P2X4-like receptors resulted in increased apical membrane insertion of ENaC. These data suggest that there is reciprocal purinergic signalling for the control of sodium transport by both apical and basolateral purinoceptors ([432]; see Fig. 5). These data highlight the complex relationship between apical and basolateral P2 receptors in the modulation of sodium transport in the collecting duct.
Fig. 5.
A summary of known effects of P2 receptor (P2R) activation on ENaC activity taken from experiments using renal principal cells (PCs) or distal nephron-derived cell lines. Relevant references are superscripted. In a, effects where 5 min or less are left between P2R activation and measurements of ENaC activity. All but apical P2X4/6 activation decreases the activity of ENaC. Apical P2X4/6 has the ability to inhibit or potentiate ENaC activity depending on the concentration of luminal Na+. Noteworthy is that basolaterally expressed P2X4 receptors have not been reported to affect ENaC activity. In b, effects where 15–30 min are left between P2R activation and measurement of ENaC activity. All apically expressed P2Rs inhibit ENaC, although the ability of P2Y receptors to inhibit ENaC is less than that in a (from 49–56 to 16 %). The potentiating effect of apical P2X4/6 receptors (when luminal Na+ is low) has not been investigated over a 30-min period, but a potentiating effect of basolaterally expressed P2X4-like receptors has been reported (reproduced from [414], with permission from Springer)
An additional layer of complexity was unveiled with the demonstration that sympathetic nerve varicosities are in close apposition to basolateral membranes of collecting duct epithelial cells of the rat kidney [230]. It was suggested that while luminal responses to autocrine or paracrine release of ATP from epithelial cells may dominate in normal physiological conditions, in pathological states, such as stress and dehydration, ATP released as a cotransmitter from sympathetic nerves may be involved in modulating collecting duct fluid and electrolyte transport via basolateral purinoceptors. It is interesting that in a recent paper, it was shown that chronic renal sympathetic denervation increased the renal tubular natriuretic and diuretic actions of ATP mediated by P2X receptors [199].
Gene deletion studies have focused on sodium homeostasis in P2Y2 null mice. Mice lacking P2Y2 receptors have hypertension and facilitated sodium and water reabsorption of the aldosterone-sensitive distal nephron [309]. Resting ENaC activity was greater in P2Y2−/− receptor mice than controls suggesting that local ATP may be involved in the regulation of basal ENaC activity in the murine collecting duct [289,290]. Additional studies from the same laboratory demonstrated that ENaC downregulation in response to sodium restriction was lost in the P2Y2 null mice, suggesting a role for the receptor in the renal response to altered sodium intake [291]. Despite this, the hypertension was not salt-sensitive, suggesting that these renal changes are compensated for elsewhere.
Extracellular ATP in the CCD not only inhibits ENaC, but also stimulates calcium-activated chloride channels (CACC). It has been shown that ATP stimulates CACC-mediated Cl− adsorption during aldosterone stimulation, and it was suggested that an interplay between purinergic signalling pathways and aldosterone may be involved in regulation of NaCl transport in CCD cells under different states of extracellular fluid volume [304].
Release and metabolism of nucleotides
Release of nucleotides from renal cells was originally suggested from studies of cell lines [328,329,387]; it is now clear that native renal tubules are also able to secrete ATP. Nucleotide release from renal epithelia is both constitutive (suggestive of a ‘purinergic tone’) and activated by mechanical or agonist-induced stimuli. Microelectrodes have measured steady-state ATP concentrations of ~400 nM in the rat kidney cortex and showed that infusion of Ang II caused a rapid and transient increase, consistent with regulated release of nucleotide [277].
In vivo micropuncture experiments indicate that luminal ATP in the PCT is 200–300 nmol/l, higher than concentrations in the glomerular filtrate [388], consistent of release into the urine from epithelial cells. In contrast, concentrations in distal tubule fluid were approximately 30 nmol/l.
In isolated perfused mouse TAL, spontaneous oscillations in [Ca2+]i were dependent on tubular nucleotide release [105]. Furthermore, flow-induced elevations of Ca2+ transients were also dependent on nucleotide release, being blocked by application of an ATP scavenger or the P2 receptor blocker suramin [174]. Agonist-induced nucleotide release has also been demonstrated in the TAL, with intraluminal AVP causing intraluminal nucleotide concentrations to rise to 200–300 nmol/l [264]. This study also showed that AVP could trigger nucleotide secretion from the mouse CCD: intraluminal ATP/UTP concentrations again reached values approaching 300 nmol/l.
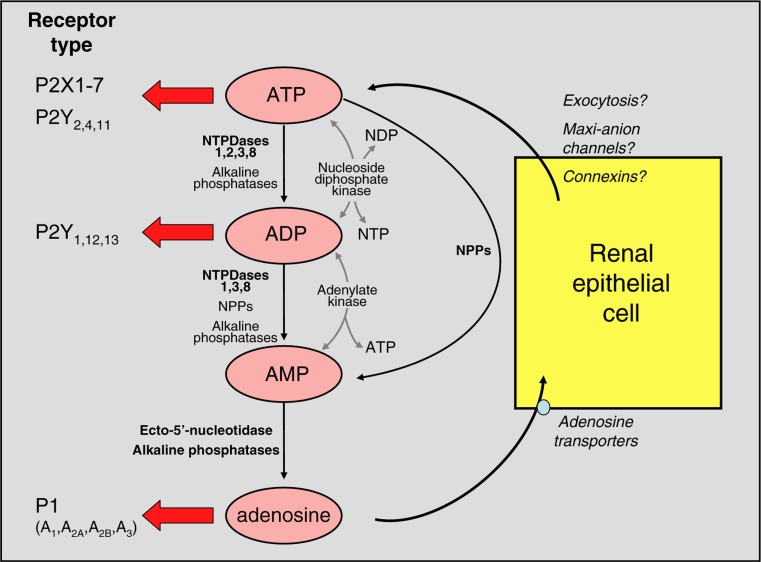
It is open to debate whether the nucleotide concentrations measured intraluminally in the above studies reflect those in the vicinity of the P2 receptors in the cell membrane. Membrane-bound and soluble ectonucleotidases (vide infra) will rapidly metabolize secreted nucleotides, and it has been estimated that bulk-phase measurements could underestimate concentrations at the cell membrane by more than 20-fold, at least in astrocytes [179]. Figure 6 summarises the distribution of ecto-nucleotidases along the nephron.
Fig. 6.
Potential effects of renal ectonucleotidases and consequences for activation of purinoceptor subtypes. The major enzymes involved in each degradative pathway are shown in bold print; for more details, see text. The information given in the figure indicates the relative potencies of ATP and ADP with respect to P2X and P2Y receptor subtypes. At sufficiently high concentrations, ATP can activate all P2 receptors other than P2Y6 and P2Y14. It is important to note that nucleotides derived from other bases are also hydrolysed/synthesised by these enzymes, but have been omitted for clarity. Uracil-based nucleotides are particularly significant: UTP is a potent agonist of P2Y2 and P2Y4 subtypes, and its dinucleotide derivative UDP is the major naturally occurring agonist of the P2Y6 subtype. The mechanism(s) of ATP exit from renal cells has/have not been defined. NDP nucleoside diphosphate, NTP nucleoside triphosphate (reproduced from [333], with permission from Springer)
Flow-induced nucleotide release
In renal cells, a primary cilium protrudes into the tubule lumen and responds to changes in flow by bending and increasing [Ca2+]i [299]. MDCK cells, which have properties of cells in the collecting duct, are ciliated and release ATP in response to increased flow [297]. Removal of the cilium or application of apyrase or suramin to the apical membrane prevented flow-induced nucleotide release.
TRPV4 channels are critical to flow-induced nucleotide release. In isolated thick ascending limbs, ATP secretion is substantially reduced following blockade or siRNA knockdown of TRPV4 [337]. Similar data were obtained in MDCK cells [198], and here TRPV4 colocalises in cilia with polycystin 2. Polycystin 2 is an ion channel devoid of intrinsic mechanosensitive properties but forms with TRPV4 in the cilium base a complex allowing Ca2+ influx when the cilium is bent. This triggers the release of nucleotides and autocrine/paracrine activation of P2 receptors [297].
Mechanism of nucleotide release
The exit route for secretion of nucleotide is not yet resolved: several pathways may contribute and this may differ from segment to segment. Exocytosis of vesicles containing ATP is well established in neurones, and a similar mechanism is observed in cell lines of proximal tubular origin [387]. A variety of channels/transporters may also contribute to nucleotide release. In the macula densa, for example, ATP transport across the basolateral membrane is mediated by maxi-anion channels [22] and contributes to tubuloglomerular feedback (see above). CFTR channels may also mediate ATP release in the kidney [348], but this has not received firm support [296].
More recently, connexin hemichannels have emerged as a route for ATP release from renal cells. Connexins are transmembrane proteins and several members of the family are expressed in the renal vasculature and tubules [123]. The homo- or heteromeric assembly of six connexins forms a connexon: two connexons from neighbouring cells can dock to form a gap junction. Undocked connexons may also function as transmembrane hemichannels and these contribute to cellular ATP secretion [60]. Evidence from Cx30 null mice suggests a similar role in the distal nephron [342]. In wild-type mice, increases in tubular flow or reductions in osmolarity of the bathing solution caused increases in ATP concentration to 10–50 μmol/l: these responses were almost absent in Cx30 knockout mice.
Although intriguing, these data must be assessed cautiously since connexin hemichannels appear to open only under non-physiological conditions [297]. In contrast, pannexins, structurally homologous to connexins [317], are permeable to ATP and can be activated by membrane depolarisations in the physiological range [229]. It now seems likely that pannexin-1 hemichannels mediate ATP release from epithelial cells involved in water and sodium reabsorption [124].
Metabolism by ectonucleotidases
Extracellular nucleotides are rapidly degraded to other nucleotides or nucleosides by surface-located and soluble enzymes (ectonucleotidases). Four families of ectonucleotidases exist: ectonucleoside triphosphate diphosphohydrolases (NTPDases), ectonucleotide pyrophosphatase phosphodiesterases (NPPs), ecto-5′-nucleotidase and alkaline phosphatises, and all are found in the kidney [333]. These enzymes play an important part in renal nucleotide signalling. They will control the availability of nucleotides agonists by hydrolysis and also dictate the signalling environment via generation of nucleotides or nucleosides that preferentially target different P2/P1 receptor subtypes.
The NTPDase family comprises eight members of which four (NTPDases 1, 2, 3 and 8) hydrolyse extracellular nucleotides. NTPDase1 hydrolyses ATP and ADP with almost equal preference, whereas NTPDase2 has a much greater preference for ATP, therefore causing accumulation of ADP; NTPDases 3 and 8 are intermediate in their preference [333]. These differential preferences for hydrolysis are functionally significant. For example, the presence of NTPDase1 will abruptly terminate all P2 receptor stimulation, whereas if NTPDase2 is expressed alone, the conversion of ATP to ADP would potentiate P2Y1, P2Y12 and P2Y13 receptor activation [382].
NTPDase1 is prominent throughout most of the renal vasculature and evident in the thin ascending limb of Henle and medullary CD [190,389]. NTPDase2 has been immunolocalised to Bowman’s capsules and to most nephron segments beyond the proximal tubule [190,389]. NTPDase3 has a similar distribution to NTPDase2 [389], whereas information on NTPDase8 is incomplete.
The NPP family comprises seven members, but only NPPs 1–3 are able to hydrolyse nucleotides. NPPs can hydrolyse ATP and ADP to AMP. Information on the intrarenal distribution of NPPs is limited. NPP1 protein is expressed in proximal tubules and in basolateral membranes of distal tubules [126]. NPP3 is localised to rat glomeruli and the proximal straight tubule but is absent in distal nephron segments [389].
Ecto-5′-nucleotidase catalyses the final stage of nucleotide hydrolysis to nucleoside and is highly expressed in the kidney; it is found in apical membranes of rat PCT and in intercalated cells throughout the distal nephron, as well as the peritubular space [98,207,389].
The physiological role of these enzymes is not clear and experimental evidence is limited. Nevertheless, NTPDase1 in the vasculature prevents ADP-induced platelet aggregation [81] and may also terminate P2X1-mediated vasoconstriction in response to ATP. In the glomerulus, NTPDase1 and NPP3 could influence the ultrafiltration coefficient by controlling P2 signalling in mesangial cells [166].
Renal pathophysiology
Renal injury and failure
It has been proposed that adenosine mediates haemodynamic changes in adult renal failure [57,344]. In human renal cortex, sympathetic nerve stimulation releases ATP and NA, and NA acting on non-neuronal cells also releases ATP. The released ATP has mitogenic effects on glomerular epithelial cells, probably via P2Y1 receptors, and ATP has the potential to contribute to remodelling of the kidney and progression to chronic renal failure, a condition that presents with sympathetic overactivity [391]. While P2X7 receptors are only weakly expressed in healthy glomerulus, following glomerular injury (for example, in diabetes and hypertension), it is significantly upregulated, mainly in podocytes, but also in endothelial and mesangial cells [393]. P2X7 receptors have been shown to participate in disturbed intracellular calcium homeostasis in peripheral blood mononuclear cells of patients with chronic kidney disease [205]. Cyclosporine has become a standard component of the immunosuppressive regime in both solid organ and bone marrow transplantation as well as for the treatment of autoimmune diseases. However, a limiting factor in its use has been the development of nephrotoxicity and hypertension in many patients. Using the adriamycin nephropathy mouse model of chronic renal injury, regulatory T cells were shown to participate in CD39-mediated protection from renal injury [397]. A Katp channel opener, nicorandil, reduces chronic renal injury by targeting podocytes and macrophages [365].
Primary idiopathic nephrotic syndrome is a source of morbidity in children; in some cases, mutations in podocyte genes explain the proteinuria, although it has been proposed that the condition is linked to T cell immunity. It has been claimed that ATP plays a key role in the regulation of innate immunity in this disease, and the effects of adenosine are reduced by the decreased expression of ectonucleotidase in this syndrome [24].
A mechanism has been proposed linking renal tubular epithelial cell death and injury to renal interstitial peritubular fibroblasts, which suggests that P2X7 receptors mediate deleterious renal epithelial–fibroblast cross-talk [293]. In another paper from this group, it was shown that necrotic renal proximal epithelial cells stimulated the expression of P2X7 receptors in renal interstitial fibroblasts through activation of the ERK signalling pathway [294]. It has been suggested that P2X7 receptor antagonists could offer innovative preventive and therapeutic modalities for the treatment of morbidity and mortality associated with kidney injury [429]. ATP binding enhanced the activity of ClC-5, the transporter mutated in Dent disease, a disease affecting the renal proximal tubule [405]. Arterial calcification is prevalent in patients with chronic kidney disease and ATP signalling appears to be involved (see review by [90]).
It has been reported that A2B receptor-mediated induction of interleukin (IL)-6 contributes to renal fibrogenesis, and the authors suggested that this receptor may have therapeutic potential for treatment of chronic kidney disease [65]. A2B receptor activation protects against acute kidney injury via inhibition of neutrophil-dependent release of tumour necrosis factor-α [116]. Dendritic cells activated by A2A receptor agonists attenuate acute renal injury [221]. A review describing adenosine generation and signalling during acute kidney injury is available [20].
Polycystic kidney disease
PKD is a genetic disorder associated with abnormal proliferation of tubular cells of the adult nephron [53]. This leads to progressive dilation of tubules, which eventually become encapsulated in fluid-filled cysts that compress and destroy neighbouring tissue. ATP is released and reaches high concentrations within cysts [329,416]. Autosomal dominant polycystic disease (ADPKD) is characterised by bilateral cyst formation in the kidneys. It is a genetic disease caused by the mutation of either PKD1 or PKD2, which encodes for polycystin. Polycystin-2 is localised to the cilia of mouse and human vascular endothelial cells, which sense fluid flow via purinergic receptor activation and NO release, but in ADPKD patients, it is absent [1]. The authors suggest that aberrant expression of polycistin-2 in cilia could promote high blood pressure because of inability to synthesise NO in response to shear stress produced by changes in blood flow. Expression of polycistin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells [137]. The nematode Caenorhabditis elegans has been used as an animal model for studying basic molecular mechanisms underlying human ADPKD; the C. elegans LOV-1 and PKD-2 proteins are homologues of human PC-1 and PC-2 proteins. Using this model, it was claimed that ciliary localised ATP synthase may play a role in polycystin signalling [141]. The involvement of nucleotide release in both fluid flow and pressure responses and its role in altered mechanosensory transduction in the aetiology of PKD is discussed in a recent review [279]. Using collecting duct principal cells derived from the Oak Ridge polycystic kidney mouse model of ARPKD, it was shown that the loss of apical monocilia impairs flow-induced ATP secretion across the apical cell surface and ATP-induced calcium signals [140].
P2X7 receptors were shown to be expressed in collecting duct cysts in the cpk/cpk mouse model of congenital PKD [132], where it mediates cyst development [133]. In a later paper from this group P2Y1, P2Y2, P2Y4, P2Y6, P2X5 and P2X7 receptors were detected on the epithelial cells lining renal cysts in the Han:SPRD cy/+ rat model of ADPKD [376]. The expression levels of mRNA and protein for P2Y2, P2Y6 and P2X7 receptors increased significantly as the disease developed, all mediating mechanisms potentially relevant to cyst growth and cell turnover. Blockade of the P2X7 receptor with oxidised ATP (or A-438079) reduced cyst formation via ERK-dependent pathways in a zebrafish model of PKD [52]. It was suggested that nucleotides present in the cyst lumen fluid could activate P2Y receptors to increase the growth of MDCK-derived cysts [377]. Attenuated flow-induced ATP release contributes to the absence of flow-sensitive, purinergic [Ca2+]i signalling in human ADPKD cyst epithelial cells [421]. Monocilia of ductal epithelia are a major focus in PKD. It has been proposed that ATP is released from monocilia to act as an autocrine regulator via P2 receptors [138]. Deficiency of PKD-1 gene expression increases A3 receptors in human renal cells [2]. The potential for cyst formation has been examined for two MDCK cell subclones, C7 cells that resemble principal cells and C11 cells that resemble α-intercalated cells [36]. It was concluded that principal rather than intercalated cells had the ability to form cysts, based on a synergism of cAMP and ATP signalling in enhancing apical fluid secretion.
Ischaemia
There is rapid early decline of proximal tubular ATP in ischaemic acute renal failure [336]. Over-expression of manganese superoxide dismutase protects against ATP depletion-mediated cell death of proximal tubule cells occurring with ischaemia/reperfusion injury during kidney transplantation [62].
Human antigen R (HuR) is a nucleocytoplasmic shuttling protein that binds and stabilizes mRNAs containing adenine- and uridine-rich elements. ATP depletion from proximal tubule cells during ischaemia results in heightened HuR protein translation and suggests a role for HuR in protecting kidney epithelia from injury during ischaemic stress [175]. STAT 3, a member of the family of signal transducers and activators of transcription, inhibits apoptosis of human proximal tubular epithelial cells induced by ATP depletion during ischaemia [395].
ATP depletion of MDCK cells has been used as an in vitro model of renal ischaemia and Rho GTPase signalling shown to regulate tight junction assembly and protect tight junctions during ATP depletion [110]. It has been claimed that in renal ischaemia, the ATP depletion-induced alteration in membrane transport function and cell viability are due to reactive oxygen species generation and cytosolic PLA2 activation in proximal tubular cells [209]. Hepatocyte growth factor has been shown to enhance recovery from renal tubular ischaemia and to promote adhesion of the ATP-depleted renal collecting duct cell line, mIMCD-3, in a MAPK-dependent manner [228]. Restoration after ischaemia by perfusion of ATP-MgCl2 was described early [104,335]. A3 receptor knock-out mice are protected against ischaemic renal failure [208]. Ischaemia remodels filamentous actin leading to desquamation of proximal tubular epithelial cells via ATP depletion-induced p38 MAPK–HSP27 signalling [72]. These findings are of potential pathophysiological importance for understanding the overall involvement of the actin cytoskeleton in cell detachment during ischaemia. Deficiency or inhibition of the ectonucleotidase CD73 protected against kidney ischaemia–reperfusion injury and the authors suggested that AMP may play a direct protective role against this type of injury [305]. In a later study, it was claimed that CD73 protects the kidney from ischaemia–reperfusion injury through adenosine production and reduction of free radicals [177]. The role of adenosine in protection from acute kidney injury has been discussed [425].
Nephritis
Glomerulonephritis is a leading cause of end-stage renal disease and its treatment is non-specific immunosuppression, which has significant adverse side effects. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptors has been demonstrated in both experimental and human glomerulonephritis and opens up the possibility that P2X7 receptor antagonists may have therapeutic potential [378]. Indeed, a later paper from this group showed that P2X7 receptor deficiency attenuated renal injury in experimental glomerulonephritis [368]. In P2Y1 receptor knock-out mice, there is protection against capillary loss, fibrosis and death by renal failure during experimental crescentic glomerulonephritis [134]. Treatment with an adenosine uptake inhibitor attenuates glomerulonephritis in mice [262]. Activation of A2A receptors has been proposed as a treatment for macrophage-mediated experimental glomerulonephritis ([100]; see also [85]). In experimental mesangial proliferative glomerulonephritis in the rat (anti-Thy-1 model), there is a pronounced mesangial cell proliferative response leading to glomerular hypercellularity. In the anti-Thy-1 model, PPADS specifically and dose-dependently reduced early (day 3) but not late (day 8) mesangial cell proliferation [313]. The authors also reported that P2Y2 and P2Y6 receptors showed a transient marked increase in expression during anti-Thy-1 disease. There are significant pro-inflammatory activities of extracellular nucleotides during anti-Thy-1 nephritis, and an anti-inflammatory role for glomerular ecto-nucleotidases suggested to convert ATP and ADP into anti-inflammatory adenosine [292]. Tuboglomerular feedback is diminished in Thy-1 nephritic rats, but this is improved by exogenous 5′-nucleotidase [363].
Lupus nephritis is a frequent and potentially fatal complication of systemic lupus erythematosus. A2A receptor activation reduced inflammation in the kidneys of MRL/lpr mice and it was suggested that this could be considered as a novel therapeutic approach for human lupus nephritis [431]. In a recent paper, it has been reported that P2X7 receptor blockade attenuates lupus nephritis by inhibiting NLRP3/ASC/caspase-1 inflammasome activation [436].
Hypertension
Hypertension is a feature of chronic renal disease and it has been claimed that this is largely due to sympathetic overactivity triggered by afferent signals emanating from the kidney and resetting sympathetic tone by stimulation of hypothalamic centres [268]. Both essential hypertensive patients and patients with renal artery stenosis show a dose-dependent vasodilation following adenosine infusion [407,408]. Enhanced increase in [Ca2+]i was found in mesangial cells in response to ATP in spontaneously hypertensive rats [226] and NO can increase P2Y receptor resensitization [227].
The kidney plays a dominant role in the development and maintenance of hypertension and the shift of renal autoregulation towards higher pressure underlying sodium-insensitive hypertension [362]. There is an enhanced P2 receptor-mediated vasoconstriction of afferent and efferent arterioles in chronic Ang II-induced hypertensive rats [94]. It was claimed that gap junction Cx37 and Cx40 transduce purinergic signals mediating renal autoregulation [364]. In a recent paper, it was shown that ATP release through Cx30 is part of a local regulatory system intrinsic to the aldosterone-sensitive distal nephron important for control of sodium excretion [246]. They showed that loss of paracrine ATP feedback regulation of ENaC to respond to changes in sodium levels contributed to salt-sensitive hypertension in Cx30−/− mice. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during Ang II-induced hypertension [114]. Renal interstitial adenosine is increased in Ang II-induced hypertensive rats [93]. Mice lacking P2Y2 receptors have salt-resistant hypertension [309]. There is an exaggerated response to adenosine in kidneys from high salt-fed rats [223]. While the P2X7 receptor is only weakly expressed in healthy kidney glomerulus, it is significantly upregulated in ren-2 transgenic hypertension [393]. In a recent paper, it was shown that P2X7 receptor antagonism prevented the development of salt-sensitive hypertension and renal injury in Dahl salt-sensitive rats [176]. ATP-induced hypotension is associated with alteration of sympathetic nerve activity mediated through vagal afferent pathways [366]. It has been reported that there is an increase in ATP hydrolysis resulting from an increase in ecto-5′-nucleotidase activity and accumulation of adenosine in the kidney of hypertensive animals [96]. A high salt diet given to Ang II hypertensive rats significantly impairs autoregulation of rat juxtamedullary afferent arterioles, which is associated with a decline in afferent arteriolar reactivity to ATP mediated by P2X1 receptors [154]. However, reactivity to UTP via P2Y2 receptors or to adenosine via P1 receptors was unchanged. It was suggested that understanding this mechanism may lead to therapeutic interventions to prevent renal decline early in the hypertension progression. The hypertensive responses to L-NG-nitroarginine methyl ester and Ang II were attenuated in A1 receptor knockout mice [99]. Adenosine, acting via A1 receptors in the proximal tubule, modulates deoxycorticosterone acetate–salt hypertension in mice [404].
Diabetic nephropathy
Diabetic nephropathy is the most common cause of end-stage renal disease. Lack of A1 receptors augments diabetic hyperfiltration and glomerular injury [83]. Treatment with adenosine receptor agonists protected diabetic rats from nephropathy by exerting hypoglycaemic and antioxidant effects as well as reducing gene expression of proinflammatory cytokines [78]. A recent review is concerned with the targeting of adenosine signalling via A2B antagonists for the treatment of diabetic nephropathy [301].
Increased ATP release induced by hyperglycaemia may contribute to mesangial extracellular matrix expansion that occurs in diabetes [346]. Increased expression of P2X7 receptors was shown in the streptozotocin diabetic rat model, mainly in podocytes but also in endothelial and mesangial cells [393]. Diabetic milieu, represented by high glucose concentrations, affects purinergic modulation of glucose transport into podocytes, which may play a role in the development of diabetic podocytopathy [182]. P2X4 receptors mediate high glucose-induced activation of the NOD-like receptor 3 inflammasome, regulate IL-1 family cytokine secretion and cause the development of tubulointerstitial inflammation in diabetic nephropathy [54].
A major limitation in chronic lithium treatment for bipolar patients is the development of nephrogenic diabetes insipidus, which manifests as polyuria, polydipsia and reduced ability to concentrate urine associated with a lack of response of the medullary collecting duct to AVP. Genetic deletion of the P2Y2 receptor offers significant resistance to the development of lithium-induced nephrogenic diabetes insipidus polyuria [435].
Inflammation
ATP, via P2Y2 receptors, inhibits inducible NOS (iNOS) in cultured mesangial cells and it has been suggested that it may play a critical role for iNOS expression and synthesis of NO during glomerular inflammatory disorders [249]. ATP has also been claimed to produce long-term pro-inflammatory effects in rat mesangial cells via it degradation to adenosine and action on A2A receptors, resulting in PLA2 and cytokine induction [322]. The P2X7 receptor plays a major role in the inflammatory process, since activation leads to release of inflammatory cytokines [55,204]. Activation of A2A receptors prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation [101] and of obstructive nephropathy [418].
Hyper- and hypothyroidism
ATP-mediated vasoconstriction of perfused rat kidney was increased in hyperthyroid kidneys and severely attenuated in kidneys from hypothyroid rats and the vasodilator response abolished [386]. Experimental hyperthyroidism modifies binding of A1 and A2A agonists in rat kidney, and this might be responsible for the incapacity for urine concentration seen in the hypothyroid kidney [92].
Nephrotoxicant injury
Reduction of drug-induced nephrotoxicity by ATP-MgCl2 was reported [355]. PKB activation increases intracellular ATP levels and decreases necrosis in proximal tubular cells injured by nephrotoxicants [330]. ATP levels in kidneys are decreased by ingestion of tullidora, a poisonous plant, which may partly explain its acute toxic effects and mortality [173]. Cisplatin is a potent anti-neoplastic drug whose clinical use is limited by its ability to induce nephrotoxicity. It has been suggested that adenosine may be involved in the haemodynamic changes in the kidney induced by cisplatin [128]. Cisplatin upregulates A1 receptors in rat kidney [25] and A1 receptor antagonists have a protective effect against cisplatin-induced acute kidney injury in rats [107]. Adenosine antagonists have protective effects against acute renal failure [156,253,358]. A1 receptor antagonists are effective against the development of nephrotoxicity by cyclosporine, an immunosuppressive agent [18]. Cyclosporine increases plasma levels of adenosine in kidney transplant patients [43]. A3 receptor antagonism is effective against acute tacrolimus toxicity [241].
Renal transplants
An ATP release assay has been used to help determine the risks of developing infection or rejection in renal transplant recipients [42]. The beneficial effects of adenosine and phosphate in kidney transplant preservation have been claimed [23].
Summary
Purines have been shown to control TGF, regulate renin release and regulate tubular ion and water transport. There is autocrine/paracrine release of ATP from epithelial and endothelial cells, as well as release as a cotransmitter from sympathetic nerves. Purinergic signalling is involved in kidney disorders, including PKD, nephritis, hypertension, diabetes and nephrotoxicant injury, and purinergic therapeutic strategies are being explored to treat these diseases.
Acknowledgments
The authors are very grateful to Dr Gillian E. Knight for her invaluable assistance in the preparation of this review article. The University/British Heart Foundation Centre for Cardiovascular Science is funded by The British Heart Foundation, Kidney Research UK.
References
- 1.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguiari G, Varani K, Bogo M, Mangolini A, Vincenzi F, Durante C, Gessi S, Sacchetto V, Catizone L, Harris P, Rizzuto R, Borea PA, Del Senno L. Deficiency of polycystic kidney disease-1 gene (PKD1) expression increases A3 adenosine receptors in human renal cells: implications for cAMP-dependent signalling and proliferation of PKD1-mutated cystic cells. Biochim Biophys Acta. 2009;1792:531–540. doi: 10.1016/j.bbadis.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Akimova AO, Bourcier N, Taurin S, Bundey RA, Grygorczyk K, Gekle M, Insel PA, Dulin NO, Orlov SN. Cl− secretion in ATP-treated renal epithelial C7-MDCK cells is mediated by activation of P2Y1 receptors, phospholipase A2 and protein kinase A. J Physiol. 2005;568:789–801. doi: 10.1113/jphysiol.2005.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimova OA, Grygorczyk A, Bundey RA, Bourcier N, Gekle M, Insel PA, Orlov SN. Transient activation and delayed inhibition of Na+, K+, Cl − cotransport in ATP-treated C11-MDCK cells involve distinct P2Y receptor subtypes and signaling mechanisms. J Biol Chem. 2006;281:31317–31325. doi: 10.1074/jbc.M602117200. [DOI] [PubMed] [Google Scholar]
- 5.Akimova OA, Taurin S, Dulin NO, Orlov SN. Purinergic inhibition of Na+, K+, Cl − cotransport in C11-MDCK cells: role of stress-activated protein kinases. Purinergic Signal. 2008;4:183–191. doi: 10.1007/s11302-007-9057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amann K, Veelken R. Mechanisms and consequences of sympathetic hyperactivity in renal disease. Clin Nephrol. 2003;60(Suppl 1):S81–S92. [PubMed] [Google Scholar]
- 7.Apicella L, Fabbretti E. P2X3 receptor expression by HEK cells conditions their survival. Purinergic Signal. 2012;8:295–300. doi: 10.1007/s11302-011-9285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assaife-Lopes N, Wengert M, Pinheiro AA, Landgraf SS, Paes-de-Carvalho R, Leao-Ferreira LR, Caruso-Neves C. Adenosine deamination to inosine in isolated basolateral membrane from kidney proximal tubule: implications for modulation of the membrane-associated protein kinase A. Arch Biochem Biophys. 2009;486:44–50. doi: 10.1016/j.abb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Awad AS, Rouse M, Liu L, Vergis AL, Rosin DL, Linden J, Sedor JR, Okusa MD. Activation of adenosine 2A receptors preserves structure and function of podocytes. J Am Soc Nephrol. 2008;19:59–68. doi: 10.1681/ASN.2007030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagorda A, Guerra L, Di SF, Helmle-Kolb C, Favia M, Jacobson KA, Casavola V, Reshkin SJ. Extracellular adenine nucleotides regulate Na+/H+ exchanger NHE3 activity in A6-NHE3 transfectants by a cAMP/PKA-dependent mechanism. J Membr Biol. 2002;188:249–259. doi: 10.1007/s00232-001-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey MA. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Ren Physiol. 2004;287:F789–F796. doi: 10.1152/ajprenal.00033.2004. [DOI] [PubMed] [Google Scholar]
- 12.Bailey MA, Shirley DG. Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signal. 2009;5:473–480. doi: 10.1007/s11302-009-9149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey MA, Hillman KA, Unwin RJ. P2 receptors in the kidney. J Auton Nerv Syst. 2000;81:264–270. doi: 10.1016/s0165-1838(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 14.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int. 2000;58:1893–1901. doi: 10.1111/j.1523-1755.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 15.Bailey MA, Imbert-Teboul M, Turner C, Srai SK, Burnstock G, Unwin RJ. Evidence for basolateral P2Y6 receptors along the rat proximal tubule: functional and molecular characterization. J Am Soc Nephrol. 2001;12:1640–1647. doi: 10.1681/ASN.V1281640. [DOI] [PubMed] [Google Scholar]
- 16.Bailey MA, Turner CM, Hus-Citharel A, Marchetti J, Imbert-Teboul M, Milner P, Burnstock G, Unwin RJ. P2Y receptors present in the native and isolated rat glomerulus. Nephron Physiol. 2004;96:79–90. doi: 10.1159/000076753. [DOI] [PubMed] [Google Scholar]
- 17.Bailey MA, Unwin RJ, Shirley DG. P2X receptors and kidney function. WIREs Memb Transplant Signal. 2012;1:503–511. [Google Scholar]
- 18.Balakrishnan VS, von Ruhland CJ, Griffiths DF, Coles GA, Williams JD. Effects of a selective adenosine A1 receptor antagonist on the development of cyclosporin nephrotoxicity. Br J Pharmacol. 1996;117:879–884. doi: 10.1111/j.1476-5381.1996.tb15275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banderali U, Brochiero E, Lindenthal S, Raschi C, Bogliolo S, Ehrenfeld J. Control of apical membrane chloride permeability in the renal A6 cell line by nucleotides. J Physiol. 1999;519(Pt 3):737–751. doi: 10.1111/j.1469-7793.1999.0737n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 21.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci U S A. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell PD, Komlosi P, Zhang ZR. ATP as a mediator of macula densa cell signalling. Purinergic Signal. 2009;5:461–471. doi: 10.1007/s11302-009-9148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belzer FO, Sollinger HW, Glass NR, Miller DT, Hoffmann RM, Southard JH. Beneficial effects of adenosine and phosphate in kidney preservation. Transplantation. 1983;36:633–635. doi: 10.1097/00007890-198336060-00008. [DOI] [PubMed] [Google Scholar]
- 24.Bertelli R, Bodria M, Nobile M, Alloisio S, Barbieri R, Montobbio G, Patrone P, Ghiggeri GM. Regulation of innate immunity by the nucleotide pathway in children with idiopathic nephrotic syndrome. Clin Exp Immunol. 2011;166:55–63. doi: 10.1111/j.1365-2249.2011.04441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhat SG, Mishra S, Mei Y, Nie Z, Whitworth CA, Rybak LP, Ramkumar V. Cisplatin up-regulates the adenosine A1 receptor in the rat kidney. Eur J Pharmacol. 2002;442:251–264. doi: 10.1016/s0014-2999(02)01510-8. [DOI] [PubMed] [Google Scholar]
- 26.Bidet M, De Renzis G, Martial S, Rubera I, Tauc M, Poujeol P. Extracellular ATP increases [CA2+]i in distal tubule cells. I. Evidence for a P2Y2 purinoceptor. Am J Physiol Ren Physiol. 2000;279:F92–F101. doi: 10.1152/ajprenal.2000.279.1.F92. [DOI] [PubMed] [Google Scholar]
- 27.Blantz RC, Singh P, Deng A, Thomson SC, Vallon V. Acute saline expansion increases nephron filtration and distal flow rate but maintains tubuloglomerular feedback responsiveness: role of adenosine A1 receptors. Am J Physiol Ren Physiol. 2012;303:F405–F411. doi: 10.1152/ajprenal.00329.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch. 2008;456:1005–1024. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boone M, Kortenoeven ML, Robben JH, Tamma G, Deen PM. Counteracting vasopressin-mediated water reabsorption by ATP, dopamine, and phorbol esters: mechanisms of action. Am J Physiol Ren Physiol. 2011;300:F761–F771. doi: 10.1152/ajprenal.00247.2010. [DOI] [PubMed] [Google Scholar]
- 30.Booth JW, Tam FW, Unwin RJ. P2 purinoceptors: Renal pathophysiology and therapeutic potential. Clin Nephrol. 2012;78:154–163. doi: 10.5414/cn107325. [DOI] [PubMed] [Google Scholar]
- 31.Bourcier N, Grygorczyk R, Gekle M, Berthiaume Y, Orlov SN. Purinergic-induced ion current in monolayers of C7-MDCK cells: role of basolateral and apical ion transporters. J Membr Biol. 2002;186:131–143. doi: 10.1007/s00232-001-0141-y. [DOI] [PubMed] [Google Scholar]
- 32.Bouyer P, Paulais M, Cougnon M, Hulin P, Anagnostopoulos T, Planelles G. Extracellular ATP raises cytosolic calcium and activates basolateral chloride conductance in Necturus proximal tubule. J Physiol. 1998;510:535–548. doi: 10.1111/j.1469-7793.1998.535bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JN, Payne BL, Smith TM, Sulya LL. Effects of ATP, NADH and aldosterone on citrate synthase from bovine kidney cortex. Int J Biochem. 1977;8:437–440. [Google Scholar]
- 34.Brown R, Ollerstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 35.Brunette MG, Mailloux J, Hilal G. ATP directly enhances calcium channels in the luminal membrane of the distal nephron. J Cell Physiol. 1999;181:416–423. doi: 10.1002/(SICI)1097-4652(199912)181:3<416::AID-JCP5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 36.Buchholz B, Teschemacher B, Schley G, Schillers H, Eckardt KU. Formation of cysts by principal-like MDCK cells depends on the synergy of cAMP- and ATP-mediated fluid secretion. J Mol Med (Berl) 2011;89:251–261. doi: 10.1007/s00109-010-0715-1. [DOI] [PubMed] [Google Scholar]
- 37.Bugaj V, Sansom SC, Wen D, Hatcher LI, Stockand JD, Mironova E. Flow-sensitive K+-coupled ATP secretion modulates activity of the epithelial Na+ channel in the distal nephron. J Biol Chem. 2012;287:38552–38558. doi: 10.1074/jbc.M112.408476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bührle CP, Scholz H, Nobiling R, Taugner R. Junctional transmission in renin-containing and smooth muscle cells of the afferent arteriole. Pflugers Arch. 1986;406:578–586. doi: 10.1007/BF00584024. [DOI] [PubMed] [Google Scholar]
- 39.Burnstock G. Purinergic signalling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 41.Cabral PD, Garvin JL. Flow-induced NO production is dependent on TRPV4 activation and ATP release in thick ascending limbs. FASEB J. 2011;25:666. [Google Scholar]
- 42.Cadillo-Chávez R, de Echegaray S, Santiago-Delpín EA, Rodríguez-Trinidad AT, Camacho-Carrazo B, Alfaro T, Saavedra-Pozo M, Carrasquillo L, González-Caraballo ZA, Morales-Otero LA. Assessing the risk of infection and rejection in Hispanic renal transplant recipients by means of an adenosine triphosphate release assay. Transplant Proc. 2006;38:918–920. doi: 10.1016/j.transproceed.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 43.Capecchi PL, Rechichi S, Lazzerini PE, Collini A, Guideri F, Ruggieri G, Carmellini M, Laghi-Pasini F. Cyclosporin and tacrolimus increase plasma levels of adenosine in kidney transplanted patients. Transpl Int. 2005;18:289–295. doi: 10.1111/j.1432-2277.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- 44.Carlström M, Wilcox CS, Welch WJ. Adenosine A2 receptors modulate tubuloglomerular feedback. Am J Physiol Ren Physiol. 2010;299:F412–F417. doi: 10.1152/ajprenal.00211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll MA, Cheng MK, Liclican EL, Li J, Doumad AB, McGiff JC. Purinoceptors in renal microvessels: adenosine-activated and cytochrome P450 monooxygenase-derived arachidonate metabolites. Pharmacol Rep. 2005;57(Suppl):191–195. [PubMed] [Google Scholar]
- 46.Castrop H. Mediators of tubuloglomerular feedback regulation of glomerular filtration: ATP and adenosine. Acta Physiol (Oxf) 2007;189:3–14. doi: 10.1111/j.1748-1716.2006.01610.x. [DOI] [PubMed] [Google Scholar]
- 47.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cha SH, Jung KY, Endou H. Effect of P2Y-purinoceptor stimulation on renal gluconeogenesis in rats. Biochem Biophys Res Commun. 1995;211:454–461. doi: 10.1006/bbrc.1995.1835. [DOI] [PubMed] [Google Scholar]
- 49.Cha SH, Sekine T, Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2. Am J Physiol. 1998;274:F1006–F1014. doi: 10.1152/ajprenal.1998.274.6.F1006. [DOI] [PubMed] [Google Scholar]
- 50.Chabardes-Garonne D, Mejean A, Aude JC, Cheval L, Di SA, Gaillard MC, Imbert-Teboul M, Wittner M, Balian C, Anthouard V, Robert C, Segurens B, Wincker P, Weissenbach J, Doucet A, Elalouf JM. A panoramic view of gene expression in the human kidney. Proc Natl Acad Sci U S A. 2003;100:13710–13715. doi: 10.1073/pnas.2234604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford APDW, Townsend-Nicholson A, Burnstock G. Localization of the P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol. 1998;274:F799–F804. doi: 10.1152/ajprenal.1998.274.4.F799. [DOI] [PubMed] [Google Scholar]
- 52.Chang MY, Lu JK, Tian YC, Chen YC, Hung CC, Huang YH, Chen YH, Wu MS, Yang CW, Cheng YC. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol. 2011;22:1696–1706. doi: 10.1681/ASN.2010070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. J Cell Biol. 2010;191:701–710. doi: 10.1083/jcb.201006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45:932–943. doi: 10.1016/j.biocel.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Chittezhath M, Frump AL, Jourquin J, Lobdell N, Eid JE. The proto-oncoprotein SYT (SS18) controls ATP release and regulates cyst formation by polarized MDCK cells. Exp Cell Res. 2008;314:3551–3562. doi: 10.1016/j.yexcr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Churchill PC, Bidani AK. Hypothesis: adenosine mediates hemodynamic changes in renal failure. Med Hypotheses. 1982;8:275–285. doi: 10.1016/0306-9877(82)90124-4. [DOI] [PubMed] [Google Scholar]
- 58.Churchill PC, Churchill MC. A1 and A2 adenosine receptor activation inhibits and stimulates renin secretion of rat renal cortical slices. J Pharmacol Exp Ther. 1985;232:589–594. [PubMed] [Google Scholar]
- 59.Churchill PC, Ellis VR. Purinergic P2Y receptors stimulate renin secretion by rat renal cortical slices. J Pharmacol Exp Ther. 1993;266:160–163. [PubMed] [Google Scholar]
- 60.Cotrina ML, Lin JH, ves-Rodrigues A, Liu S, Li J, zmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95(ves-Rodrigues A):15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crawford C, Kennedy-Lydon TM, Callaghan H, Sprott C, Simmons RL, Sawbridge L, Syme HM, Unwin RJ, Wildman SS, Peppiatt-Wildman CM. Extracellular nucleotides affect pericyte-mediated regulation of rat in situ vasa recta diameter. Acta Physiol (Oxf) 2011;202:241–251. doi: 10.1111/j.1748-1716.2011.02310.x. [DOI] [PubMed] [Google Scholar]
- 62.Cruthirds DL, Saba H, Millan-Crow LA. Overexpression of manganese superoxide dismutase protects against ATP depletion-mediated cell death of proximal tubule cells. Arch Biochem Biophys. 2005;437:96–105. doi: 10.1016/j.abb.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 63.Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl− secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. J Physiol. 2000;524:77–90. doi: 10.1111/j.1469-7793.2000.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Ren Physiol. 2007;292:F1105–F1123. doi: 10.1152/ajprenal.00194.2006. [DOI] [PubMed] [Google Scholar]
- 65.Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011;22:890–901. doi: 10.1681/ASN.2010080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies JP, Robson L. Pharmacological properties and physiological function of a P2X-like current in single proximal tubule cells isolated from frog kidney. J Membr Biol. 2010;237:79–91. doi: 10.1007/s00232-010-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deetjen P, Thomas J, Lehrmann H, Kim SJ, Leipziger J. The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J Am Soc Nephrol. 2000;11:1798–1806. doi: 10.1681/ASN.V11101798. [DOI] [PubMed] [Google Scholar]
- 68.Di Garbo A, Alloisio S, Nobile M. P2X7 receptor-mediated calcium dynamics in HEK293 cells: experimental characterization and modelling approach. Phys Biol. 2012;9:026001. doi: 10.1088/1478-3975/9/2/026001. [DOI] [PubMed] [Google Scholar]
- 69.Di Sole F. Adenosine and renal tubular function. Curr Opin Nephrol Hypertens. 2008;17:399–407. doi: 10.1097/MNH.0b013e32830321e1. [DOI] [PubMed] [Google Scholar]
- 70.Díaz-Sylvester P, Mac Laughlin M, Amorena C. Peritubular fluid viscosity modulates H+ flux in proximal tubules through NO release. Am J Physiol Ren Physiol. 2001;280:F239–F243. doi: 10.1152/ajprenal.2001.280.2.F239. [DOI] [PubMed] [Google Scholar]
- 71.Dobrowolski L, Walkowska A, Kompanowska-Jezierska E, Kuczeriszka M, Sadowski J. Effects of ATP on rat renal haemodynamics and excretion: role of sodium intake, nitric oxide and cytochrome P450. Acta Physiol (Oxf) 2007;189:77–85. doi: 10.1111/j.1748-1716.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 72.Du J, Zhang L, Yang Y, Li W, Chen L, Ge Y, Sun C, Zhu Y, Gu L. ATP depletion-induced actin rearrangement reduces cell adhesion via p38 MAPK–HSP27 signaling in renal proximal tubule cells. Cell Physiol Biochem. 2010;25:501–510. doi: 10.1159/000303055. [DOI] [PubMed] [Google Scholar]
- 73.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Adenosine inhibits PDGF-induced growth of human glomerular mesangial cells via A2B receptors. Hypertension. 2005;46:628–634. doi: 10.1161/01.HYP.0000178464.63393.88. [DOI] [PubMed] [Google Scholar]
- 74.Ecelbarger CA, Maeda Y, Gibson CC, Knepper MA. Extracellular ATP increases intracellular calcium in rat terminal collecting duct via a nucleotide receptor. Am J Physiol. 1994;267:F998–F1006. doi: 10.1152/ajprenal.1994.267.6.F998. [DOI] [PubMed] [Google Scholar]
- 75.Edgecombe M, Craddock HS, Smith DC, McLennan AG, Fisher MJ. Diadenosine polyphosphate-stimulated gluconeogenesis in isolated rat proximal tubules. Biochem J. 1997;323(Pt 2):451–456. doi: 10.1042/bj3230451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edwards RM. Basolateral, but not apical, ATP inhibits vasopressin action in rat inner medullary collecting duct. Eur J Pharmacol. 2002;438:179–181. doi: 10.1016/s0014-2999(02)01293-1. [DOI] [PubMed] [Google Scholar]
- 77.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol. 2012;74:325–349. doi: 10.1146/annurev-physiol-020911-153225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elsherbiny NM, Abd El Galil KH, Gabr MM, Al-Gayyar MM, Eissa LA, El-Shishtawy MM. Reno-protective effect of NECA in diabetic nephropathy: implication of IL-18 and ICAM-1. Eur Cytokine Netw. 2012;23:78–86. doi: 10.1684/ecn.2012.0309. [DOI] [PubMed] [Google Scholar]
- 79.Eltze M, Ullrich B. Characterization of vascular P2 purinoceptors in the rat isolated perfused kidney. Eur J Pharmacol. 1996;306:139–152. doi: 10.1016/0014-2999(96)00244-0. [DOI] [PubMed] [Google Scholar]
- 80.Elwi AN, Damaraju VL, Kuzma ML, Mowles DA, Baldwin SA, Young JD, Sawyer MB, Cass CE. Transepithelial fluxes of adenosine and 2′-deoxyadenosine across human renal proximal tubule cells: roles of nucleoside transporters hENT1, hENT2, and hCNT3. Am J Physiol Ren Physiol. 2009;296:F1439–F1451. doi: 10.1152/ajprenal.90411.2008. [DOI] [PubMed] [Google Scholar]
- 81.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 82.Eppel GA, Ventura S, Evans RG. Regional vascular responses to ATP and ATP analogues in the rabbit kidney in vivo: roles for adenosine receptors and prostanoids. Br J Pharmacol. 2006;149:523–531. doi: 10.1038/sj.bjp.0706901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol. 2008;19:722–730. doi: 10.1681/ASN.2007060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng MG, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Ren Physiol. 2010;299:F310–F315. doi: 10.1152/ajprenal.00149.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferenbach DA, Hughes J. Adenosine A2A agonists as therapy for glomerulonephritis. Kidney Int. 2011;80:329–331. doi: 10.1038/ki.2011.167. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez O, Wangensteen R, Osuna A, Vargas F. Renal vascular reactivity to P2-purinoceptor activation in spontaneously hypertensive rats. Pharmacology. 2000;60:47–50. doi: 10.1159/000028346. [DOI] [PubMed] [Google Scholar]
- 87.Fischer KG, Saueressig U, Jacobshagen C, Wichelmann A, Pavenstädt H. Extracellular nucleotides regulate cellular functions of podocytes in culture. Am J Physiol Ren Physiol. 2001;281:F1075–F1081. doi: 10.1152/ajprenal.2001.281.6.F1075. [DOI] [PubMed] [Google Scholar]
- 88.Fischer W, Wirkner K, Weber M, Eberts C, Köles L, Reinhardt R, Franke H, Allgaier C, Gillen C, Illes P. Characterization of P2X3, P2Y1 and P2Y4 receptors in cultured HEK293-hP2X3 cells and their inhibition by ethanol and trichloroethanol. J Neurochem. 2003;85:779–790. doi: 10.1046/j.1471-4159.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 89.Fischer W, Franke H, Gröger-Arndt H, Illes P. Evidence for the existence of P2Y1,2,4 receptor subtypes in HEK-293 cells: reactivation of P2Y1 receptors after repetitive agonist application. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:466–472. doi: 10.1007/s00210-005-1070-6. [DOI] [PubMed] [Google Scholar]
- 90.Fish RS, Klootwijk E, Tam FW, Kleta R, Wheeler DC, Unwin RJ, Norman J. ATP and arterial calcification. Eur J Clin Investig. 2013;43:405–412. doi: 10.1111/eci.12055. [DOI] [PubMed] [Google Scholar]
- 91.Franco M, Bell PD, Navar LG. Effect of adenosine A1 analogue on tubuloglomerular feedback mechanism. Am J Physiol. 1989;257:F231–F236. doi: 10.1152/ajprenal.1989.257.2.F231. [DOI] [PubMed] [Google Scholar]
- 92.Franco M, Galicia O, Quintana A, Martínez F. Experimental hypothyroidism modifies specific binding of A1 and A2A analogues to adenosine receptors in the rat kidney. Br J Pharmacol. 2004;142:461–468. doi: 10.1038/sj.bjp.0705799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Franco M, Bautista R, Perez-Mendez O, Gonzalez L, Pacheco U, Sanchez-Lozada LG, Santamaria J, Tapia E, Monreal R, Martinez F. Renal interstitial adenosine is increased in angiotensin II-induced hypertensive rats. Am J Physiol Ren Physiol. 2008;294:F84–F92. doi: 10.1152/ajprenal.00123.2007. [DOI] [PubMed] [Google Scholar]
- 94.Franco M, Bautista R, Tapia E, Soto V, Santamaría J, Osorio H, Pacheco U, Sánchez-Lozada LG, Kobori H, Navar LG. Contribution of renal purinergic receptors to renal vasoconstriction in angiotensin II-induced hypertensive rats. Am J Physiol Ren Physiol. 2011;300:F1301–F1309. doi: 10.1152/ajprenal.00367.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedrich F, Weiss H, Paulmichl M, Lang F. Activation of potassium channels in renal epithelioid cells (MDCK) by extracellular ATP. Am J Physiol. 1989;256:C1016–C1021. doi: 10.1152/ajpcell.1989.256.5.C1016. [DOI] [PubMed] [Google Scholar]
- 96.Fürstenau CR, Ramos DB, Vuaden FC, Casali EA, PdS M, DdS T, Gossenheimer AN, Bogo MR, Bonan CD, Barreto-Chaves ML, Sarkis JJ, Wofchuk ST. L-NAME-treatment alters ectonucleotidase activities in kidney membranes of rats. Life Sci. 2010;87:325–332. doi: 10.1016/j.lfs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 97.Gaál K, Forgács I, Bácsalmásy Z. Effect of adenosine compounds (ATP, cAMP) on renin release in vitro. Acta Physiol Acad Sci Hung. 1976;47:49–54. [PubMed] [Google Scholar]
- 98.Gandhi R, Le Hir M, Kaissling B. Immunolocalization of ecto-5′-nucleotidase in the kidney by a monoclonal antibody. Histochemistry. 1990;95:165–174. doi: 10.1007/BF00266589. [DOI] [PubMed] [Google Scholar]
- 99.Gao X, Patzak A, Sendeski M, Scheffer PG, Teerlink T, Sällstrom J, Fredholm BB, Persson AE, Carlström M. Adenosine A1-receptor deficiency diminishes afferent arteriolar and blood pressure responses during nitric oxide inhibition and angiotensin II treatment. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1669–R1681. doi: 10.1152/ajpregu.00268.2011. [DOI] [PubMed] [Google Scholar]
- 100.Garcia GE, Truong LD, Li P, Zhang P, Du J, Chen JF, Feng L. Adenosine A2A receptor activation and macrophage-mediated experimental glomerulonephritis. FASEB J. 2008;22:445–454. doi: 10.1096/fj.07-8430com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garcia GE, Truong LD, Chen JF, Johnson RJ, Feng L. Adenosine A2A receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int. 2011;80:378–388. doi: 10.1038/ki.2011.101. [DOI] [PubMed] [Google Scholar]
- 102.Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am J Physiol. 1999;277:F377–F382. doi: 10.1152/ajprenal.1999.277.3.F377. [DOI] [PubMed] [Google Scholar]
- 103.Garvin JL, Herrera M, Ortiz PA. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: clinical implications. Annu Rev Physiol. 2011;73:359–376. doi: 10.1146/annurev-physiol-012110-142247. [DOI] [PubMed] [Google Scholar]
- 104.Garvin PJ, Jellinek M, Morgan R, Codd JE. Renal cortical levels of adenosine triphosphate: restoration after prolonged ischemia by in situ perfusion of ATP-MgCl2. Arch Surg. 1981;116:221–224. doi: 10.1001/archsurg.1981.01380140067015. [DOI] [PubMed] [Google Scholar]
- 105.Geyti CS, Odgaard E, Overgaard MT, Jensen ME, Leipziger J, Praetorius HA. Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflugers Arch. 2008;455:1105–1117. doi: 10.1007/s00424-007-0366-4. [DOI] [PubMed] [Google Scholar]
- 106.Gheorghiu M, Van Driessche W. Modeling of basolateral ATP release induced by hypotonic treatment in A6 cells. Eur Biophys J. 2004;33:412–420. doi: 10.1007/s00249-003-0375-y. [DOI] [PubMed] [Google Scholar]
- 107.Gill A, Wortham K, Costa D, Davis W, Ticho B, Whalley E. Protective effect of tonapofylline (BG9928), an adenosine A1 receptor antagonist, against cisplatin-induced acute kidney injury in rats. Am J Nephrol. 2009;30:521–526. doi: 10.1159/000248762. [DOI] [PubMed] [Google Scholar]
- 108.Goel M, Schilling WP. Role of TRPC3 channels in ATP-induced Ca2+ signaling in principal cells of the inner medullary collecting duct. Am J Physiol Ren Physiol. 2010;299:F225–F233. doi: 10.1152/ajprenal.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomes CP, Leão-Ferreira LR, Pinheiro AA, Gomes-Quintana E, Wengert M, Lopes AG, Caruso-Neves C. Crosstalk between the signaling pathways triggered by angiotensin II and adenosine in the renal proximal tubules: implications for modulation of Na+-ATPase activity. Peptides. 2008;29:2033–2038. doi: 10.1016/j.peptides.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 110.Gopalakrishnan S, Raman N, Atkinson SJ, Marrs JA. Rho GTPase signaling regulates tight junction assembly and protects tight junctions during ATP depletion. Am J Physiol. 1998;275:C798–C809. doi: 10.1152/ajpcell.1998.275.3.C798. [DOI] [PubMed] [Google Scholar]
- 111.Gopalakrishnan S, Hallett MA, Atkinson SJ, Marrs JA. aPKC–PAR complex dysfunction and tight junction disassembly in renal epithelial cells during ATP depletion. Am J Physiol Cell Physiol. 2007;292:C1094–C1102. doi: 10.1152/ajpcell.00099.2006. [DOI] [PubMed] [Google Scholar]
- 112.Gorelik J, Zhang Y, Sánchez D, Shevchuk A, Frolenkov G, Lab M, Klenerman D, Edwards C, Korchev Y. Aldosterone acts via an ATP autocrine/paracrine system: the Edelman ATP hypothesis revisited. Proc Natl Acad Sci U S A. 2005;102:15000–15005. doi: 10.1073/pnas.0507008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gottlieb SS. Are all beta-blockers the same for chronic heart failure? Curr Cardiol Rep. 2001;3:124–129. doi: 10.1007/s11886-001-0038-5. [DOI] [PubMed] [Google Scholar]
- 114.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Ren Physiol. 2008;294:F161–F169. doi: 10.1152/ajprenal.00281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greiber S, Münzel T, Kästner S, Müller B, Schollmeyer P, Pavenstädt H. NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphate. Kidney Int. 1998;53:654–663. doi: 10.1046/j.1523-1755.1998.00796.x. [DOI] [PubMed] [Google Scholar]
- 116.Grenz A, Kim JH, Bauerle JD, Tak E, Eltzschig HK, Clambey ET. Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-α release. J Immunol. 2012;189:4566–4573. doi: 10.4049/jimmunol.1201651. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Guan Z, Inscho EW. Role of adenosine 5′-triphosphate in regulating renal microvascular function and in hypertension. Hypertension. 2011;58:333–340. doi: 10.1161/HYPERTENSIONAHA.110.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guan Z, Osmond DA, Inscho EW. Purinoceptors in the kidney. Exp Biol Med (Maywood) 2007;232:715–726. [PubMed] [Google Scholar]
- 119.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in ANG II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Ren Physiol. 2010;298:F1276–F1284. doi: 10.1152/ajprenal.00743.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guerra L, Favia M, Fanelli T, Calamita G, Svetlo M, Bagorda A, Jacobson KA, Reshkin SJ, Casavola V. Stimulation of Xenopus P2Y1 receptor activates CFTR in A6 cells. Pflugers Arch. 2004;449:66–75. doi: 10.1007/s00424-004-1293-2. [DOI] [PubMed] [Google Scholar]
- 121.Gutierrez AM, Lou X, Erik A, Persson G, Ring A. Ca2+ response of rat mesangial cells to ATP analogues. Eur J Pharmacol. 1999;369:107–112. doi: 10.1016/s0014-2999(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 122.Gutierrez AM, Lou X, Erik A, Persson G, Ring A. Growth hormones reverse desensitization of P2Y2 receptors in rat mesangial cells. Biochem Biophys Res Commun. 2000;270:594–599. doi: 10.1006/bbrc.2000.2461. [DOI] [PubMed] [Google Scholar]
- 123.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1143–R1155. doi: 10.1152/ajpregu.00808.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Ren Physiol. 2012;303:F1454–F1459. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harada H, Tsukimoto M, Ikari A, Suketa Y. P2X7 receptor-induced generation of reactive oxygen species in rat mesangial cells. Drug Dev Res. 2003;59:112–117. [Google Scholar]
- 126.Harahap AR, Goding JW. Distribution of the murine plasma cell antigen PC-1 in non-lymphoid tissues. J Immunol. 1988;141:2317–2320. [PubMed] [Google Scholar]
- 127.Harhun M, Povstyan O, Gordienko D. P2X receptor-mediated current in myocytes from renal resistance arteries. J Gen Physiol. 2009;134:7a. doi: 10.1111/j.1476-5381.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heidemann HT, Müller S, Mertins L, Stepan G, Hoffmann K, Ohnhaus EE. Effect of aminophylline on cisplatin nephrotoxicity in the rat. Br J Pharmacol. 1989;97:313–318. doi: 10.1111/j.1476-5381.1989.tb11956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heidenreich S, Tepel M, Schlüter H, Harrach B, Zidek W. Regulation of rat mesangial cell growth by diadenosine phosphates. J Clin Invest. 1995;95:2862–2867. doi: 10.1172/JCI117992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heyeraas KJ, Aukland K. Interlobular arterial resistance: influence of renal arterial pressure and angiotensin II. Kidney Int. 1987;31:1291–1298. doi: 10.1038/ki.1987.142. [DOI] [PubMed] [Google Scholar]
- 131.Hill C, Parker AJ, White SJ. Expression of P2X purinergic receptors in cultured mouse mesangial cells. J Physiol. 2000;527:15P–16P. [Google Scholar]
- 132.Hillman KA, Johnson TM, Winyard PJD, Burnstock G, Unwin RJ, Woolf AS. P2X7 receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse. Exp Nephrol. 2002;10:34–42. doi: 10.1159/000049896. [DOI] [PubMed] [Google Scholar]
- 133.Hillman KA, Woolf AS, Johnson TM, Wade A, Unwin RJ, Winyard PJ. The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem Biophys Res Commun. 2004;322:434–439. doi: 10.1016/j.bbrc.2004.07.148. [DOI] [PubMed] [Google Scholar]
- 134.Hohenstein B, Renk S, Lang K, Daniel C, Freund M, Léon C, Amann KU, Gachet C, Hugo CP. P2Y1 gene deficiency protects from renal disease progression and capillary rarefaction during passive crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:494–505. doi: 10.1681/ASN.2006050439. [DOI] [PubMed] [Google Scholar]
- 135.Holstein-Rathlou NH. Synchronization of proximal intratubular pressure oscillations: evidence for interaction between nephrons. Pflugers Arch. 1987;408:438–443. doi: 10.1007/BF00585066. [DOI] [PubMed] [Google Scholar]
- 136.Holtzclaw JD, Cornelius RJ, Hatcher LI, Sansom SC. Coupled ATP and potassium efflux from intercalated cells. Am J Physiol Ren Physiol. 2011;300:F1319–F1326. doi: 10.1152/ajprenal.00112.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hooper KM, Boletta A, Germino GG, Hu Q, Ziegelstein RC, Sutters M. Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am J Physiol Ren Physiol. 2005;289:F521–F530. doi: 10.1152/ajprenal.00355.2004. [DOI] [PubMed] [Google Scholar]
- 138.Hovater MB, Olteanu D, Yoder BK, Schwiebert EM. Autocrine purinergic signaling is required for monocilium-driven signaling. FASEB J. 2007;21:596.1. [Google Scholar]
- 139.Hovater MB, Olteanu D, Welty EA, Schwiebert EM. Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts. Purinergic Signal. 2008;4:109–124. doi: 10.1007/s11302-008-9102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal. 2008;4:155–170. doi: 10.1007/s11302-007-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu J, Barr MM. ATP-2 interacts with the PLAT domain of LOV-1 and is involved in Caenorhabditis elegans polycystin signaling. Mol Biol Cell. 2005;16:458–469. doi: 10.1091/mbc.E04-09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hughes RJ, Torres B, Zambon A, Arthur D, Bohmann C, Rump LC, Insel PA. Expression of multiple P2Y receptors by MDCK-D1 cells: P2Y1 receptor cloning and signaling. Drug Dev Res. 2003;59:1–7. [Google Scholar]
- 143.Huwiler A, Pfeilschifter J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br J Pharmacol. 1994;113:1455–1463. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huwiler A, Wartmann M, van den Bosch H, Pfeilschifter J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br J Pharmacol. 2000;129:612–618. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huwiler A, Rölz W, Dorsch S, Ren S, Pfeilschifter J. Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y2 purinoceptor in renal mesangial cells. Br J Pharmacol. 2002;136:520–529. doi: 10.1038/sj.bjp.0704748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Ren Physiol. 2001;280:F927–F944. doi: 10.1152/ajprenal.2001.280.6.F927. [DOI] [PubMed] [Google Scholar]
- 147.Inscho EW. Purinoceptor regulation of renal tubular transport is coming of age. Am J Physiol Ren Physiol. 2009;297:F1166–F1167. doi: 10.1152/ajprenal.00506.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Inscho EW. Lewis K. Dahl Memorial Lecture. Mysteries of renal autoregulation. Hypertension. 2009;53:299–306. doi: 10.1161/HYPERTENSIONAHA.108.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Inscho EW, Ohishi K, Navar LG. Effects of ATP on pre- and postglomerular juxtamedullary microvasculature. Am J Physiol. 1992;263:F886–F893. doi: 10.1152/ajprenal.1992.263.5.F886. [DOI] [PubMed] [Google Scholar]
- 150.Inscho EW, Mitchell KD, Navar LG. Extracellular ATP in the regulation of renal microvascular function. FASEB J. 1994;8:319–328. doi: 10.1096/fasebj.8.3.8143938. [DOI] [PubMed] [Google Scholar]
- 151.Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol. 1996;271:F1077–F1085. doi: 10.1152/ajprenal.1996.271.5.F1077. [DOI] [PubMed] [Google Scholar]
- 152.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand. 2004;181:445–453. doi: 10.1111/j.1365-201X.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 154.Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension. 2011;57:780–787. doi: 10.1161/HYPERTENSIONAHA.110.168955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Insel PA, Ostrom RS, Zambon AC, Hughes RJ, Balboa MA, Shehnaz D, Gregorian C, Torres B, Firestein BL, Xing M, Post SR. P2Y receptors of MDCK cells: epithelial cell regulation by extracellular nucleotides. Clin Exp Pharmacol Physiol. 2001;28:351–354. doi: 10.1046/j.1440-1681.2001.03452.x. [DOI] [PubMed] [Google Scholar]
- 156.Ishikawa I, Shikura N, Takada K. Amelioration of glycerol-induced acute renal failure in rats by an adenosine A1 receptor antagonist (FR-113453) Ren Fail. 1993;15:1–5. doi: 10.3109/08860229309065565. [DOI] [PubMed] [Google Scholar]
- 157.Ishikawa S, Kawasumi M, Kusaka I, Komatsu N, Iwao N, Saito T. Extracellular ATP promotes cellular growth of glomerular mesangial cells mediated via phospholipase C. Biochem Biophys Res Commun. 1994;202:234–240. doi: 10.1006/bbrc.1994.1917. [DOI] [PubMed] [Google Scholar]
- 158.Itoh S, Carretero OA, Murray RD. Possible role of adenosine in the macula densa mechanism of renin release in rabbits. J Clin Invest. 1985;76:1412–1417. doi: 10.1172/JCI112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jackson EK. Adenosine: a physiological brake on renin release. Annu Rev Pharmacol Toxicol. 1991;31:1–35. doi: 10.1146/annurev.pa.31.040191.000245. [DOI] [PubMed] [Google Scholar]
- 160.Jackson EK. Renal actions of purines. In: Jacobson KA, Jarvis MF, editors. Purinergic approaches in experimental therapeutics. New York: Wiley-Liss; 1997. pp. 217–250. [Google Scholar]
- 161.Jackson EK. P1 and P2 receptors in the renal system. In: Abbracchio MP, Williams M, editors. Purinergic and pyrimidinergic signalling II. Berlin: Springer; 2001. pp. 33–71. [Google Scholar]
- 162.Jackson EK, Cheng D, Tofovic SP, Mi Z. Endogenous adenosine contributes to renal sympathetic neurotransmission via postjunctional A1 receptor-mediated coincident signaling. Am J Physiol Ren Physiol. 2012;302:F466–F476. doi: 10.1152/ajprenal.00495.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jalilian I, Spildrejorde M, Seavers A, Curtis BL, McArthur JD, Sluyter R. Functional expression of the damage-associated molecular pattern receptor P2X7 on canine kidney epithelial cells. Vet Immunol Immunopathol. 2012;150:228–233. doi: 10.1016/j.vetimm.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 164.Jan CR, Ho CM, Wu SN, Tseng CJ. Mechanisms of rise and decay of ADP-evoked calcium signal in MDCK cells. Chin J Physiol. 1998;41:67–73. [PubMed] [Google Scholar]
- 165.Jankowski M. Purinergic regulation of glomerular microvasculature and tubular function. J Physiol Pharmacol. 2008;59(Suppl 9):121–135. [PubMed] [Google Scholar]
- 166.Jankowski M, Szczepanska-Konkel M, Kalinowski L, Angielski S. Cyclic GMP-dependent relaxation of isolated rat renal glomeruli induced by extracellular ATP. J Physiol. 2001;530:123–130. doi: 10.1111/j.1469-7793.2001.0123m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jankowski M, Angielski S, Szczepanska-Konkel M. Dissociation between the effects of P1, P4-diadenosine tetraphosphate (Ap4A) on renal haemodynamics and tubular function in anaesthetized rats. J Physiol Pharmacol. 2008;59:129–137. [PubMed] [Google Scholar]
- 168.Jankowski M, Szamocka E, Kowalski R, Angielski S, Szczepanska-Konkel M. The effects of P2X receptor agonists on renal sodium and water excretion in anaesthetized rats. Acta Physiol (Oxf) 2011;202:193–201. doi: 10.1111/j.1748-1716.2011.02276.x. [DOI] [PubMed] [Google Scholar]
- 169.Jankowski V, Karadogan S, Vanholder R, Nofer JR, Herget-Rosenthal S, van der Giet M, Tölle M, Tran TN, Zidek W, Jankowski J. Paracrine stimulation of vascular smooth muscle proliferation by diadenosine polyphosphates released from proximal tubule epithelial cells. Kidney Int. 2007;71:994–1000. doi: 10.1038/sj.ki.5002186. [DOI] [PubMed] [Google Scholar]
- 170.Jankowski V, Patzak A, Herget-Rosenthal S, Tran TN, Lai EY, Gunthner T, Buschmann I, Zidek W, Jankowski J. Uridine adenosine tetraphosphate acts as an autocrine hormone affecting glomerular filtration rate. J Mol Med. 2008;86:333–340. doi: 10.1007/s00109-008-0306-6. [DOI] [PubMed] [Google Scholar]
- 171.Jankowski V, Günthner T, Herget-Rosenthal S, Zidek W, Jankowski J. Dinucleoside polyphosphates and uremia. Semin Dial. 2009;22:396–399. doi: 10.1111/j.1525-139X.2009.00588.x. [DOI] [PubMed] [Google Scholar]
- 172.Jans D, Srinivas SP, Waelkens E, Segal A, Larivière E, Simaels J, Van Driessche W. Hypotonic treatment evokes biphasic ATP release across the basolateral membrane of cultured renal epithelia (A6) J Physiol. 2002;545:543–555. doi: 10.1113/jphysiol.2002.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jaramillo-Juárez F, Rodríguez-Vázquez ML, Muñoz-Martínez J, Quezada-Tristán T, Posadas del Río FA, Llamas-Viramontes J, Ortíz GG, Feria-Velasco A, Reyes JL. The ATP levels in kidneys and blood are mainly decreased by acute ingestion of tullidora (Karwinskia humboldtiana) Toxicon. 2005;46:99–103. doi: 10.1016/j.toxicon.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 174.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol. 2007;18:2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- 175.Jeyaraj SC, Dakhlallah D, Hill SR, Lee BS. Expression and distribution of HuR during ATP depletion and recovery in proximal tubule cells. Am J Physiol Ren Physiol. 2006;291:F1255–F1263. doi: 10.1152/ajprenal.00440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ji X, Naito Y, Hirokawa G, Weng H, Hiura Y, Takahashi R, Iwai N. P2X7 receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res. 2012;35:173–179. doi: 10.1038/hr.2011.153. [DOI] [PubMed] [Google Scholar]
- 177.Jian R, Sun Y, Wang Y, Yu J, Zhong L, Zhou P. CD73 protects kidney from ischemia–reperfusion injury through reduction of free radicals. APMIS. 2012;120:130–138. doi: 10.1111/j.1600-0463.2011.02827.x. [DOI] [PubMed] [Google Scholar]
- 178.Jin W, Hopfer U. Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: elevated cytosolic Ca2+ is not required. Am J Physiol. 1997;272:C1169–C1177. doi: 10.1152/ajpcell.1997.272.4.C1169. [DOI] [PubMed] [Google Scholar]
- 179.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 180.Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, Malinski T. Third-generation β-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107:2747–2752. doi: 10.1161/01.CIR.0000066912.58385.DE. [DOI] [PubMed] [Google Scholar]
- 181.Kang HS, Kerstan D, Dai LJ, Ritchie G, Quamme GA. Adenosine modulates Mg2+ uptake in distal convoluted tubule cells via A1 and A2 purinoceptors. Am J Physiol Ren Physiol. 2001;281:F1141–F1147. doi: 10.1152/ajprenal.2001.281.6.F1141. [DOI] [PubMed] [Google Scholar]
- 182.Karczewska J, Piwkowska A, Rogacka D, Stepinski J, Angielski S, Jankowski M. Purinergic modulation of glucose uptake into cultured rat podocytes: effect of diabetic milieu. Biochem Biophys Res Commun. 2011;404:723–727. doi: 10.1016/j.bbrc.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 183.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, Orleans-Juste P, Marceau F, Thorin E, Sevigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kawabata M, Ogawa T, Takabatake T. Control of rat glomerular microcirculation by juxtaglomerular adenosine A1 receptors. Kidney Int Suppl. 1998;67:S228–S230. doi: 10.1046/j.1523-1755.1998.06757.x. [DOI] [PubMed] [Google Scholar]
- 185.Kempson SA, Edwards JM, Osborn A, Sturek M. Acute inhibition of the betaine transporter by ATP and adenosine in renal MDCK cells. Am J Physiol Ren Physiol. 2008;295:F108–F117. doi: 10.1152/ajprenal.00108.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim YH, Choi YJ, Bae HR, Woo JS. P2 Receptor-mediated inhibition of vasopressin-stimulated fluid transport and cAMP responses in AQP2-transfected MDCK cells. Korean J Physiol Pharmacol. 2009;13:9–14. doi: 10.4196/kjpp.2009.13.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol. 1995;269:F863–F869. doi: 10.1152/ajprenal.1995.269.6.F863. [DOI] [PubMed] [Google Scholar]
- 188.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA. Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol Ren Physiol. 2000;278:F43–F51. doi: 10.1152/ajprenal.2000.278.1.F43. [DOI] [PubMed] [Google Scholar]
- 189.Kishore BK, Kran CM, Reif M, Menon AG (2001) Molecular physiology of urinary concentration defect in elderly population. Int Urol Nephrol 33:235–248 [DOI] [PubMed]
- 190.Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sévigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Ren Physiol. 2005;288:F1032–F1043. doi: 10.1152/ajprenal.00108.2004. [DOI] [PubMed] [Google Scholar]
- 191.Kishore BK, Krane CM, Miller RL, Shi H, Zhang P, Hemmert A, Sun R, Nelson RD. P2Y2 receptor mRNA and protein expression is altered in inner medullas of hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Ren Physiol. 2005;288:F1164–F1172. doi: 10.1152/ajprenal.00199.2004. [DOI] [PubMed] [Google Scholar]
- 192.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptors and water transport in the kidney. Purinergic Signal. 2009;5:491–499. doi: 10.1007/s11302-009-9151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Klawitter S, Hofmann LP, Pfeilschifter J, Huwiler A. Extracellular nucleotides induce migration of renal mesangial cells by upregulating sphingosine kinase-1 expression and activity. Br J Pharmacol. 2007;150:271–280. doi: 10.1038/sj.bjp.0706983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kleta R, Hirsch J, Heidenreich S, Schlüter H, Zidek W, Schlatter E. Effects of diadenosine polyphosphates, ATP and angiotensin II on membrane voltage and membrane conductances of rat mesangial cells. Pflugers Arch. 1995;430:713–720. doi: 10.1007/BF00386166. [DOI] [PubMed] [Google Scholar]
- 195.Koltsova SV, Platonova A, Maksimov GV, Mongin AA, Grygorczyk R, Orlov SN. Activation of P2Y receptors causes strong and persistent shrinkage of C11-MDCK renal epithelial cells. Am J Physiol Cell Physiol. 2011;301:C403–C412. doi: 10.1152/ajpcell.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol. 2004;286:F1054–F1058. doi: 10.1152/ajprenal.00336.2003. [DOI] [PubMed] [Google Scholar]
- 197.Koster HP, Hartog A, van Os CH, Bindels RJ. Inhibition of Na+ and Ca2+ reabsorption by P2u purinoceptors requires PKC but not Ca2+ signaling. Am J Physiol. 1996;270:F53–F60. doi: 10.1152/ajprenal.1996.270.1.F53. [DOI] [PubMed] [Google Scholar]
- 198.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kowalski R, Kreft E, Kasztan M, Jankowski M, Szczepanska-Konkel M. Chronic renal denervation increases renal tubular response to P2X receptor agonists in rats: implication for renal sympathetic nerve ablation. Nephrol Dial Transplant. 2012;27:3443–3448. doi: 10.1093/ndt/gfs087. [DOI] [PubMed] [Google Scholar]
- 200.Kriz W. Adenosine and ATP: traffic regulators in the kidney. J Clin Invest. 2004;114:611–613. doi: 10.1172/JCI22669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kuczeriszka M, Dobrowolski L, Walkowska A, Sadowski J, Kompanowska-Jezierska E. Adenosine effects on renal function in the rat: role of sodium intake and cytochrome P450. Nephron Physiol. 2013;123:1–5. doi: 10.1159/000353705. [DOI] [PubMed] [Google Scholar]
- 202.Kulick A, Panico C, Gill P, Welch WJ. Low salt intake increases adenosine type 1 receptor expression and function in the rat proximal tubule. Am J Physiol Ren Physiol. 2008;295:F37–F41. doi: 10.1152/ajprenal.00061.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kurtz A, Kaissling B, Busse R, Baier W. Endothelial cells modulate renin secretion from isolated mouse juxtaglomerular cells. J Clin Invest. 1991;88:1147–1154. doi: 10.1172/JCI115415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 205.Lajdova I, Oksa A, Chorvat D, Jr, Topor P, Spustova V. Purinergic P2X7 receptors participate in disturbed intracellular calcium homeostasis in peripheral blood mononuclear cells of patients with chronic kidney disease. Kidney Blood Press Res. 2012;35:48–57. doi: 10.1159/000330349. [DOI] [PubMed] [Google Scholar]
- 206.Lang F, Plöckinger B, Häussinger D, Paulmichl M. Effects of extracellular nucleotides on electrical properties of subconfluent Madin Darby canine kidney cells. Biochim Biophys Acta. 1988;943:471–476. doi: 10.1016/0005-2736(88)90379-3. [DOI] [PubMed] [Google Scholar]
- 207.Le Hir M, Gandhi R, Dubach UC. Purification and properties of a 5′-nucleotidase from rat renal membranes. Enzyme. 1989;41:87–93. doi: 10.1159/000469058. [DOI] [PubMed] [Google Scholar]
- 208.Lee HT, Ota-Setlik A, Xu H, D’Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Ren Physiol. 2003;284:F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- 209.Lee SJ, Kwon CH, Kim YK. Alterations in membrane transport function and cell viability induced by ATP depletion in primary cultured rabbit renal proximal tubular cells. Korean J Physiol Pharmacol. 2009;13:15–22. doi: 10.4196/kjpp.2009.13.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee YJ, Park SH, Han HJ. ATP stimulates Na+-glucose cotransporter activity via cAMP and p38 MAPK in renal proximal tubule cells. Am J Physiol Cell Physiol. 2005;289:C1268–C1276. doi: 10.1152/ajpcell.00002.2005. [DOI] [PubMed] [Google Scholar]
- 211.Lee YJ, Park SH, Jeung TO, Kim KW, Lee JH, Han HJ. Effect of adenosine triphosphate on phosphate uptake in renal proximal tubule cells: involvement of PKC and p38 MAPK. J Cell Physiol. 2005;205:68–76. doi: 10.1002/jcp.20367. [DOI] [PubMed] [Google Scholar]
- 212.Lee YJ, Han HJ. Role of ATP in DNA synthesis of renal proximal tubule cells: involvement of calcium, MAPKs, and CDKs. Am J Physiol Ren Physiol. 2006;291:F98–F106. doi: 10.1152/ajprenal.00486.2005. [DOI] [PubMed] [Google Scholar]
- 213.Lee YJ, Lee JH, Han HJ. Extracellular adenosine triphosphate protects oxidative stress-induced increase of p21WAF1/Cip1 and p27Kip1 expression in primary cultured renal proximal tubule cells: role of PI3K and Akt signaling. J Cell Physiol. 2006;209:802–810. doi: 10.1002/jcp.20763. [DOI] [PubMed] [Google Scholar]
- 214.Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol. 2002;13:10–18. doi: 10.1681/ASN.V13110. [DOI] [PubMed] [Google Scholar]
- 215.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Ren Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 216.Leipziger J. Luminal nucleotides are tonic inhibitors of renal tubular transport. Curr Opin Nephrol Hypertens. 2011;20:518–522. doi: 10.1097/MNH.0b013e3283487393. [DOI] [PubMed] [Google Scholar]
- 217.Leipziger J, Bailey MA, Unwin RJ. Purinergic (P2) receptors in the Kidney. Curr Top Membr. 2003;54:36–394. [Google Scholar]
- 218.Lewis CJ, Evans RJ. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res. 2001;38:332–340. doi: 10.1159/000051064. [DOI] [PubMed] [Google Scholar]
- 219.Li L, Jeanette Lynch I, Zheng W, Cash MN, Teng X, Wingo CS, Verlander JW, Xia SL. Apical P2XR contribute to [Ca2+]i signaling and Isc in mouse renal MCD. Biochem Biophys Res Commun. 2007;359:438–444. doi: 10.1016/j.bbrc.2007.05.143. [DOI] [PubMed] [Google Scholar]
- 220.Li L, Wingo CS, Xia SL. Downregulation of SGK1 by nucleotides in renal tubular epithelial cells. Am J Physiol Ren Physiol. 2007;293:F1751–F1757. doi: 10.1152/ajprenal.00091.2007. [DOI] [PubMed] [Google Scholar]
- 221.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD. Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J Clin Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li L, Lai EY, Huang Y, Eisner C, Mizel D, Wilcox CS, Schnermann J. Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am J Physiol Ren Physiol. 2012;303:F1166–F1175. doi: 10.1152/ajprenal.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liclican EL, McGiff JC, Pedraza PL, Ferreri NR, Falck JR, Carroll MA. Exaggerated response to adenosine in kidneys from high salt-fed rats: role of epoxyeicosatrienoic acids. Am J Physiol Ren Physiol. 2005;289:F386–F392. doi: 10.1152/ajprenal.00421.2004. [DOI] [PubMed] [Google Scholar]
- 224.Lieberthal W, Menza SA, Levine JS. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol. 1998;274:F315–F327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- 225.Listhrop R, Nelson R, Ecelbarger CA, Kohan DE, Kishore B. Genetic deletion of P2Y2 receptor (P2Y2-R) alters the protein abundances of renal sodium transporters and channels. FASEB J. 2007;21:A1328. [Google Scholar]
- 226.Liu R, Persson AE. Effects of nitric oxide on P2Y receptor resensitization in spontaneously hypertensive rat mesangial cells. J Hypertens. 2002;20:1835–1842. doi: 10.1097/00004872-200209000-00030. [DOI] [PubMed] [Google Scholar]
- 227.Liu R, Gutierrez AM, Ring A, Persson AE. Nitric oxide induces resensitization of P2Y nucleotide receptors in cultured rat mesangial cells. J Am Soc Nephrol. 2002;13:313–321. doi: 10.1681/ASN.V132313. [DOI] [PubMed] [Google Scholar]
- 228.Liu ZX, Nickel CH, Cantley LG. HGF promotes adhesion of ATP-depleted renal tubular epithelial cells in a MAPK-dependent manner. Am J Physiol Ren Physiol. 2001;281:F62–F70. doi: 10.1152/ajprenal.2001.281.1.F62. [DOI] [PubMed] [Google Scholar]
- 229.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 230.Loesch A, Unwin R, Gandhi V, Burnstock G. Sympathetic nerve varicosities in close apposition with basolateral membranes of collecting duct epithelial cells of rat kidney. Nephron Physiol. 2009;113:15–21. doi: 10.1159/000235246. [DOI] [PubMed] [Google Scholar]
- 231.Lu Y, Ge Y, Manning RD., Jr Expression and function of adenosine A3 receptor in the afferent arteriole. Hypertens. 2011;58:E139. [Google Scholar]
- 232.Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG. ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Ren Physiol. 2002;282:F501–F505. doi: 10.1152/ajprenal.00147.2001. [DOI] [PubMed] [Google Scholar]
- 233.Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch. 2007;455:169–180. doi: 10.1007/s00424-007-0294-3. [DOI] [PubMed] [Google Scholar]
- 234.Macala LJ, Hayslett JP. Basolateral and apical A1 adenosine receptors mediate sodium transport in cultured renal epithelial (A6) cells. Am J Physiol Ren Physiol. 2002;283:F1216–F1225. doi: 10.1152/ajprenal.00085.2002. [DOI] [PubMed] [Google Scholar]
- 235.MacLaughlin M, Martinez-Salgado C, Eleno N, Olivera A, Lopez-Novoa JM. Adenosine activates mesangial cell proliferation. Cell Signal. 1997;9:59–63. doi: 10.1016/s0898-6568(96)00091-5. [DOI] [PubMed] [Google Scholar]
- 236.Mamenko M, Zaika O, Jin M, O’Neil RG, Pochynyuk O. Purinergic activation of Ca2+-permeable TRPV4 channels is essential for mechano-sensitivity in the aldosterone-sensitive distal nephron. PLoS One. 2011;6:e22824. doi: 10.1371/journal.pone.0022824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marques RD, de Bruijn PI, Sorensen MV, Bleich M, Praetorius HA, Leipziger J. Basolateral P2X receptors mediate inhibition of NaCl transport in mouse medullary thick ascending limb (mTAL) Am J Physiol Ren Physiol. 2012;302:F487–F494. doi: 10.1152/ajprenal.00570.2011. [DOI] [PubMed] [Google Scholar]
- 238.McCoy DE, Taylor AL, Kudlow BA, Karlson K, Slattery MJ, Schwiebert LM, Schwiebert EM, Stanton BA. Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol. 1999;277:F552–F559. doi: 10.1152/ajprenal.1999.277.4.F552. [DOI] [PubMed] [Google Scholar]
- 239.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Ren Physiol. 2005;289:F1304–F1312. doi: 10.1152/ajprenal.00203.2005. [DOI] [PubMed] [Google Scholar]
- 240.McKinney TD, Hosford MA. ATP-stimulated tetraethylammonium transport by rabbit renal brush border membrane vesicles. J Biol Chem. 1993;268:6886–6895. [PubMed] [Google Scholar]
- 241.McLaughlin GE, Alva MD, Egea M. Adenosine receptor antagonism in acute tacrolimus toxicity. Nephrol Dial Transplant. 2006;21:1961–1965. doi: 10.1093/ndt/gfl082. [DOI] [PubMed] [Google Scholar]
- 242.Menzies RI, Unwin RJ, Dash RK, Beard DA, Cowley AW, Jr., Carlson BE, Mullins JJ, Bailey MA (2013) Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Front Physiol 4:305 [DOI] [PMC free article] [PubMed]
- 243.Middleton JP, Mangel AW, Basavappa S, Fitz JG. Nucleotide receptors regulate membrane ion transport in renal epithelial cells. Am J Physiol. 1993;264:F867–F873. doi: 10.1152/ajprenal.1993.264.5.F867. [DOI] [PubMed] [Google Scholar]
- 244.Migita K, Lu L, Zhao Y, Honda K, Iwamoto T, Kita S, Katsuragi T. Adenosine induces ATP release via an inositol 1,4,5-trisphosphate signaling pathway in MDCK cells. Biochem Biophys Res Commun. 2005;328:1211–1215. doi: 10.1016/j.bbrc.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 245.Migita K, Zhao Y, Katsuragi T. Mitochondria play an important role in adenosine-induced ATP release from Madin–Darby canine kidney cells. Biochem Pharmacol. 2007;73:1676–1682. doi: 10.1016/j.bcp.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 246.Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem. 2011;286:1054–1060. doi: 10.1074/jbc.M110.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mo J, Fisher MJ. Uridine nucleotide-induced stimulation of gluconeogenesis in isolated rat proximal tubules. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:151–157. doi: 10.1007/s00210-002-0571-9. [DOI] [PubMed] [Google Scholar]
- 248.Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens. 2003;12:497–502. doi: 10.1097/00041552-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 249.Mohaupt MG, Fischer T, Schwöbel J, Sterzel RB, Schulze-Lohoff E. Activation of purinergic P2Y2 receptors inhibits inducible NO synthase in cultured rat mesangial cells. Am J Physiol. 1998;275:F103–F110. doi: 10.1152/ajprenal.1998.275.1.F103. [DOI] [PubMed] [Google Scholar]
- 250.Mori M, Nishizaki T, Kawahara K, Okada Y. ATP-activated cation conductance in a Xenopus renal epithelial cell line. J Physiol. 1996;491:281–290. doi: 10.1113/jphysiol.1996.sp021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mori M, Hosomi H, Nishizaki T, Kawahara K, Okada Y. Calcium release from intracellular stores evoked by extracellular ATP in a Xenopus renal epithelial cell line. J Physiol. 1997;502:365–373. doi: 10.1111/j.1469-7793.1997.365bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Murray RD, Churchill PC. Effects of adenosine receptor agonists in the isolated, perfused rat kidney. Am J Physiol. 1984;247:H343–H348. doi: 10.1152/ajpheart.1984.247.3.H343. [DOI] [PubMed] [Google Scholar]
- 253.Nagashima K, Kusaka H, Sato K, Karasawa A. Effects of KW-3902, a novel adenosine A1-receptor antagonist, on cephaloridine-induced acute renal failure in rats. Jpn J Pharmacol. 1994;64:9–17. doi: 10.1254/jjp.64.9. [DOI] [PubMed] [Google Scholar]
- 254.Nanoff C, Freissmuth M, Tuisl E, Schütz W. P2-, but not P1-purinoceptors mediate formation of 1,4,5-inositol trisphosphate and its metabolites via a pertussis toxin-insensitive pathway in the rat renal cortex. Br J Pharmacol. 1990;100:63–68. doi: 10.1111/j.1476-5381.1990.tb12052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Navar LG, Bell PD (2004) Romancing the macula densa at UAB. Kidney Int Suppl S34-S40 [DOI] [PubMed]
- 256.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 257.Nilius B, Sehrer J, Heinke S, Droogmans G. Ca2+ release and activation of K+ and Cl− currents by extracellular ATP in distal nephron epithelial cells. Am J Physiol. 1995;269:C376–C384. doi: 10.1152/ajpcell.1995.269.2.C376. [DOI] [PubMed] [Google Scholar]
- 258.Nishiyama A, Navar LG. ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol. 2002;283:R273–R275. doi: 10.1152/ajpregu.00071.2002. [DOI] [PubMed] [Google Scholar]
- 259.Nishiyama A, Abe Y. Potential roles of interstitial fluid adenosine in the regulation of renal hemodynamics. Biogenic Amines. 2003;17:459–474. [Google Scholar]
- 260.Nishiyama A, Majid DS, Walker M, III, Miyatake A, Navar LG. Renal interstitial ATP responses to changes in arterial pressure during alterations in tubuloglomerular feedback activity. Hypertension. 2001;37:753–759. doi: 10.1161/01.hyp.37.2.753. [DOI] [PubMed] [Google Scholar]
- 261.Nishiyama A, Rahman M, Inscho EW. Role of interstitial ATP and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertens Res. 2004;27:791–804. doi: 10.1291/hypres.27.791. [DOI] [PubMed] [Google Scholar]
- 262.Noji T, Sato H, Sano J, Nishikawa S, Kusaka H, Karasawa A. Treatment with an adenosine uptake inhibitor attenuates glomerulonephritis in mice. Eur J Pharmacol. 2002;449:293–300. doi: 10.1016/s0014-2999(02)02039-3. [DOI] [PubMed] [Google Scholar]
- 263.O’Connor PM, Cowley AW., Jr Medullary thick ascending limb buffer vasoconstriction of renal outer-medullary vasa recta in salt-resistant but not salt-sensitive rats. Hypertension. 2012;60:965–972. doi: 10.1161/HYPERTENSIONAHA.112.195214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Odgaard E, Praetorius HA, Leipziger J. AVP-stimulated nucleotide secretion in perfused mouse medullary thick ascending limb and cortical collecting duct. J Med Invest. 2009;56(Suppl):262–263. doi: 10.2152/jmi.56.262. [DOI] [PubMed] [Google Scholar]
- 265.Olivera A, Lopez-Novoa JM. Effect of adenosine and adenosine analogues on cyclic AMP accumulation in cultured mesangial cells and isolated glomeruli of the rat. Br J Pharmacol. 1992;107:341–346. doi: 10.1111/j.1476-5381.1992.tb12748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Olivetti G, Anversa P, Melissari M, Loud AV. Morphometry of the renal corpuscle during postnatal growth and compensatory hypertrophy. Kidney Int. 1980;17:438–454. doi: 10.1038/ki.1980.52. [DOI] [PubMed] [Google Scholar]
- 267.Olteanu D, Hovater MB, Schwiebert EM. Intraluminal autocrine purinergic signaling within cysts: implications for the progression of diseases that involve encapsulated cyst formation. Am J Physiol Ren Physiol. 2007;292:F11–F14. doi: 10.1152/ajprenal.00291.2006. [DOI] [PubMed] [Google Scholar]
- 268.Orth SR, Amann K, Strojek K, Ritz E. Sympathetic overactivity and arterial hypertension in renal failure. Nephrol Dial Transplant. 2001;16(Suppl 1):67–69. doi: 10.1093/ndt/16.suppl_1.67. [DOI] [PubMed] [Google Scholar]
- 269.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+–K+–2Cl− cotransporter activity. Am J Physiol Ren Physiol. 2001;281:F819–F825. doi: 10.1152/ajprenal.2001.281.5.F819. [DOI] [PubMed] [Google Scholar]
- 270.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Ren Physiol. 2004;287:F274–F280. doi: 10.1152/ajprenal.00382.2003. [DOI] [PubMed] [Google Scholar]
- 271.Ortiz-Capisano MC, Mendez M, Ortiz PA, Beierwaltes WH. Adenosine 1 receptors are expressed by juxtaglomerular cells and interact with calcium signaling to decrease renin release. FASEB J. 2011;25:824. [Google Scholar]
- 272.Osmond DA, Inscho EW. P2X1 receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Ren Physiol. 2010;298:F1360–F1368. doi: 10.1152/ajprenal.00016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Osswald H. The role of adenosine in the regulation of glomerular filtration rate and renin secretion. Trends Pharmacol Sci. 1984;5:94–97. [Google Scholar]
- 274.Osswald H, Schmitz HJ, Kemper R. Renal action of adenosine: effect on renin secretion in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1978;303:95–99. doi: 10.1007/BF00496190. [DOI] [PubMed] [Google Scholar]
- 275.Ostrom RS, Gregorian C, Insel PA. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem. 2000;275:11735–11739. doi: 10.1074/jbc.275.16.11735. [DOI] [PubMed] [Google Scholar]
- 276.Ostrom RS, Gregorian C, Drenan RM, Gabot K, Rana BK, Insel PA. Key role for constitutive cyclooxygenase-2 of MDCK cells in basal signaling and response to released ATP. Am J Physiol Cell Physiol. 2001;281:C524–C531. doi: 10.1152/ajpcell.2001.281.2.C524. [DOI] [PubMed] [Google Scholar]
- 277.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Ryan RP, Cowley AW, Jr, Staruschenko A. Real-time electrochemical detection of ATP and H2O2 release in freshly isolated kidneys. Am J Physiol Ren Physiol. 2013;305:F134–F141. doi: 10.1152/ajprenal.00129.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Panico C, Luo Z, Welch WJ. Inhibition of adenosine type 2 receptors increases fluid uptake in the proximal tubule. FASEB J. 2011;25:665. [Google Scholar]
- 279.Patel A, Honoré E. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol. 2010;6:530–538. doi: 10.1038/nrneph.2010.97. [DOI] [PubMed] [Google Scholar]
- 280.Paulais M, Baudouin-Legros M, Teulon J. Extracellular ATP and UTP trigger calcium entry in mouse cortical thick ascending limbs. Am J Physiol. 1995;268:F496–F502. doi: 10.1152/ajprenal.1995.268.3.F496. [DOI] [PubMed] [Google Scholar]
- 281.Paulmichl M, Pfeilschifter J, Wöll E, Lang F. Cellular mechanisms of ATP-induced hyperpolarization in renal epitheloid MDCK-cells. J Cell Physiol. 1991;147:68–75. doi: 10.1002/jcp.1041470110. [DOI] [PubMed] [Google Scholar]
- 282.Pavenstädt H, Gloy J, Leipziger J, Klär B, Pfeilschifter J, Schollmeyer P, Greger R. Effect of extracellular ATP on contraction, cytosolic calcium activity, membrane voltage and ion currents of rat mesangial cells in primary culture. Br J Pharmacol. 1993;109:953–959. doi: 10.1111/j.1476-5381.1993.tb13713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pavenstädt H, Ruh J, Greger R, Schollmeyer P. Adenosine-induced hyperpolarization of the membrane voltage in rat mesangial cells in primary culture. Br J Pharmacol. 1994;113:7–12. doi: 10.1111/j.1476-5381.1994.tb16166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pellerin I, Leclerc M, Claveau D, Mailloux J, Brunette MG. Roles of ATP and cytoskeleton in the regulation of Na+/H+ exchanger along the nephron luminal membrane. J Cell Physiol. 2001;187:109–116. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1054>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 285.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Ren Physiol. 2006;291:F473–F480. doi: 10.1152/ajprenal.00425.2005. [DOI] [PubMed] [Google Scholar]
- 286.Pfeilschifter J. Comparison of extracellular ATP and UTP signalling in rat renal mesangial cells. Biochem J. 1990;272:469–472. doi: 10.1042/bj2720469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Piwkowska A, Rogacka D, Jankowski M, Angielski S. Extracellular ATP through P2 receptors activates AMP-activated protein kinase and suppresses superoxide generation in cultured mouse podocytes. Exp Cell Res. 2011;317:1904–1913. doi: 10.1016/j.yexcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 288.Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens. 2008;17:533–540. doi: 10.1097/MNH.0b013e328308fff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Ren Physiol. 2008;294:F38–F46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- 290.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283:36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J. 2010;24:2056–2065. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Poelstra K, Heynen ER, Baller JF, Hardonk MJ, Bakker WW. Modulation of anti-Thy1 nephritis in the rat by adenine nucleotides. Evidence for an anti-inflammatory role for nucleotidases. Lab Invest. 1992;66:555–563. [PubMed] [Google Scholar]
- 293.Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial–fibroblast cross talk. Am J Physiol Ren Physiol. 2011;300:F62–F70. doi: 10.1152/ajprenal.00473.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ponnusamy M, Liu N, Gong R, Yan H, Zhuang S. ERK pathway mediates P2X7 expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells. Am J Physiol Ren Physiol. 2011;301:F650–F659. doi: 10.1152/ajprenal.00215.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Post SR, Rump LC, Zambon A, Hughes RJ, Buda MD, Jacobson JP, Kao CC, Insel PA. ATP activates cAMP production via multiple purinergic receptors in MDCK-D1 epithelial cells. Blockade of an autocrine/paracrine pathway to define receptor preference of an agonist. J Biol Chem. 1998;273:23093–23097. doi: 10.1074/jbc.273.36.23093. [DOI] [PubMed] [Google Scholar]
- 296.Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol (Oxf) 2009;197:241–251. doi: 10.1111/j.1748-1716.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 297.Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol. 2010;72:377–393. doi: 10.1146/annurev-physiol-021909-135825. [DOI] [PubMed] [Google Scholar]
- 298.Praetorius HA, Leipziger J. Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin Cell Dev Biol. 2013;24:3–10. doi: 10.1016/j.semcdb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 299.Praetorius HA, Frøkiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Ren Physiol. 2005;288:F133–F141. doi: 10.1152/ajprenal.00238.2004. [DOI] [PubMed] [Google Scholar]
- 300.Qu LP, Xue H, Yuan P, Zhou L, Yao T, Huang Y, Lu LM. Adenosine 5′-triphosphate stimulates the increase of TGF-β1 in rat mesangial cells under high-glucose conditions via reactive oxygen species and ERK1/2. Acta Pharmacol Sin. 2009;30:1601–1606. doi: 10.1038/aps.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Quezada C, Alarcón S, Jaramillo C, Muñoz D, Oyarzún C, San Martín R. Targeting adenosine signaling to treatment of diabetic nephropathy. Curr Drug Targets. 2013;14:490–496. doi: 10.2174/1389450111314040010. [DOI] [PubMed] [Google Scholar]
- 302.Rajagopal M, Pao AC. Adenosine activates AS2b receptors and enhances chloride secretion in kidney inner medullary collecting duct cells. Hypertension. 2010;55:1123–1128. doi: 10.1161/HYPERTENSIONAHA.109.143404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rajagopal M, Kathpalia PP, Thomas SV, Pao AC. Activation of P2Y1 and P2Y2 receptors induces chloride secretion via calcium-activated chloride channels in kidney inner medullary collecting duct cells. Am J Physiol Ren Physiol. 2011;301:F544–F553. doi: 10.1152/ajprenal.00709.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rajagopal M, Kathpalia PP, Widdicombe JH, Pao AC. Differential effects of extracellular ATP on chloride transport in cortical collecting duct cells. Am J Physiol Ren Physiol. 2012;303:F483–F491. doi: 10.1152/ajprenal.00062.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Rajakumar SV, Lu B, Crikis S, Robson SC, d’Apice AJ, Cowan PJ, Dwyer KM. Deficiency or inhibition of CD73 protects in mild kidney ischemia–reperfusion injury. Transplantation. 2010;90:1260–1264. doi: 10.1097/TP.0b013e3182003d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ren J, Mi Z, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther. 2008;325:920–926. doi: 10.1124/jpet.108.137752. [DOI] [PubMed] [Google Scholar]
- 307.Ren Y, Carretero OA, Garvin JL. Role of mesangial cells and gap junctions in tubuloglomerular feedback. Kidney Int. 2002;62:525–531. doi: 10.1046/j.1523-1755.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- 308.Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. Am J Physiol Regul Integr Comp Physiol. 2009;296:R419–R427. doi: 10.1152/ajpregu.90784.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na + and water reabsorption. FASEB J. 2007;21:3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- 310.Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y2 receptor activation decreases blood pressure and increases renal Na+ excretion. Am J Physiol Regul Integr Comp Physiol. 2011;301:R510–R518. doi: 10.1152/ajpregu.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Rivera I, Zhang S, Fuller BS, Edwards B, Seki T, Wang MH, Marrero MB, Inscho EW. P2 receptor regulation of [Ca2+]i in cultured mouse mesangial cells. Am J Physiol Ren Physiol. 2007;292:F1380–F1389. doi: 10.1152/ajprenal.00349.2006. [DOI] [PubMed] [Google Scholar]
- 312.Robson L, Hunter M. Two K+-selective conductances in single proximal tubule cells isolated from frog kidney are regulated by ATP. J Physiol. 1997;500:605–616. doi: 10.1113/jphysiol.1997.sp022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rost S, Daniel C, Schulze-Lohoff E, Bäumert HG, Lambrecht G, Hugo C. P2 receptor antagonist PPADS inhibits mesangial cell proliferation in experimental mesangial proliferative glomerulonephritis. Kidney Int. 2002;62:1659–1671. doi: 10.1046/j.1523-1755.2002.00621.x. [DOI] [PubMed] [Google Scholar]
- 314.Rouse D, Leite M, Suki WN. ATP inhibits the hydrosmotic effect of AVP in rabbit CCT: evidence for a nucleotide P2u receptor. Am J Physiol. 1994;267:F289–F295. doi: 10.1152/ajprenal.1994.267.2.F289. [DOI] [PubMed] [Google Scholar]
- 315.Rubera I, Tauc M, Bidet M, Verheecke-Mauze C, De Renzis G, Poujeol C, Cuiller B, Poujeol P. Extracellular ATP increases [Ca2+]i in distal tubule cells. II. Activation of a Ca2+-dependent Cl− conductance. Am J Physiol Ren Physiol. 2000;279:F102–F111. doi: 10.1152/ajprenal.2000.279.1.F102. [DOI] [PubMed] [Google Scholar]
- 316.Rubera I, Barrière H, Tauc M, Bidet M, Verheecke-Mauze C, Poujeol C, Cuiller B, Poujeol P. Extracellular adenosine modulates a volume-sensitive-like chloride conductance in immortalized rabbit DC1 cells. Am J Physiol Ren Physiol. 2001;280:F126–F145. doi: 10.1152/ajprenal.2001.280.1.F126. [DOI] [PubMed] [Google Scholar]
- 317.Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Schlatter E, Ankorina I, Haxelmans S, Kleta R. Effects of diadenosine polyphosphates, ATP and angiotensin II on cytosolic Ca2+ activity and contraction of rat mesangial cells. Pflugers Arch. 1995;430:721–728. doi: 10.1007/BF00386167. [DOI] [PubMed] [Google Scholar]
- 319.Schnermann J. Maintained tubuloglomerular feedback responses during acute inhibition of P2 purinergic receptors in mice. Am J Physiol Ren Physiol. 2011;300:F339–F344. doi: 10.1152/ajprenal.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 321.Schnermann J, Briggs JP. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int. 2008;74:418–426. doi: 10.1038/ki.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Scholz-Pedretti K, Pfeilschifter J, Kaszkin M. Potentiation of cytokine induction of group IIA phospholipase A2 in rat mesangial cells by ATP and adenosine via the A2A adenosine receptor. Br J Pharmacol. 2001;132:37–46. doi: 10.1038/sj.bjp.0703774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Schulze-Lohoff E, Zanner S, Ogilvie A, Sterzel RB. Extracellular ATP stimulates proliferation of cultured mesangial cells via P2-purinergic receptors. Am J Physiol. 1992;263:F374–F383. doi: 10.1152/ajprenal.1992.263.3.F374. [DOI] [PubMed] [Google Scholar]
- 324.Schulze-Lohoff E, Schagerl S, Ogilvie A, Sterzel RB. Extracellular ATP augments mesangial cell growth induced by multiple growth factors. Nephrol Dial Transplant. 1995;10:2027–2034. [PubMed] [Google Scholar]
- 325.Schulze-Lohoff E, Zanner S, Ogilvie A, Sterzel RB. Vasoactive diadenosine polyphosphates promote growth of cultured renal mesangial cells. Hypertension. 1995;26:899–904. doi: 10.1161/01.hyp.26.6.899. [DOI] [PubMed] [Google Scholar]
- 326.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brüne B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol. 1998;275:F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- 327.Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. Blood pressure-dependent inhibition of renin secretion requires A1 adenosine receptors. Hypertension. 2005;46:780–786. doi: 10.1161/01.HYP.0000183963.07801.65. [DOI] [PubMed] [Google Scholar]
- 328.Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol. 2001;28:340–350. doi: 10.1046/j.1440-1681.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 329.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Ren Physiol. 2002;282:F763–F775. doi: 10.1152/ajprenal.0337.2000. [DOI] [PubMed] [Google Scholar]
- 330.Shaik ZP, Fifer EK, Nowak G. Protein kinase B/Akt modulates nephrotoxicant-induced necrosis in renal cells. Am J Physiol Ren Physiol. 2007;292:F292–F303. doi: 10.1152/ajprenal.00082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Shaver SR, Rideout JL, Pendergast W, Douglass JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M, Yerxa BR. Structure–activity relationships of dinucleotides: potent and selective agonists of P2Y receptors. Purinergic Signal. 2005;1:183–191. doi: 10.1007/s11302-005-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Shirley DG, Bailey MA, Unwin RJ. In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Ren Physiol. 2005;288:F1243–F1248. doi: 10.1152/ajprenal.00152.2004. [DOI] [PubMed] [Google Scholar]
- 333.Shirley DG, Vekaria RM, Sévigny J. Ectonucleotidases in the kidney. Purinergic Signal. 2009;5:501–511. doi: 10.1007/s11302-009-9152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Shrivastava AN, Triller A, Sieghart W, Sarto-Jackson I. Regulation of GABAA receptor dynamics by interaction with purinergic P2X2 receptors. J Biol Chem. 2011;286:14455–14468. doi: 10.1074/jbc.M110.165282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Siegel NJ, Glazier WB, Chaudry IH, Gaudio KM, Lytton B, Baue AE, Kashgarian M. Enhanced recovery from acute renal failure by the postischemic infusion of adenine nucleotides and magnesium chloride in rats. Kidney Int. 1980;17:338–349. doi: 10.1038/ki.1980.39. [DOI] [PubMed] [Google Scholar]
- 336.Siegel NJ, Avison MJ, Reilly HF, Alger JR, Shulman RG. Enhanced recovery of renal ATP with postischemic infusion of ATP-MgCl2 determined by 31P-NMR. Am J Physiol. 1983;245:F530–F534. doi: 10.1152/ajprenal.1983.245.4.F530. [DOI] [PubMed] [Google Scholar]
- 337.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Ren Physiol. 2008;295:F1090–F1095. doi: 10.1152/ajprenal.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Silva GB, Garvin JL. Akt1 mediates purinergic-dependent NOS3 activation in thick ascending limbs. Am J Physiol Ren Physiol. 2009;297:F646–F652. doi: 10.1152/ajprenal.00270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Silva GB, Garvin JL. Extracellular ATP inhibits transport in medullary thick ascending limbs: role of P2X receptors. Am J Physiol Ren Physiol. 2009;297:F1168–F1173. doi: 10.1152/ajprenal.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Silva GB, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension. 2006;47:563–567. doi: 10.1161/01.HYP.0000197954.93874.ef. [DOI] [PubMed] [Google Scholar]
- 341.Simmons NL. Identification of a purine (P2) receptor linked to ion transport in a cultured renal (MDCK) epithelium. Br J Pharmacol. 1981;73:379–384. doi: 10.1111/j.1476-5381.1981.tb10432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20:1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Skøtt O, Baumbach L. Effects of adenosine on renin release from isolated rat glomeruli and kidney slices. Pflugers Arch. 1985;404:232–237. doi: 10.1007/BF00581244. [DOI] [PubMed] [Google Scholar]
- 344.Smith JA, Whitaker EM, Bowmer CJ, Yates MS. Differential expression of renal adenosine A1 receptors induced by acute renal failure. Biochem Pharmacol. 2000;59:727–732. doi: 10.1016/s0006-2952(99)00369-x. [DOI] [PubMed] [Google Scholar]
- 345.Smith JA, Sivaprasadarao A, Munsey TS, Bowmer CJ, Yates MS. Immunolocalisation of adenosine A1 receptors in the rat kidney. Biochem Pharmacol. 2001;61:237–244. doi: 10.1016/s0006-2952(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 346.Solini A, Iacobini C, Ricci C, Chiozzi P, Amadio L, Pricci F, Di MU, Di Virgilio F, Pugliese G. Purinergic modulation of mesangial extracellular matrix production: role in diabetic and other glomerular diseases. Kidney Int. 2005;67:875–885. doi: 10.1111/j.1523-1755.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- 347.Solini A, Santini E, Chimenti D, Chiozzi P, Pratesi F, Cuccato S, Falzoni S, Lupi R, Ferrannini E, Pugliese G, Di Virgilio F. Multiple P2X receptors are involved in the modulation of apoptosis in human mesangial cells: evidence for a role of P2X4. Am J Physiol Ren Physiol. 2007;292:F1537–F1547. doi: 10.1152/ajprenal.00440.2006. [DOI] [PubMed] [Google Scholar]
- 348.Souza-Menezes J, Morales M. CFTR structure and function: is there a role in the kidney? Biophys Rev. 2009;1:3–12. doi: 10.1007/s12551-008-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Spielman WS, Arend LJ. Adenosine receptors and signaling in the kidney. Hypertension. 1991;17:117–130. doi: 10.1161/01.hyp.17.2.117. [DOI] [PubMed] [Google Scholar]
- 350.Stefanovic V, Vlahovic P. A2 adenosine receptors in human glomerular mesangial cells. Experientia. 1995;51:360–362. doi: 10.1007/BF01928895. [DOI] [PubMed] [Google Scholar]
- 351.Steinhausen M, Blum M, Fleming JT, Holz FG, Parekh N, Wiegman DL. Visualization of renal autoregulation in the split hydronephrotic kidney of rats. Kidney Int. 1989;35:1151–1160. doi: 10.1038/ki.1989.104. [DOI] [PubMed] [Google Scholar]
- 352.Stewart GS, Glanville M, Aziz O, Simmons NL, Gray MA. Regulation of an outwardly rectifying chloride conductance in renal epithelial cells by external and internal calcium. J Membr Biol. 2001;180:49–64. doi: 10.1007/s002320010058. [DOI] [PubMed] [Google Scholar]
- 353.Stiepanow-Trzeciak A, Jankowski M, Angielski S, Szczepanska-Konkel M. P1, P4-diadenosine tetraphosphate (Ap4A) inhibits proximal tubular reabsorption of sodium in rats. Nephron Physiol. 2007;106:13–18. doi: 10.1159/000101488. [DOI] [PubMed] [Google Scholar]
- 354.Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol. 2010;21:1903–1911. doi: 10.1681/ASN.2010040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sumpio BE, Chaudry IH, Baue AE. Reduction of the drug-induced nephrotoxicity by ATP-MgCl2. 1. Effects on the cis-diamminedichloroplatinum-treated isolated perfused kidneys. J Surg Res. 1985;38:429–437. doi: 10.1016/0022-4804(85)90058-7. [DOI] [PubMed] [Google Scholar]
- 356.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Sun R, Carlson NG, Hemmert AC, Kishore BK. P2Y 2 receptor-mediated release of prostaglandin E2 by IMCD is altered in hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Ren Physiol. 2005;289:F585–F592. doi: 10.1152/ajprenal.00050.2005. [DOI] [PubMed] [Google Scholar]
- 358.Suzuki F, Shimada J, Mizumoto H, Karasawa A, Kubo K, Nonaka H, Ishii A, Kawakita T. Adenosine A1 antagonists. 2. Structure–activity relationships on diuretic activities and protective effects against acute renal failure. J Med Chem. 1992;35:3066–3075. doi: 10.1021/jm00094a022. [DOI] [PubMed] [Google Scholar]
- 359.Szczepanska-Konkel M, Jankowski M, Stiepanow-Trzeciak A, Angielski S. Effects of diadenosine polyphosphates on glomerular volume. Br J Pharmacol. 2005;144:1109–1117. doi: 10.1038/sj.bjp.0706149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Takeda M, Yoshitomi K, Imai M. Regulation of Na+-3HCO3- cotransport in rabbit proximal convoluted tubule via adenosine A1 receptor. Am J Physiol. 1993;265:F511–F519. doi: 10.1152/ajprenal.1993.265.4.F511. [DOI] [PubMed] [Google Scholar]
- 361.Takeda M, Kawamura T, Kobayashi M, Endou H. ATP-induced calcium mobilization in glomerular mesangial cells is mediated by P2U purinoceptor. Biochem Mol Biol Int. 1996;39:1193–1200. doi: 10.1080/15216549600201382. [DOI] [PubMed] [Google Scholar]
- 362.Takenaka T, Hayashi K, Ikenaga H. Blood pressure regulation and renal microcirculation. Contrib Nephrol. 2004;143:46–64. doi: 10.1159/000078711. [DOI] [PubMed] [Google Scholar]
- 363.Takenaka T, Okada H, Kanno Y, Inoue T, Ryuzaki M, Nakamoto H, Kawachi H, Shimizu F, Suzuki H. Exogenous 5′-nucleotidase improves glomerular autoregulation in Thy-1 nephritic rats. Am J Physiol Ren Physiol. 2006;290:F844–F853. doi: 10.1152/ajprenal.00112.2005. [DOI] [PubMed] [Google Scholar]
- 364.Takenaka T, Inoue T, Kanno Y, Okada H, Hill CE, Suzuki H. Connexins 37 and 40 transduce purinergic signals mediating renal autoregulation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1–R11. doi: 10.1152/ajpregu.00269.2007. [DOI] [PubMed] [Google Scholar]
- 365.Tamura Y, Tanabe K, Kitagawa W, Uchida S, Schreiner GF, Johnson RJ, Nakagawa T. Nicorandil, a Katp channel opener, alleviates chronic renal injury by targeting podocytes and macrophages. Am J Physiol Ren Physiol. 2012;303:F339–F349. doi: 10.1152/ajprenal.00158.2012. [DOI] [PubMed] [Google Scholar]
- 366.Taneyama C, Goto H, Benson KT, Unruh GK, Arakawa K. Vagal involvement in the action of exogenous adenosine triphosphate on reflex renal sympathetic nerve activity. Anesth Analg. 1991;72:351–358. doi: 10.1213/00000539-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 367.Tang Y, Zhou L. Characterization of adenosine A1 receptors in human proximal tubule epithelial (HK-2) cells. Receptors Channels. 2003;9:67–75. [PubMed] [Google Scholar]
- 368.Taylor SRJ, Turner CM, Elliott JI, Hewitt R, Pickering M, Cook HT, Burnstock G, Pusey CD, Unwin R, Tam FWK. P2X7-deficiency ameliorates accelerated nephrotoxic nephritis in mice. J Am Soc Nephrol. 2009;20:1275–1281. doi: 10.1681/ASN.2008060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Teitelbaum I. Hormone signaling systems in inner medullary collecting ducts. Am J Physiol. 1992;263:F985–F990. doi: 10.1152/ajprenal.1992.263.6.F985. [DOI] [PubMed] [Google Scholar]
- 370.Thomas J, Deetjen P, Ko WH, Jacobi C, Leipziger J. P2Y2 receptor-mediated inhibition of amiloride-sensitive short circuit current in M-1 mouse cortical collecting duct cells. J Membr Biol. 2001;183:115–124. doi: 10.1007/s00232-001-0059-4. [DOI] [PubMed] [Google Scholar]
- 371.Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1769–R1776. doi: 10.1152/ajpregu.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Toney GM, Vallon V, Stockand JD. Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial Na+ channel. Curr Opin Nephrol Hypertens. 2012;21:52–60. doi: 10.1097/MNH.0b013e32834db4a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Torres B, Zambon AC, Insel PA. P2Y11 receptors activate adenylyl cyclase and contribute to nucleotide-promoted cAMP formation in MDCK-D1 cells. A mechanism for nucleotide-mediated autocrine–paracrine regulation. J Biol Chem. 2002;277:7761–7765. doi: 10.1074/jbc.M110352200. [DOI] [PubMed] [Google Scholar]
- 374.Tran E, Sun H, Fang Y. Dynamic mass redistribution assays decode surface influence on signaling of endogenous purinergic P2Y receptors. Assay Drug Dev Technol. 2012;10:37–45. doi: 10.1089/adt.2011.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Turner C, Vonend O, Chan CM, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Org. 2003;175:105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 376.Turner C, Ramesh B, Srai SKS, Burnstock G, Unwin R. Altered P2 receptor expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Org. 2004;178:168–179. doi: 10.1159/000082247. [DOI] [PubMed] [Google Scholar]
- 377.Turner CM, King BF, Srai KS, Unwin RJ. Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am J Physiol Ren Physiol. 2007;292:F15–F25. doi: 10.1152/ajprenal.00103.2006. [DOI] [PubMed] [Google Scholar]
- 378.Turner CM, Tam F, Lai P-C, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin RJ. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant. 2007;22:386–395. doi: 10.1093/ndt/gfl589. [DOI] [PubMed] [Google Scholar]
- 379.Turner CM, Elliott JI, Tam FW. P2 receptors in renal pathophysiology. Purinergic Signal. 2009;5:513–520. doi: 10.1007/s11302-009-9153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Udagawa T, Hanaoka K, Kawamura M, Hosoya T. Characteristics of spontaneous calcium oscillations in renal tubular epithelial cells. Clin Exp Nephrol. 2012;16:389–398. doi: 10.1007/s10157-012-0588-4. [DOI] [PubMed] [Google Scholar]
- 381.Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci. 2003;18:237–241. doi: 10.1152/nips.01436.2003. [DOI] [PubMed] [Google Scholar]
- 382.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Ren Physiol. 2008;294:F10–F27. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- 383.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Ren Physiol. 2011;301:F463–F475. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Vallon V, Mühlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 385.van der Weyden L, Adams DJ, Morris BJ. Capacity for purinergic control of renin promoter via P2Y11 receptor and cAMP pathways. Hypertension. 2000;36:1093–1098. doi: 10.1161/01.hyp.36.6.1093. [DOI] [PubMed] [Google Scholar]
- 386.Vargas F, Osuna A, Fernández-Rivas A. Renal vascular reactivity to ATP in hyper- and hypothyroid rats. Experientia. 1996;52:225–229. doi: 10.1007/BF01920711. [DOI] [PubMed] [Google Scholar]
- 387.Vekaria RM. Vesicular storage and release of ATP in a rat proximal tubule cell line. J Physiol. 2004;560:C17. [Google Scholar]
- 388.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol. 2006;17:1841–1847. doi: 10.1681/ASN.2005111171. [DOI] [PubMed] [Google Scholar]
- 389.Vekaria RM, Shirley DG, Sevigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Ren Physiol. 2006;290:F550–F560. doi: 10.1152/ajprenal.00151.2005. [DOI] [PubMed] [Google Scholar]
- 390.Vitzthum H, Weiss B, Bachleitner W, Krämer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int. 2004;65:1180–1190. doi: 10.1111/j.1523-1755.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 391.Vonend O, Oberhauser V, von Kügelgen I, Apel TW, Amann K, Ritz E, Rump LC. ATP release in human kidney cortex and its mitogenic effects in visceral glomerular epithelial cells. Kidney Int. 2002;61:1617–1626. doi: 10.1046/j.1523-1755.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- 392.Vonend O, Grote T, Oberhauser V, von Kügelgen I, Rump LC. P2Y-receptors stimulating the proliferation of human mesangial cells through the MAPK42/44 pathway. Br J Pharmacol. 2003;139:1119–1126. doi: 10.1038/sj.bjp.0705358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Vonend O, Turner C, Chan CM, Loesch A, Dell’Anna GC, Srai SK, Burnstock G, Unwin R. Glomerular expression of the ATP-sensitive P2X7 receptor in diabetic and hypertensive rat models. Kidney Int. 2004;66:157–166. doi: 10.1111/j.1523-1755.2004.00717.x. [DOI] [PubMed] [Google Scholar]
- 394.Walker M, III, Harrison-Bernard LM, Cook AK, Navar LG. Dynamic interaction between myogenic and TGF mechanisms in afferent arteriolar blood flow autoregulation. Am J Physiol Ren Physiol. 2000;279:F858–F865. doi: 10.1152/ajprenal.2000.279.5.F858. [DOI] [PubMed] [Google Scholar]
- 395.Wang J, Ouyang C, Chen X, Fu B, Lu Y, Hong Q. STAT3 inhibits apoptosis of human renal tubular epithelial cells induced by ATP depletion/recovery. Nephron Exp Nephrol. 2008;108:e11–e18. doi: 10.1159/000112557. [DOI] [PubMed] [Google Scholar]
- 396.Wang Y, Knowlton AA, Christensen TG, Shih T, Borkan SC. Prior heat stress inhibits apoptosis in adenosine triphosphate-depleted renal tubular cells. Kidney Int. 1999;55:2224–2235. doi: 10.1046/j.1523-1755.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 397.Wang YM, McRae JL, Robson SC, Cowan PJ, Zhang GY, Hu M, Polhill T, Wang Y, Zheng G, Wang Y, Lee VW, Unwin RJ, Harris DC, Dwyer KM, Alexander SI. Regulatory T cells participate in CD39-mediated protection from renal injury. Eur J Immunol. 2012;42:2441–2451. doi: 10.1002/eji.201242434. [DOI] [PubMed] [Google Scholar]
- 398.Weaver DR, Reppert SM. Adenosine receptor gene expression in rat kidney. Am J Physiol. 1992;263:F991–F995. doi: 10.1152/ajprenal.1992.263.6.F991. [DOI] [PubMed] [Google Scholar]
- 399.Wei Q, Wang J, Wang MH, Yu F, Dong Z. Inhibition of apoptosis by Zn2+ in renal tubular cells following ATP depletion. Am J Physiol Ren Physiol. 2004;287:F492–F500. doi: 10.1152/ajprenal.00083.2004. [DOI] [PubMed] [Google Scholar]
- 400.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Ren Physiol. 2010;299:F1220–F1236. doi: 10.1152/ajprenal.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Welch BD, Carlson NG, Shi H, Myatt L, Kishore BK. P2Y2 receptor-stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. Am J Physiol Ren Physiol. 2003;285:F711–F721. doi: 10.1152/ajprenal.00096.2003. [DOI] [PubMed] [Google Scholar]
- 402.Welch WJ. Adenosine A1 receptor antagonists in the kidney: effects in fluid-retaining disorders. Curr Opin Pharmacol. 2002;2:165–170. doi: 10.1016/s1471-4892(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 403.Welch WJ. Adenosine type 1 receptor antagonists in fluid retaining disorders. Expert Opin Inv Drugs. 2002;11:1553–1562. doi: 10.1517/13543784.11.11.1553. [DOI] [PubMed] [Google Scholar]
- 404.Welch WJ, Solis G, Wilcox CS. Adenosine receptors in the proximal tubule modulate deoxycorticosterone acetate (DOCA)-salt hypertension in mice. Hypertension. 2011;58:e60. [Google Scholar]
- 405.Wellhauser L, Luna-Chavez C, D’Antonio C, Tainer J, Bear CE. ATP induces conformational changes in the carboxyl-terminal region of ClC-5. J Biol Chem. 2011;286:6733–6741. doi: 10.1074/jbc.M110.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wengert M, Berto C, Jr, Kaufman J, Leão-Ferreira LR, Paes-de-Carvalho R, Lopes AG, Caruso-Neves C. Stimulation of the proximal tubule Na+-ATPase activity by adenosine A2A receptor. Int J Biochem Cell Biol. 2005;37:155–165. doi: 10.1016/j.biocel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 407.Wierema TK, Postma CT, Houben AJ, Kroon AA, Thien T, Smits P, de Leeuw PW. Adenosine-induced renal vasodilatation is prolonged in renal artery stenosis. J Hypertens. 1998;16:2109–2112. [PubMed] [Google Scholar]
- 408.Wierema TK, Houben AJ, Kroon AA, Postma CT, Koster D, van Engelshoven JM, Smits P, de Leeuw PW. Mechanisms of adenosine-induced renal vasodilatation in hypertensive patients. J Hypertens. 2005;23:1731–1736. doi: 10.1097/01.hjh.0000180160.89264.9d. [DOI] [PubMed] [Google Scholar]
- 409.Wildman SS, King BF. P2X receptors: epithelial ion channels and regulators of salt and water transport. Nephron Physiol. 2008;108:60–67. doi: 10.1159/000122028. [DOI] [PubMed] [Google Scholar]
- 410.Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Wildman SS, Marks J, Churchill LJ, Peppiatt CM, Chraibi A, Shirley DG, Horisberger JD, King BF, Unwin RJ. Regulatory interdependence of cloned epithelial Na+ channels and P2X receptors. J Am Soc Nephrol. 2005;16:2586–2597. doi: 10.1681/ASN.2005020130. [DOI] [PubMed] [Google Scholar]
- 412.Wildman SS, Turner CM, Marks J, Shirley DG, King BF, Unwin RJ. Differential regulation of renal ENaC by apical P2X and P2Y receptors. FASEB J. 2007;21:937.3. [Google Scholar]
- 413.Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol. 2008;19:731–742. doi: 10.1681/ASN.2007040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wildman SS, Kang ES, King BF. ENaC, renal sodium excretion and extracellular ATP. Purinergic Signal. 2009;5:481–489. doi: 10.1007/s11302-009-9150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wildman SS, Boone M, Peppiatt-Wildman CM, Contreras-Sanz A, King BF, Shirley DG, Deen PM, Unwin RJ. Nucleotides downregulate aquaporin 2 via activation of apical P2 receptors. J Am Soc Nephrol. 2009;20:1480–1490. doi: 10.1681/ASN.2008070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol. 1999;10:218–229. doi: 10.1681/ASN.V102218. [DOI] [PubMed] [Google Scholar]
- 417.Xia SL, Wang L, Cash MN, Teng X, Schwalbe RA, Wingo CS. Extracellular ATP-induced calcium signaling in mIMCD-3 cells requires both P2X and P2Y purinoceptors. Am J Physiol Ren Physiol. 2004;287:F204–F214. doi: 10.1152/ajprenal.00281.2003. [DOI] [PubMed] [Google Scholar]
- 418.Xiao H, Shen HY, Liu W, Xiong RP, Li P, Meng G, Yang N, Chen X, Si LY, Zhou YG. Adenosine A2A receptor: a target for regulating renal interstitial fibrosis in obstructive nephropathy. PLoS One. 2013;8:e60173. doi: 10.1371/journal.pone.0060173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Xie Y, Schafer JA. Endogenous ATP release inhibits electrogenic Na+ absorption and stimulates Cl− secretion in MDCK cells. Purinergic Signal. 2008;4:125–137. doi: 10.1007/s11302-007-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Xin C, Ren S, Pfeilschifter J, Huwiler A. Heterologous desensitization of the sphingosine-1-phosphate receptors by purinoceptor activation in renal mesangial cells. Br J Pharmacol. 2004;143:581–589. doi: 10.1038/sj.bjp.0705980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Ren Physiol. 2009;296:F1464–F1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Yamada H, Seki G, Taniguchi S, Uwatoko S, Suzuki K, Kurokawa K. Mechanism of [Ca2+]i increase by extracellular ATP in isolated rabbit renal proximal tubules. Am J Physiol. 1996;270:C1096–C1104. doi: 10.1152/ajpcell.1996.270.4.C1096. [DOI] [PubMed] [Google Scholar]
- 423.Yamaguchi S, Umemura S, Tamura K, Iwamoto T, Nyui N, Ishigami T, Ishii M. Adenosine A1 receptor mRNA in microdissected rat nephron segments. Hypertension. 1995;26:1181–1185. doi: 10.1161/01.hyp.26.6.1181. [DOI] [PubMed] [Google Scholar]
- 424.Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T. ATP-dependent mechanism for coordination of intercellular Ca2+ signaling and renin secretion in rat juxtaglomerular cells. Circ Res. 2003;93:338–345. doi: 10.1161/01.RES.0000086802.21850.5D. [DOI] [PubMed] [Google Scholar]
- 425.Yap SC, Lee HT. Adenosine and protection from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:24–32. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Zambon AC, Hughes RJ, Meszaros JG, Wu JJ, Torres B, Brunton LL, Insel PA. P2Y2 receptor of MDCK cells: cloning, expression, and cell-specific signaling. Am J Physiol Ren Physiol. 2000;279:F1045–F1052. doi: 10.1152/ajprenal.2000.279.6.F1045. [DOI] [PubMed] [Google Scholar]
- 427.Zambon AC, Brunton LL, Barrett KE, Hughes RJ, Torres B, Insel PA. Cloning, expression, signaling mechanisms, and membrane targeting of P2Y11 receptors in Madin Darby canine kidney cells. Mol Pharmacol. 2001;60:26–35. doi: 10.1124/mol.60.1.26. [DOI] [PubMed] [Google Scholar]
- 428.Zamir M, Phipps S. Morphometric analysis of the distributing vessels of the kidney. Can J Physiol Pharmacol. 1987;65:2433–2440. doi: 10.1139/y87-386. [DOI] [PubMed] [Google Scholar]
- 429.Zarjou A, Agarwal A. ATP as a death factor: purinergic signaling in renal epithelial–fibroblast cross talk. Am J Physiol Ren Physiol. 2011;300:F60–F61. doi: 10.1152/ajprenal.00593.2010. [DOI] [PubMed] [Google Scholar]
- 430.Zegarra-Moran O, Romeo G, Galietta LJ. Regulation of transepithelial ion transport by two different purinoceptors in the apical membrane of canine kidney (MDCK) cells. Br J Pharmacol. 1995;114:1052–1056. doi: 10.1111/j.1476-5381.1995.tb13312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Zhang L, Yang N, Wang S, Huang B, Li F, Tan H, Liang Y, Chen M, Li Y, Yu X. Adenosine 2A receptor is protective against renal injury in MRL/lpr mice. Lupus. 2011;20:667–677. doi: 10.1177/0961203310393262. [DOI] [PubMed] [Google Scholar]
- 432.Zhang Y, Sanchez D, Gorelik J, Klenerman D, Lab M, Edwards C, Korchev Y. Basolateral P2X4-like receptors regulate the extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. Am J Physiol Ren Physiol. 2007;292:F1734–F1740. doi: 10.1152/ajprenal.00382.2006. [DOI] [PubMed] [Google Scholar]
- 433.Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Ren Physiol. 2008;295:F1715–F1724. doi: 10.1152/ajprenal.90311.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Ren Physiol. 2011;300:F657–F668. doi: 10.1152/ajprenal.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zhang Y, Pop IL, Carlson NG, Kishore BK. Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Ren Physiol. 2012;302:F70–F77. doi: 10.1152/ajprenal.00444.2011. [DOI] [PubMed] [Google Scholar]
- 436.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, Wang S, Gaskin F, Yang N, Fu SM (2013) P2X7 blockade attenuates lupus nephritis by inhibiting NLRP3/ASC/caspase-1 activation. Arthritis Rheum [DOI] [PMC free article] [PubMed]
- 437.Zhao Z, Kapoian T, Shepard M, Lianos EA. Adenosine-induced apoptosis in glomerular mesangial cells. Kidney Int. 2002;61:1276–1285. doi: 10.1046/j.1523-1755.2002.00256.x. [DOI] [PubMed] [Google Scholar]