Abstract
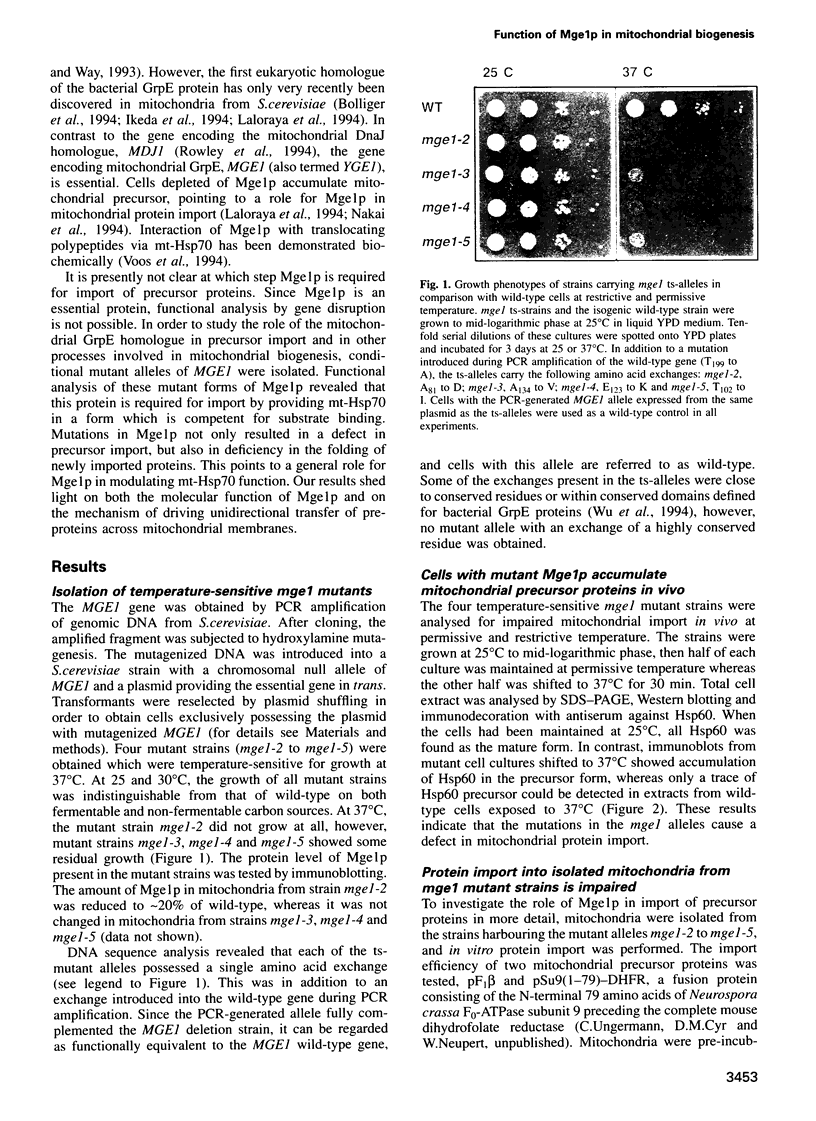
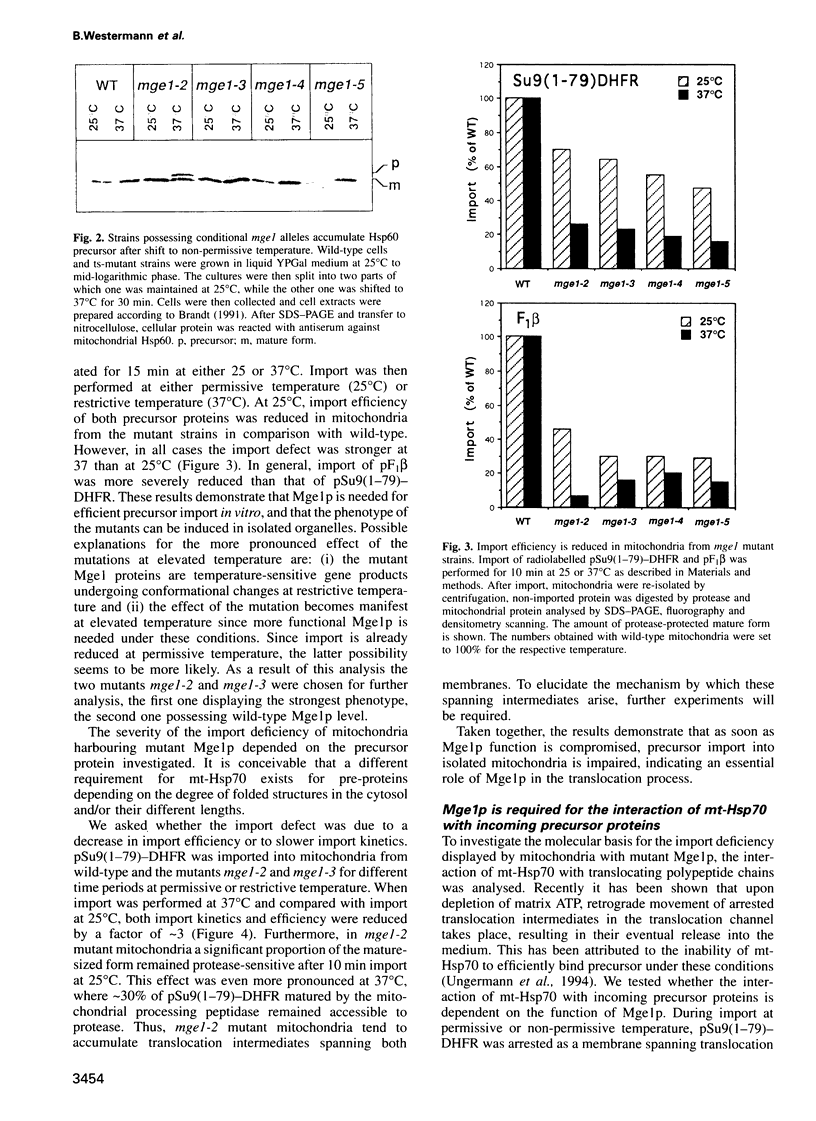
Mge1p, a mitochondrial GrpE homologue, has recently been identified in the yeast Saccharomyces cerevisiae and a role for this protein in precursor import has been reported. To dissect the molecular mechanism of Mge1p function, conditional mge1 mutants were constructed. Cells harbouring mutant mge1 accumulated precursor proteins at restrictive temperature. Both kinetics and efficiency of import were reduced in mitochondria isolated from strains possessing mutant mge1. Binding of mitochondrial-Hsp70 (mt-Hsp70) to incoming precursor proteins was abolished at restrictive temperature. Nucleotide-dependent dissociation of mt-Hsp70 from the import component MIM44 was reduced in mitochondria from mutant mge1 strains. Furthermore, at restrictive temperature an increase of incompletely folded, newly imported protein and enhanced protein aggregation was observed in mitochondria isolated from the mutant strains. We conclude that Mge1p exerts an essential function in import and folding of proteins by controlling the nucleotide-dependent binding of mt-Hsp70 to substrate proteins and the association of mt-Hsp70 with MIM44.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bolliger L., Deloche O., Glick B. S., Georgopoulos C., Jenö P., Kronidou N., Horst M., Morishima N., Schatz G. A mitochondrial homolog of bacterial GrpE interacts with mitochondrial hsp70 and is essential for viability. EMBO J. 1994 Apr 15;13(8):1998–2006. doi: 10.1002/j.1460-2075.1994.tb06469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A. Pulse labeling of yeast cells as a tool to study mitochondrial protein import. Methods Cell Biol. 1991;34:369–376. doi: 10.1016/s0091-679x(08)61691-x. [DOI] [PubMed] [Google Scholar]
- Buchberger A., Schröder H., Büttner M., Valencia A., Bukau B. A conserved loop in the ATPase domain of the DnaK chaperone is essential for stable binding of GrpE. Nat Struct Biol. 1994 Feb;1(2):95–101. doi: 10.1038/nsb0294-95. [DOI] [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. Eukaryotic homologues of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol Biol Cell. 1993 Jun;4(6):555–563. doi: 10.1091/mbc.4.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992 Jan 2;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Weissman J. S., Horwich A. L. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994 Aug 12;78(3):365–372. doi: 10.1016/0092-8674(94)90416-2. [DOI] [PubMed] [Google Scholar]
- Cyr D. M., Stuart R. A., Neupert W. A matrix ATP requirement for presequence translocation across the inner membrane of mitochondria. J Biol Chem. 1993 Nov 15;268(32):23751–23754. [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Ellis R. J. The general concept of molecular chaperones. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):257–261. doi: 10.1098/rstb.1993.0023. [DOI] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993 Oct;123(1):109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992 Mar 25;20(6):1425–1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S. Can Hsp70 proteins act as force-generating motors? Cell. 1995 Jan 13;80(1):11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Hwang S. T., Wachter C., Schatz G. Protein import into the yeast mitochondrial matrix. A new translocation intermediate between the two mitochondrial membranes. J Biol Chem. 1991 Nov 5;266(31):21083–21089. [PubMed] [Google Scholar]
- Ikeda E., Yoshida S., Mitsuzawa H., Uno I., Toh-e A. YGE1 is a yeast homologue of Escherichia coli grpE and is required for maintenance of mitochondrial functions. FEBS Lett. 1994 Feb 21;339(3):265–268. doi: 10.1016/0014-5793(94)80428-1. [DOI] [PubMed] [Google Scholar]
- Jascur T., Goldenberg D. P., Vestweber D., Schatz G. Sequential translocation of an artificial precursor protein across the two mitochondrial membranes. J Biol Chem. 1992 Jul 5;267(19):13636–13641. [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kronidou N. G., Oppliger W., Bolliger L., Hannavy K., Glick B. S., Schatz G., Horst M. Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12818–12822. doi: 10.1073/pnas.91.26.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloraya S., Gambill B. D., Craig E. A. A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6481–6485. doi: 10.1073/pnas.91.14.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Liberek K., Marszalek J., Ang D., Georgopoulos C., Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M., Kato Y., Ikeda E., Toh-e A., Endo T. Yge1p, a eukaryotic Grp-E homolog, is localized in the mitochondrial matrix and interacts with mitochondrial Hsp70. Biochem Biophys Res Commun. 1994 Apr 15;200(1):435–442. doi: 10.1006/bbrc.1994.1468. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Reid K. L., Shi L., Welch W. J., Fink A. L. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993 Oct 14;365(6447):664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Rassow J., Maarse A. C., Krainer E., Kübrich M., Müller H., Meijer M., Craig E. A., Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994 Dec;127(6 Pt 1):1547–1556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Pfanner N. Mitochondrial preproteins en route from the outer membrane to the inner membrane are exposed to the intermembrane space. FEBS Lett. 1991 Nov 18;293(1-2):85–88. doi: 10.1016/0014-5793(91)81157-4. [DOI] [PubMed] [Google Scholar]
- Rowley N., Prip-Buus C., Westermann B., Brown C., Schwarz E., Barrell B., Neupert W. Mdj1p, a novel chaperone of the DnaJ family, is involved in mitochondrial biogenesis and protein folding. Cell. 1994 Apr 22;77(2):249–259. doi: 10.1016/0092-8674(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Schneider H. C., Berthold J., Bauer M. F., Dietmeier K., Guiard B., Brunner M., Neupert W. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature. 1994 Oct 27;371(6500):768–774. doi: 10.1038/371768a0. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Seytter T., Guiard B., Neupert W. Targeting of cytochrome b2 into the mitochondrial intermembrane space: specific recognition of the sorting signal. EMBO J. 1993 Jun;12(6):2295–2302. doi: 10.1002/j.1460-2075.1993.tb05883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Boeke J. D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. A., Way J. C. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell. 1993 Jul 16;74(1):5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Cyr D. M., Craig E. A., Neupert W. Mitochondrial molecular chaperones: their role in protein translocation. Trends Biochem Sci. 1994 Feb;19(2):87–92. doi: 10.1016/0968-0004(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Gruhler A., van der Klei I., Guiard B., Koll H., Neupert W. The requirement of matrix ATP for the import of precursor proteins into the mitochondrial matrix and intermembrane space. Eur J Biochem. 1994 Feb 15;220(1):9–18. doi: 10.1111/j.1432-1033.1994.tb18593.x. [DOI] [PubMed] [Google Scholar]
- Szabo A., Langer T., Schröder H., Flanagan J., Bukau B., Hartl F. U. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system DnaK, DnaJ, and GrpE. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10345–10349. doi: 10.1073/pnas.91.22.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Neupert W., Cyr D. M. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994 Nov 18;266(5188):1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- Voos W., Gambill B. D., Guiard B., Pfanner N., Craig E. A. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J Cell Biol. 1993 Oct;123(1):119–126. doi: 10.1083/jcb.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W., Gambill B. D., Laloraya S., Ang D., Craig E. A., Pfanner N. Mitochondrial GrpE is present in a complex with hsp70 and preproteins in transit across membranes. Mol Cell Biol. 1994 Oct;14(10):6627–6634. doi: 10.1128/mcb.14.10.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner I., Arlt H., van Dyck L., Langer T., Neupert W. Molecular chaperones cooperate with PIM1 protease in the degradation of misfolded proteins in mitochondria. EMBO J. 1994 Nov 1;13(21):5135–5145. doi: 10.1002/j.1460-2075.1994.tb06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hoskins J., McKenney K. Function of DnaJ and DnaK as chaperones in origin-specific DNA binding by RepA. Nature. 1991 Mar 14;350(6314):165–167. doi: 10.1038/350165a0. [DOI] [PubMed] [Google Scholar]
- Wickner W. T. How ATP drives proteins across membranes. Science. 1994 Nov 18;266(5188):1197–1198. doi: 10.1126/science.7973701. [DOI] [PubMed] [Google Scholar]
- Wu B., Ang D., Snavely M., Georgopoulos C. Isolation and characterization of point mutations in the Escherichia coli grpE heat shock gene. J Bacteriol. 1994 Nov;176(22):6965–6973. doi: 10.1128/jb.176.22.6965-6973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]