Abstract
Background
Infantile hemangioma (IH) is the most common tumor of infancy. Hemangioma stem cells (HemSC) are a mesenchymal subpopulation isolated from IH CD133+ cells. HemSC can differentiate into endothelial and pericyte/smooth muscle cells and form vascular networks when injected in immune-deficient mice. α6-Integrin subunit has been implicated in the tumorgenicity of glioblastoma stem cells and the homing properties of hematopoietic, endothelial and mesenchymal progenitor cells. Therefore, we investigated the possible function(s) of α6-integrin in HemSC.
Methods/Results
We documented α6-integrin expression in IH tumor specimens and HemSC by RT-qPCR and flow cytometry. We examined the effect of blocking or silencing α6-integrin on the adhesive and proliferative properties of HemSCin vitro and the vasculogenic and homing properties of HemSCin vivo. Targeting α6-integrin in cultured HemSC inhibited adhesion to laminin but had no effect on proliferation. Vessel-forming ability in Matrigel implants and hepatic homing after intravenous delivery were significantly decreased in α6-integrin siRNA transfected HemSC.
Conclusion
α6-Integrin is required for HemSC adherence to laminin, vessel formation in vivo and for homing to the liver. Thus, we uncovered an important role for α6 integrin in the vasculogenic properties of HemSC. Our results suggest that α6-integrin expression on HemSC could be a new target for anti-hemangioma therapy.
Keywords: hemangioma stem cells, α6-integrin, adhesion, vasculogenesis, Fluorescence Molecular Tomography
Introduction
Integrins are receptors important for cellular adhesion to extracellular matrix (ECM) and to other cells. The integrin family consists of α- (18 types) and β- (8 types) subunits that can form 24 distinct heterodimers of which the composition dictates ligand specificity 1. The α6-integrin subunit (α6) is a 140-kDa protein that can associate with β1- or β4-integrin subunits. α6-integrin is expressed during the early murine developmental stages in which laminin-containing basement membrane is produced 2. Integrin α6β1 is a receptor for laminin and is expressed on platelets, monocytes/macrophages, neutrophils, endothelial cells and their progenitors 3, 4. Integrin α6β4 also binds to laminin and is responsible for intercellular adherens junctions called hemidesmosomes5. In humans, defects in α6-integrin result in deficient hemidesmosomes, cause skin and mucous membrane disorders (epidermolysisbullosa, pyloric atresia) and, in most cases, early postnatal death 6, 7. The α6-integrin subunit is also involved in angiogenesis; it is required for human brain microvascular endothelial cells 8 or endothelial progenitor cells 3 to form vascular networks in vitro. Recently, glioblastoma stem cells have been shown to express high levels of α6-integrin. Targeting α6-integrin decreased self-renewal and proliferation of glioblastoma stem cells and reduced their ability to form tumors9. Moreover, α6-integrin subunit is important in myogenic stem cell differentiation 10 and has been associated with CD117-positive cells in the sub-epicardium of adult human heart 11. Hematopoietic stem cell homing to bone marrow has been shown to be dependent on α6-integrin subunit 12. In summary, α6-integrin plays a myriad of roles in specific cellular contexts.
The hallmark of infantile hemangioma (IH) is its unique life cycle: neonatal proliferation followed by slow regression and cessation of growth during childhood 13. Hemangioma stem cells (HemSC) have been isolated from specimens of proliferating IH 14-16. These cells are multi-potent, exhibit a mesenchymal morphology and proliferate rapidly in vitro 14. HemSC can form blood vessels with the immunophenotype and dynamics of IH when injected subcutaneously into nude mice 14. Laminin has been detected in the thickened basement membranes of hemangioma17, 18 and α6-integrin subunit can contribute to tumor angiogenesis and invasive properties of tumor cells 19-21, Therefore, we analyzed expression of α6-integrin subunit in HemSC isolated from proliferating IH, and tested its role in their vasculogenic potential. Our findings implicate α6-integrin in the formation of hemangioma blood vessels and suggest that blocking α6-integrin subunit could be a way to treat hemangioma.
Materials and Methods
Cell Isolation and Culture
Specimens of IH were obtained under a human subject protocol approved by the Committee on Clinical Investigation, Boston Children's Hospital. Informed consent was obtained for the specimens, according to the Declaration of Helsinki. The clinical diagnosis was confirmed by histopathology. Single cell suspensions were prepared from the proliferating phase specimens by collagenase digestion and anti-CD133-coated magnetic beads were used to isolate HemSC14, 22-24. The three HemSCs used in this paper were from two different female infants and one male infant who were 3 months, 10 months and 2 years old, respectively, at time of IH removal.
Real-Time quantitative PCR (RT-qPCR)
RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA synthesis was performed with the iScriptcDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). To measure the input target, the parameter Ct (threshold cycle), defined as the fractional cycle number at which fluorescence generated by SYBR green dye–amplicon complex formation passes a fixed threshold above baseline. As an endogenous RNA control, we quantified transcripts of the TBP gene encoding the TATA box-binding protein (a component of the DNA-binding protein complex TFIID). The amount of target transcript (Ntarget) was normalized on the basis of TBP content of each sample and was subsequently normalized to a basal mRNA level with the equation: Ntarget = 2ΔCtsample where ΔCt is the Ct value of the target gene minus the Ct value of the TBP gene. The results are presented as ‘normalized mRNA levels’, i.e., the N target value divided by the Ntarget value of the smallest quantifiable amount of target gene mRNA (i.e. target gene Ct value = 35). PCR efficiency was optimal and ranged from 90% to 100% in the different target gene RT-PCR assays. Thus, normalized mRNA levels were compared to mRNA content of the genes of interest (CD31, von Willebrand Factor (vWF), α6-integrin (ITGa6) normalized to CD31 content, CD133, N-cadherin and vimentin) in each proliferating and involuting IH. Primers for TBP and the target genes were chosen with the assistance of Oligo 5.0 software (National Biosciences, Plymouth, MN, USA). The nucleotide sequences of the primers are shown in Table 1.
Table 1. Primers Used for Quantitative Real-Time PCR.
| Gene name | Primer Sequences | |
|---|---|---|
| 5′ U 3′ | 5′ L 3′ | |
|
| ||
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA |
| CD31 | AAGTCGGACAGTGGGACGTATATC | GGCTGGGAGAGCATTTCACAT |
| CD133 | TGGTCCAACAGGGCTATCAATC | TTCAAGACCCTTTTGATACCTGCTA |
| N-CADH | GAGGGATCAAAGCCTGGAACAT | CGATTCTGTACCTCAACATCCCAT |
| VIM | CTCCCTCTGGTTGATACCCACTC | AGAAGTTTCGTTGATAACCTGTCCA |
| ITGa6 | CACATCTCCTCCCTGAGCACAT | TATATCTTGCCACCCATCCTTGTT |
Flow Cytometry
HemSC were labeled with PE-conjugated rat anti-human α6-integrin (BD Bioscience) or PE-conjugated isotype-matched control rat anti-human IgG2a (BD Bioscience). Flow cytometry was performed on a BD FACScan. Data were analyzed using FlowJo software (version 8.7).
In Vitro Assays for Cellular Proliferation and Viability
HemSC (1 × 104) were seeded on fibronectin or laminin-coated 24-well plates and cultured in growth medium (Endothelial Basal Cell Medium (EBM), SingleQuot Kit with 20% fetal bovine serum (FBS) (Lonza® Allendale, NJ, USA). All components from the Single Quot Kit, which contains growth factors, cytokines and supplements, were included in the growth medium except for hydrocortisone. Cell numbers on days 1-to-11 were counted by phase-contrast microscopy using disposable hemocytometers (from Digital Bio®, Seoul, Korea). Proliferation was also measured by cellular alkaline phosphatase activity using the substrate para-nitrophenol phosphate (pNPP) (Sigma). The released pNPP was measured by spectrophotometry, OD 405 nm, after 2, 3 or 4 days of growth.
Adhesion Assay
HemSC (1×104/well) were plated on 96-well polystyrene plates coated with fibronectin or laminin. After 20 minutes, non-adherent cells were washed off and the number of adherent cells determined in an alkaline phosphatase assay using pNPP. Each data point represents the average of three wells and each experiment was performed at least three times. For inhibition experiments, anti-human α6-integrin (clone GoH3, R&D Systems) was added to the HemSC at a concentration of 10 μg/ml, 2 hours before the HemSC were added to the matrix-coated wells.
In Vivo Model of Infantile Hemangioma and Microvessel Density
Experiments were performed with 3×106 cells per implant, as described previously 16, 23, 24. HemSC were suspended in 200 μL of Matrigel (BD Bioscience, Bedford, MA – catalog number 356237) and injected subcutaneously on the back of 6- to 7-week-old male athymic nu/nu mice (Massachusetts General Hospital, Boston, MA). To assess microvessel density (MVD), luminal structures containing red blood cells were counted in 4 fields of a mid-Matrigelhematoxylin and eosin (H&E)–stained section from each animal in every group (n=5 mice per groups). MVD was expressed as vessels/mm2 +/− standard error of the mean.
Cell Transfection with siRNA against α6-Integrin
siRNA, previously shown to silence α6-integrin (sc- 43129, Santa Cruz Biotechnology, Santa Cruz, CA, USA), was mixed with the Primefect reagent (LONZA) at 10 μM to obtain the transfection complexes, which were added to 1.5×105HemSC in complete EGM2 medium in 6-well plates. Scrambled siRNA (Allstars Neg. control siRNA, Qiagen, Cambridge, MA, USA) was used as a control.
VEGF-A Protein Quantification
VEGF-A secreted in the culture medium of HemSC was quantified using the Quantikine Human VEGF enzyme-linked immunosorbent assay (R&D Systems), as previously described 23, 25.
HemSC-to-Pericyte Differentiation Assay
HemSC-to-pericyte differentiation was performed, as previously described 16, by seeding siRNA transfected HemSC together with cord blood endothelial colony forming cells (ECFC), formerly called endothelial progenitor cells (EPC), at a ratio of 1:1 and a total density of 104 cells/cm2 on fibronectin-coated plates in EGM-2/20% fetal bovine serum.
Fluorescence Molecular Tomography
The fate of intravenously delivered HemSC in vivo was followed by labeling HemSC, transfected with control-siRNA or α6-siRNA, with a near-infrared cell tracker reagent VivoTag 680 XL (VT680; Ex: 670±5 nm, Em: 688±5 nm; MW: 1856 g.mol−1), obtained from Perkin Elmer (Boston, MA). Mice were imaged on the Fluorescence Molecular Tomography™ (FMT) system (VisEn Medical, Perkin Elmer) 26 to determine the location and quantity of HemSC labeled with VT680. VT680 was dissolved in DMSO and stored in aliquots of 5 mM at −20°C. Sufficient volume to ensure a final VT680 concentration of 100 μM was added to 1×106 cells/ml suspended in PBS. Cells were incubated with VT680 at 37°C for 30 min at 5% CO2, washed twice after labeling and resuspended to 1×106 cells/ml in PBS. Efficacy of labeling was determined by flow cytometry.
The FMT 2500™ LX system provides non-invasive, whole body, deep tissue quantitative imaging. Mice were imaged using the FMT system (VisEn Medical, Perkin Elmer) 6 hours and 2 days after retro-orbital i.v. injection of labeled cells. Mice were anesthetized by inhalation of isoflurane and placed in the imaging chamber, where anesthesia was maintained in the system by isoflurane diffusion. Reflectance images were taken in white light and fluorescent modes. Non-invasive fluorescent tomographic imaging was carried out in the 680 channel. FMT software allows for 3D reconstruction of the imaging data utilizing a normalized born equation. Following the reconstruction, volumes of interest (VOI's) were selected by drawing regions of interest (ROI's) in all 3 imaging planes (X, Y, Z). Because food chlorophyl is auto-fluorescent, for the final analysis, nude mice were euthanized 48 hours after injection of HemSC for organ fluorescent and flow cytometric analysis (n=7 per group). Liver, lung, spleen and kidney were excised, and analyzed in white light and fluorescent modes with FMT.
Liver was the only organ showing a fluorescent signal after 48 hours; this was documented with classic optic photomicrography. Livers were weighted and disrupted on a 50 μm cell strainer (BD Bioscience, Bedford, MA), washed once in PBS and resuspended in 1950 μl of PBS. 50μl of green fluorescent counting beads (FlowCount, Beckman Coulter), with a known concentration per volume, was added to obtain a 2 ml final volume suspension. The hepatic cell suspension with counting beads was processed by flow cytometry on a multicolor flow cytometer (Fortessa, BD Biosciences) and VT 680 expression was collected in a 660/60 band pass filter emission. Non-injected control HemSC labeled with VT680-XL were used as positive control to define the gate of analysis. To confirm the absolute HemSC size, we used a flow cytometry size calibration kit (Invitrogen, Reference F13838), which uses microsphere suspensions (6-15 μm) to serve as reliable size references for flow cytometric analyses.
Statistical Analysis
Data were expressed as mean ± SEM and were analyzed by Mann-Whitney. Differences were considered significant at P values <0.05.
Results
α6-Integrin is increased in proliferating phase of IH and expressed by hemangioma stem cells
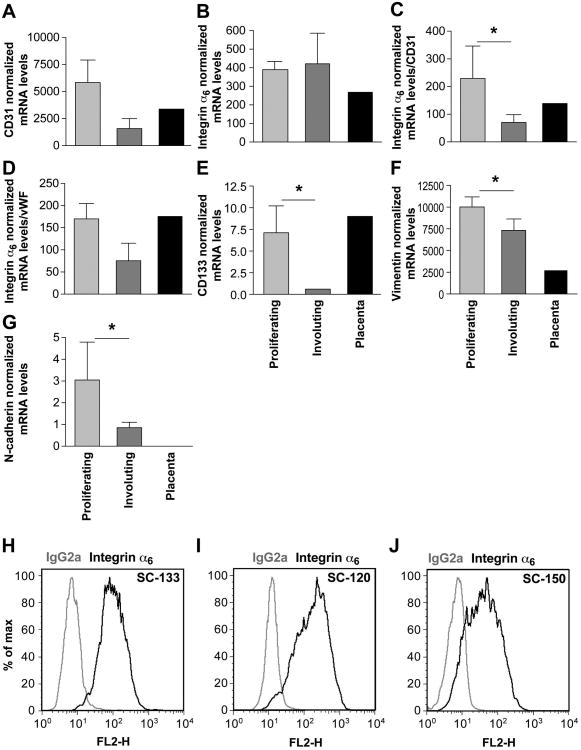
To evaluate a potential relationship between α6-integrin and the life cycle of hemangioma, we analyzed six IH tissues, three proliferating phase and three involuting phase, for mRNA expression of α6-integrin (Figure 1A-G, light and dark grey bars). Placental tissue was analyzed for comparison (Figure 1A-G, black bars). As shown in Figure 1A, proliferating IH had higher CD31 expression than involuting IH. However, the difference did not reach significance (p = 0.08), indicating that the involuting tissues are still vascular. α6-Integrin levels were similar between proliferating and involuting phase IH (p = 0.83) (Figure 1B). Because α6-integrin is expressed on endothelial cells and their progenitors, we normalized α6-integrin to the mRNA transcript levels of CD31 and vWF in the IH samples (Figure 1C, D). This provides a measure of the degree to which α6-integrin is expressed relative to the vascularity of the individual IH specimen. This RT-qPCR analysis revealed higher expression of α6-integrin mRNA in proliferating versus involuting phase IH. The stem cell antigen CD133 and mesenchymal markers vimentin and N-cadherin were also significantly decreased in involuting phase (Figure 1E-G), confirming importance of stemness and mesenchymal phenotype cells in proliferating phase. Because we previously showed that hemangioma stem cells (HemSC) can form hemangioma-like blood vessels in mice 14, we tested α6-integrin expression in HemSC isolated from three different IH tumors. Figure 1H-J represent cell surface profiles of α6-integrin protein on three different HemSC.
Figure 1. α6-integrin is expressed in proliferating IH and in HemSC.
A-B-C-D-E-F-G RT-qPCR analysis of CD31, α6-integrin, CD133, vimentin and N-cadherin in proliferating and involuting IH specimens. mRNA levels were normalized to the housekeeping gene TBP in A, B, E, F and G. mRNA levels in C and D were normalized again to CD31 and vWF, respectively. Mean and SEM values of 3 different samples are shown at each point. (* indicates P values <0.05. C, P=0.04; E, P=0.03; F, P=0.05; G, P=0.04. H/I/J - Flow cytometric analysis of HemSC isolated from three different proliferating IH. Black lines: cells labeled with PE-conjugated anti-α6-integrin. Gray lines: cells labeled with PE-conjugated isotype-matched control antibodies. The MFI values observed for these 3 samples were of 91, 110 and 88.7 respectively. The MFI values for the isotype-matched IgG controls were 8.7, 6.3 and 5.02.
Blocking α6-integrin inhibits HemSC adhesion to laminin in vitro and ability to form vessels in vivo
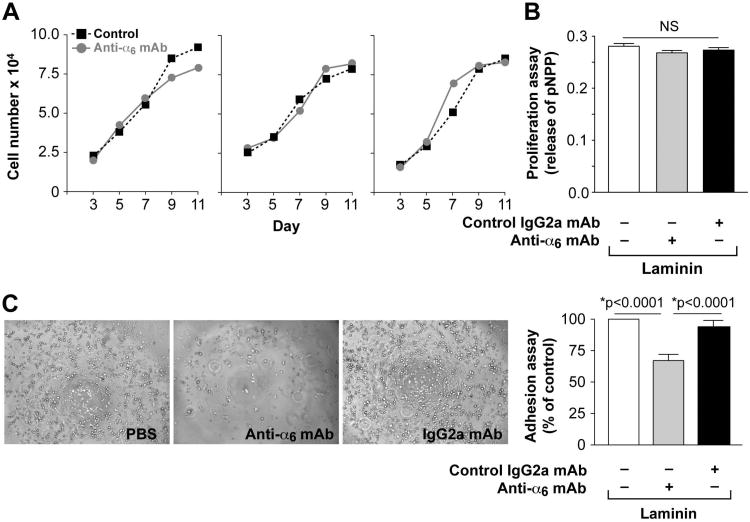
α6-integrin has been described as a marker of proliferation, self-renewal and tumor formation in glioblastoma stem cells 9; as well as a target for adhesion and differentiation of endothelial progenitor cells 3, 4. Therefore, we tested the hypothesis that α6-integrin acts as a modulator of one or more of these steps in HemSC. At a basal level, without blocking antibody, HemSCs have the same proliferative properties on fibronectin or laminin (Figure S1A). Moreover, HemSCs have the same adhesive properties to fibronectin or laminin in the absence of blocking antibody (Figure S1C). To test the effect of α6 integrin blocking mAb on cellular proliferation, the cells were allowed to adhere to the matrix-coated wells 24 hours before adding blocking mAb or the control mAb. The blocking anti-α6-integrin mAb had no effect on the proliferative capacity of HemSC, plated on laminin or fibronectin, in two different assays (Figure 2A, 2B and Supplemental Figure 1). For the adhesion test, the antibody was incubated with the HemSC for 2 hours before the cells were added to the matrix-coated wells. HemSC adhered to both laminin- and fibronectin-coated wells, but laminin-mediated adhesion was inhibited by anti-α6-integrin mAb (Figure 2C, Supplemental Figure 1D).
Figure 2. Blocking mAb against α6-integrin had no effect on proliferation of HemSC but decreased adhesion of HemSC to laminin.
A- Proliferation of 3 different HemSC cultured in EBM-2/20% FBS in presence (closed grey circles) or absence (closed black squares) of α6-integrin blocking mAb over 11 days evaluated by counting cells.
B- Proliferation of HemSC cultured in EBM-2/20% FBS in presence or absence of α6-integrin blocking mAb over 4 days evaluated by measuring cellular phosphatase activity.
C- α6-integrin blocking mAb decreased HemSC adhesion to laminin-coated wells. Adherent cells in the phase images appear round and translucent (original magnification, ×10).
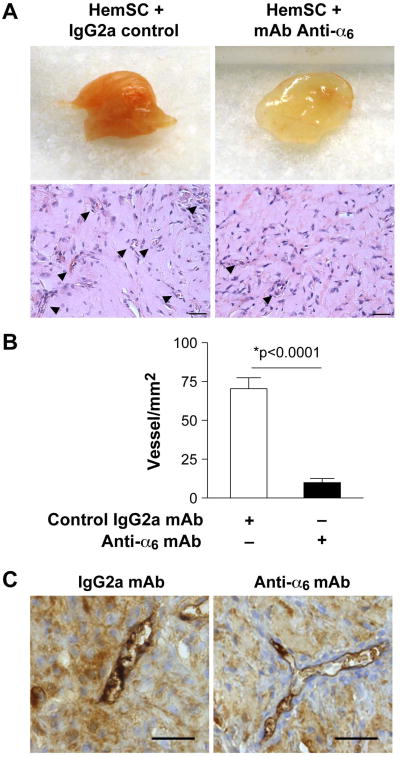
We tested the possible role of α6-integrin in vessel-forming ability of HemSC by adding the blocking anti-α6-integrin mAb to HemSC suspended in Matrigel prior to injection into immune-deficient mice. Anti-α6-integrin mAb reduced microvessel density (MVD) in the HemSC/Matrigel implants by 60%, (P<0.0001)(Figure 3A, B). To confirm anti- α6 integrin GoH3 antibody clone block vascularisation induced by human HemSC and not by murine cells in the Matrigel implant, we immunostained implant sections with an anti-human CD31 mAb and found that vessels in both conditions have a human origin (Figure 3C).
Figure 3. Blocking mAb against α6-integrin decreasedvasculogenic potential of HemSC.
A- HemSC suspended in Matrigel with α6-integrin blocking mAb or control mAb and injected s.c. into nude mice. Representative photographs of Matrigel explants at day10 after injection with corresponding histological sections stained with H&E. Black arrowheads point to luminal structures with red blood cells. Scale bar = 50 μm.
B- Quantification of lumens with red blood cells, reported as vessels/mm2. Data are mean ± SEM.
C- Immunohistochemical staining for human CD31 in implants after 10 days (scale bars, 50 μm).
α6-Integrin knockdown reduces cell adhesion in vitro and vessel formation in vivo
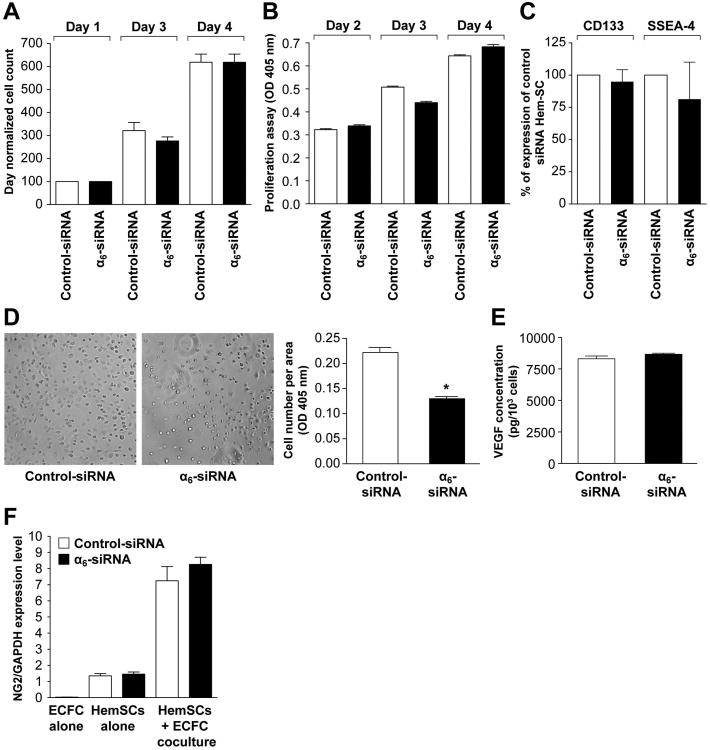
siRNA-mediated knockdown of α6 integrin in HemSC was used to confirm the results observed with anti-α6 integrin mAb. We validated α6-integrin knockdown by RT-qPCR and the integrin specificity in HemSC by flow cytometry of α1, α2, α3, α4, α5, α6 and αvβ3 after α6 silencing by siRNA (Supplemental Figure 2). Targeting α6-integrin did not affect proliferation of HemSC (Figure 4A and 4B) and had no effect on cellular expression of the stem cells markers CD133 and SSEA-4 as determined by flow cytometry (Figure 4C). Adhesion of HemSC to laminin-coated dishes was significantly reduced after α6-siRNA transfection compared to the control siRNA (*p<0.001, Figure 4D).
Figure 4. Effect of α6-integrin knock-down on proliferation, stem cell antigen expression, adhesion and differentiation of HemSC.
A- Proliferation of siRNA transfected HemSC cultured in EBM-2/20% FBS over 4 days evaluated by counting cells.
B- Proliferation of transfected HemSC cultured in EBM-2/20% over 4 days evaluated by measuring cellular phosphatase activity.
C- Expression of stem cell antigens CD133 and SSEA-4 inHemSC determined by flow cytometry 3 days after siRNA transfection with control or α6-integrin siRNA.
D- Adhesion assay: cells allowed adhering to laminin-coated wells for 20 minutes. Number of adherent cells determined by the p-NPP colorimetric assay (right panel: original magnification, ×10).
E- VEGF-A secreted by siRNA transfected HemSC measured after 3 days by ELISA on HemSC supernatant.
F- siRNA transfected HemSC co-cultured with cord blood ECFC for 5 days and separated into endothelial and non-endothelial cells using anti-CD31-coated magnetic beads. CD31-negative (non-endothelial) fraction analyzed by RT-qPCR for pericytic marker NG2 (neural glial antigen-2) and results compared with HemSC and cord blood ECFC cultured alone. No difference observed between control-siRNA and α6-integrin siRNAHemSC.
Before evaluating whether disruption in α6-integrin function would affect blood vessel-forming ability in vivo, we tested two properties of HemSC that we had previously shown as a requirement for vasculogenic capability. The first was to analyze VEGF-A secretion from HemSC, because suppression of VEGF-A produced by HemSC blocked their ability to form vessels in vivo23. α6-siRNA transfection had no effect on VEGF secretion from HemSC (Figure 4E). The second property was to assess the ability of HemSC to differentiate into pericytes when in direct contact with endothelial cells 16. Pericytic differentiation, assessed by expression of the pericyte marker NG-2, was not affected in α6-siRNA-treated HemSC after five days of coculture with ECFC. These assays ruled out a role for α6-integrin in VEGF-A expression or HemSC-to-pericyte differentiation. We tested α6-siRNA transfected HemSC for ability to form vessels when implanted subcutaneously in mice for 10 days. The knockdown of α6-integrin in HemSC was maintained for at least 7 days (Supplemental Figure 2B). The α6-siRNA HemSC showed a significantly reduced vascularity compared to control-siRNAHemSC (Figure 5A and 5B), and a significantly decreased MVD (P<0.0001, Figure 5C).
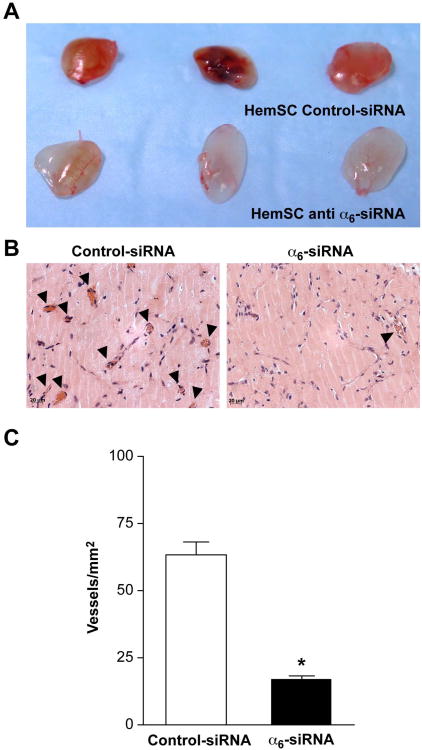
Figure 5. α6-Integrin knock-down decreased vasculogenic potential of HemSC.
A- Representative photographs of Matrigel explants at day 10 after injection of transfected HemSC.
B- Sections of implants in A stained with H&E. Black arrowheads point to lumens filled with red blood cells.
D- Quantification of lumens filled with red blood cells, reported as vessels/mm2, * indicates P<0.0001. Scale bar = 20 μm. Data are mean ± SEM.
α6-Integrin knockdown reduces homing of HemSC to liver
To further explore the role of α6 integrin in HemSC and in hemangioma-genesis, we injected HemSC intravenously into nude mice (n=7/group) and followed distribution of the cells using FMT™. Before injection in nude mice, control siRNA and α6 integrin siRNA-transfected HemSC were labeled using a near-infrared fluorochrome VT680-XL the day after transfection. At 48 hours after intravenous delivery, bright near-infrared fluorescence (NIRF) was evident in liver, indicating HemSC homing; there was no fluorescent signal in lung, skin, spleen or kidney (Figure 6A). Quantitative analysis showed a significantly reduced fluorescent signal in liver from mice that received α6-siRNA HemSC (*p=0.026) (Figure 6B).
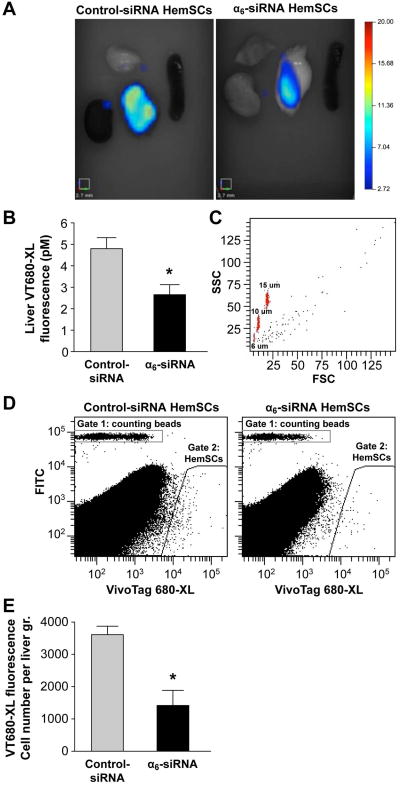
Figure 6. α6-Integrin knock-downdecreased HemSC hepatic homing after intravenous injection.
A- Representative photomicrographs of fluorescence observed in isolated organs
B- Quantitative analysis 2 days after intravenous injection. 12 hours after transfection, HemSCstained with near-infrared cell tracker reagent VivoTag 680 XLand injected. 7 mice per group injected intravenously with 1×105 cells suspended in 100 μl of PBS, as follows: HemSC transfected with controlsiRNA or HemSC transfected with siRNA α6. Data are given as mean±SEM. * indicates P=0.026.
C- Cells were recovered from the liver and analysed using the Flow Cytometry Size Calibration Kit (Invitrogen, reference F13838) with calibration beads of 6, 10 and 15 μM. Representative gated density plot indicating forward scatter (FSC) versus side scatter (SSC) of cells within Gate 2 – see Figure 6D (VT-680-labeled HemSC).
D- Analysis by flow cytometry after liver weighted and disrupted, with counting beads included to quantify the number of cells. Gate 2 was set by running VT-680 labeled HemSC prior to injection.
E- Number of fluorescent cells/gram of liver weight. Data are given as mean±SEM. * indicates P=0.021.
To verify that cells were recovered from the liver and quantified, we included size calibration beads composed of microsphere suspensions ranging in size from 6 to 15 μm to serve as reliable size references (Figure 6C). These 3 beads population were mixed with the cells and appear on Figure 6C. As shown in Figure 6C, cells were greater than 10 μM. This rules out the possibility that the fluorescent signal was due to debris or microparticles whose size is less than 6 μM. Flow cytometric analysis of single cell suspensions prepared from the livers from both groups of mice allowed us to confirm the decrease of α6-siRNA HemSC in the liver 48 hours after intravenous delivery (*p=0.021, Figure 6D,E).
Discussion
This study showed that an extracellular matrix receptor, α6-integrin, is expressed in IH tissue and the hemangioma stem cells (HemSC). Silencing α6-integrin expression or blocking its function with anti-α6-integrin monoclonal antibody resulted in diminished adhesion of HemSC to laminin and decreased vessel formation in a murine model of IH. α6-integrin did not appear to be required for proliferation of HemSC, VEGF-A expression in HemSC or differentiation of HemSC into pericyte/smooth muscle cells. We suggest that α6-integrin's adhesive properties are needed for the de novo assembly of blood vessels from HemSC.
HemSC are vasculogenic: clonally-derived HemSC can differentiate into endothelial cells and pericytes, and form a perfused vascular network within 7 days after implantation in vivo 14-16. HemSC secrete high levels of VEGF-A, which is suppressed by corticosteroid 23, a mainstay treatment for IH. VEGF-mediated angiogenic potential in endothelial progenitor cells has been attributed to increased α6-integrin3 and laminin, its ligand, has been detected in the basement membranes of IH specimens 17, 18. These findings constitute the basis for our hypothesis that α6-integrin subunit might contribute to vasculogenic properties of HemSC.
To investigate the role of α6-integrin in vasculogenesis that drives the growth of IH, we used stem-cell isolation techniques to purify and expand HemSC from 3 IH tumor specimens. The HemSC were implanted in immune-deficient mice as reported previously 14. We found that blocking α6-integrin with GoH3 neutralizing antibody or silencing α6-integrin with siRNAinhibited vasculogenesis mediated by HemSCin vivo. We also observed that inhibition of α6-integrin decreased the adhesive properties of HemSCin vitro. We had previously shown that differentiation of HemSC into both endothelial cells and pericytes is required for formation of hemangioma-like blood vessels 15, 16. Here, we demonstrate that without an apparent role in HemSC-to-pericyte differentiation, α6-integrin expression on HemSC is an important contributor to the vasculogenic capability of HemSC. In vitro, we investigated the contribution of α6-integrin to proliferation and adhesion of HemSC, which are critical requirements for vasculogenesis and angiogenesis. Silencing α6-integrin or applying a blocking mAb had no effect on proliferation of HemSC whereas these treatments blocked adhesion to laminin.
The final experiment in this study was to inject HemSC intravenously into nude mice and follow their path using FMT™. The human HemSCs were labelled with VivoTag 680 (VT680), an amine reactive N-hydroxysuccinimide (NHS) ester of a (benz) indolium-derived far red fluorescent probe previously described by Swirski and collaborators 26. This VivoTag 680 (VT680) has been shown to diffuse into leukocytes within minutes, to covalently bind cellular components, to remain internalized for days in vitro and in vivo, to not transfer fluorescence to adjacent cells and to keep cells fully functional, and emit fluorescence at high intensities. Thus, in summary, based on the absence of secretion previously described for this VT-680 and the size and the cellular aspect of flow cytometry analysis, we are confident that we are detecting the VT-680-labeled HemSC and not bi-products of detoxification in the liver. However, we cannot rule out the possibility that a small amount of detoxification contributes to the signal.
At the level of detection afforded by this technology, HemSC appear to home exclusively to liver in an α6-integrin-dependent manner. HemSC were detected in liver 48 hours after intravenous injection and significantly decreased levels were measured in HemSC transfected with α6-integrin specific siRNA (60% inhibition was observed). This apparent decrease in homing is consistent with previous studies showing a role for α6-integrin in homing of hematopoietic stem cells to bone marrow 12 and endothelial or mesenchymal progenitor cells to ischemic muscle 4, 27.
The hepatic localization of intravenously injected HemSC is interesting in light of pathophysiology of IH. Hepatic hemangiomas are commonly found as either focal, multifocal or diffuse lesions 28 and often small tumors are detected in the evaluation of asymptomatic infants 29, 30. Large hepatic IHs are life-threatening due to cardiac overload and can cause irreversible intellectual deficits 31. Infants with five or more cutaneous IH are at increased risk of having hepatic hemangioma32.
There are some limitations to our use of the murine model of IH. First, although the dissection of IH into purified cellular components has enabled the investigation of their specific roles, it prevents us from studying the tumor as a complex of multiple cell types, tumoral and normal. HemSC may promote vasculogenesis via indirect effects, such as interactions with other cell types. Second, the implant microenvironment is composed of Matrigel, a commercially prepared murine basement membrane extract particularly rich in laminin; this could alter the behavior of HemSC. Interestingly, α6-integrin can mediate cell-cell interactions independently of laminin. For example, α6β1 has a key role in gamete fusion 33resulting from an interaction with membrane-anchored cell surface ligands from the A Disintegrin and Metalloproteinase (ADAM) family. Interaction with ADAM-9 is also responsible for the induction of fibroblast motility 34. The ability of HemSC to interact with other cell types is an important topic for further studies.
α6-Integrin, like other integrins, is likely to have precisely tailored functions depending on the cell in which it is expressed. Indeed, in endothelial progenitor cells (as defined by consensus classification: ECFC for endothelial colony forming cells 35), α6-integrin appears to mediate adhesion and tube formation 3, 4 without affecting proliferation. In glioblastoma stem cells, α6-integrin regulates cell renewal, proliferation and CD133 expression 9. Integrin activation is induced by several growth factors including VEGF, and also by mechanical stress. For example, the role of two FERM (F for 4.1 protein, E for ezrin, R for radixin and M for moesin) domain-containing proteins talin and kindlin has been demonstrated 36. α6-Integrin has also been shown to be a marker of the invasive potential of prostatic tumor cells. The human prostatic carcinoma cell line DU145 was characterized for its adhesive properties and integrin profile 20: α6-integrin expression was correlated with prostate cell migration on laminin and invasion through stroma. Moreover, α6 overexpression on hepatocarcinoma cells causes them to acquire an invasive phenotype 37. The same phenomenon has been observed in breast or prostate cancer, wherein α6-integrin promotes carcinoma survival and progression 19, 20, 38, 39. Thus, our findings support the hypothesis that α6-integrin could be involved in adhesive and possibly the invasive properties of HemSC.
In conclusion, in vitro and in vivo data show that α6-integrin plays a functional role in the vasculogenic properties of HemSC. α6-integrin is involved in HemSC adhesion to laminin, their ability to form blood vessels in vivo, and their homing to liver. A better understanding of the origin and contributions of HemSC to the formation and growth of IH and the participation of α6-integrin could lead to new strategies for treatment.
Supplementary Material
Supplemental Figure 1: HemSC proliferation (A,B) and adhesion (C,D)
A- Proliferation of HemSC cultured in EBM-2/20% over 4 days. Cellular phosphatase activity was the same when the cells were plated on fibronectin or laminin.
B- Proliferation of HemSC cultured in EBM-2/20% over 4 days evaluated by measuring cellular phosphatase activity. Proliferation on fibronectin-coated wells not affected by blocking mAb against α6-integrin.
C- Adhesion assay: cells allowed to adhere to fibronectin or laminin for 20 minutes. Number of adherent cells determined by p-NPP assay.
D- Adhesion assay: cells allowed to adhere to fibronectin for 20 minutes. Number of adherent cells determined by p-NPP assay. In contrast to laminin (Figure 2C), adhesion on fibronectin was not affected by blocking mAb against α6-integrin.
Supplemental Figure 2: Assessment of siRNA efficiency and specificity by qRT-PCR (A) and flow cytometry (B-I)
A -HemSC cultured and transfected as described in Materials and Methods. At various times after transfection, quantity of α6-integrin subunit mRNA was determined by RT-qPCR.
B - α6-integrin protein expression at cell surface measured by flow cytometry from 2-to-7 days. Results for HemSC transfected siRNA α6 integrin normalized to control siRNA-transfected HemSC. Results reported as means ± S.E.M. of three different experiments. α6-Integrin subunit protein expression reduced on HemSC transfected with siRNA α6 (about 10% of control value from day 2 to day 5 and 20% after 5 days).
C-H - At day 3 after siRNA transfection, quantity of α6-integrin subunit measured by flow cytometry (H) and compared to cell surface expression of integrins α1 (C), α2 (D), α3 (E), α4 (F), α5 (G), αvβ3 (I) to verify the specificity of siRNA α6 integrin transfection. No reduction in cell surface expression of other integrins observed. Isotype-matched control IgG2a binding to control siRNA-transfected shown as a red line and to α6-siRNA transfected HemSC as a green line. Integrin mAb binding to control siRNA transfected HemSC shown as a black line and to α6-siRNA transfected HemSC as blue line.
Acknowledgments
David M. Smadja was supported by NIH grant HL096384 (J.B.) and Université Paris Descartes. We thank the Dana-Farber/Harvard Cancer Center for Specialized Histopathology Core, Lan Huang for helpful discussions and Kristin Johnson for preparation of the figures.
Footnotes
Author Contributions: D. Smadja designed and performed the experiments and wrote the manuscript, C. Guerin and I. Bieche performed and analyzed the in vivo homing of hemangioma stem cells, E. Boscolo provided critical insights and expertise on hemangioma stem cells, J. Mulliken provided hemangioma specimens and clinical expertise on the pathogenesis of hemangioma, J. Bischoff provided insight on experimental design, data analysis and interpretation, and edited the manuscript.
Conflicts of interest: none
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Hierck BP, Thorsteinsdottir S, Niessen CM, et al. Variants of the alpha 6 beta 1 laminin receptor in early murine development: distribution, molecular cloning and chromosomal localization of the mouse integrin alpha 6 subunit. Cell Adhes Commun. 1993;1:33–53. doi: 10.3109/15419069309095680. [DOI] [PubMed] [Google Scholar]
- 3.Smadja DM, Bieche I, Helley D, et al. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6) J Cell Mol Med. 2007;11:1149–1161. doi: 10.1111/j.1582-4934.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvard C, Gafsou B, Dizier B, et al. alpha6-integrin subunit plays a major role in the proangiogenic properties of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1569–1575. doi: 10.1161/ATVBAHA.110.209163. [DOI] [PubMed] [Google Scholar]
- 5.Georges-Labouesse E, Messaddeq N, Yehia G, et al. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 6.Pulkkinen L, Kimonis VE, Xu Y, et al. Homozygous alpha6 integrin mutation in junctional epidermolysis bullosa with congenital duodenal atresia. Hum Mol Genet. 1997;6:669–674. doi: 10.1093/hmg/6.5.669. [DOI] [PubMed] [Google Scholar]
- 7.Allegra M, Gagnoux-Palacios L, Gache Y, et al. Rapid decay of alpha6 integrin caused by a mis-sense mutation in the propeller domain results in severe junctional epidermolysis bullosa with pyloric atresia. J Invest Dermatol. 2003;121:1336–1343. doi: 10.1111/j.1523-1747.2003.12625.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Seng S, Li H, et al. Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: role of alpha6beta1 integrin in angiogenesis. J Biol Chem. 2006;281:40450–40460. doi: 10.1074/jbc.M607525200. [DOI] [PubMed] [Google Scholar]
- 9.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilschut KJ, van Tol HT, Arkesteijn GJ, et al. Alpha 6 integrin is important for myogenic stem cell differentiation. Stem Cell Res. 2011;7:112–123. doi: 10.1016/j.scr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Castaldo C, Di Meglio F, Nurzynska D, et al. CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with laminin-1 and alpha6 integrin expression. Stem Cells. 2008;26:1723–1731. doi: 10.1634/stemcells.2007-0732. [DOI] [PubMed] [Google Scholar]
- 12.Qian H, Tryggvason K, Jacobsen SE, et al. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 13.Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000;37:517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 14.Khan ZA, Boscolo E, Picard A, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boscolo E, Mulliken JB, Bischoff J. VEGFR-1 mediates endothelial differentiation and formation of blood vessels in a murine model of infantile hemangioma. Am J Pathol. 2011;179:2266–2277. doi: 10.1016/j.ajpath.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boscolo E, Stewart CL, Greenberger S, et al. JAGGED1 Signaling Regulates Hemangioma Stem Cell-to-Pericyte/Vascular Smooth Muscle Cell Differentiation. Arterioscler Thromb Vasc Biol. 2011;31:2181–2192. doi: 10.1161/ATVBAHA.111.232934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Padura I, De Castellarnau C, Uccini S, et al. Expression of VE (vascular endothelial)-cadherin and other endothelial-specific markers in haemangiomas. J Pathol. 1995;175:51–57. doi: 10.1002/path.1711750109. [DOI] [PubMed] [Google Scholar]
- 18.Tan ST, Velickovic M, Ruger BM, et al. Cellular and extracellular markers of hemangioma. Plast Reconstr Surg. 2000;106:529–538. doi: 10.1097/00006534-200009030-00001. [DOI] [PubMed] [Google Scholar]
- 19.Chung J, Mercurio AM. Contributions of the alpha6 integrins to breast carcinoma survival and progression. Mol Cells. 2004;17:203–209. [PubMed] [Google Scholar]
- 20.Rabinovitz I, Nagle RB, Cress AE. Integrin alpha 6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metastasis. 1995;13:481–491. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Primo L, Seano G, Roca C, et al. Increased expression of alpha6 integrin in endothelial cells unveils a proangiogenic role for basement membrane. Cancer Res. 2010;70:5759–5769. doi: 10.1158/0008-5472.CAN-10-0507. [DOI] [PubMed] [Google Scholar]
- 22.Greenberger S, Adini I, Boscolo E, et al. Targeting NF-kappaB in infantile hemangioma-derived stem cells reduces VEGF-A expression. Angiogenesis. 2010;13:327–335. doi: 10.1007/s10456-010-9189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberger S, Boscolo E, Adini I, et al. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005–1013. doi: 10.1056/NEJMoa0903036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smadja DM, Mulliken JB, Bischoff J. E-selectin mediates stem cell adhesion and formation of blood vessels in a murine model of infantile hemangioma. Am J Pathol. 2012;181:2239–2247. doi: 10.1016/j.ajpath.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smadja D, Gaussem P, Roncal C, et al. Arterial and venous thrombosis is associated with different angiogenic cytokine patterns in patients with antiphospholipid syndrome. Lupus. 2010;19:837–843. doi: 10.1177/0961203309360985. [DOI] [PubMed] [Google Scholar]
- 26.Swirski FK, Berger CR, Figueiredo JL, et al. A near-infrared cell tracker reagent for multiscopic in vivo imaging and quantification of leukocyte immune responses. PLoS One. 2007;2:e1075. doi: 10.1371/journal.pone.0001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee RH, Seo MJ, Pulin AA, et al. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulungowski AM, Alomari AI, Chawla A, et al. Lessons from a liver hemangioma registry: subtype classification. Journal of pediatric surgery. 2012;47:165–170. doi: 10.1016/j.jpedsurg.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Caseiro-Alves F, Brito J, Araujo AE, et al. Liver haemangioma: common and uncommon findings and how to improve the differential diagnosis. Eur Radiol. 2007;17:1544–1554. doi: 10.1007/s00330-006-0503-z. [DOI] [PubMed] [Google Scholar]
- 30.Kulungowski AM, Alomari AI, Chawla A, et al. Lessons from a liver hemangioma registry: subtype classification. J Pediatr Surg. 2012;47:165–170. doi: 10.1016/j.jpedsurg.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 31.Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–189. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 32.Horii KA, Drolet BA, Frieden IJ, et al. Prospective study of the frequency of hepatic hemangiomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol. 2011;28:245–253. doi: 10.1111/j.1525-1470.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen MS, Tung KS, Coonrod SA, et al. Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc Natl Acad Sci U S A. 1999;96:11830–11835. doi: 10.1073/pnas.96.21.11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nath D, Slocombe PM, Webster A, et al. Meltrin gamma(ADAM-9) mediates cellular adhesion through alpha(6)beta(1)integrin, leading to a marked induction of fibroblast cell motility. J Cell Sci. 2000;113(Pt 12):2319–2328. doi: 10.1242/jcs.113.12.2319. [DOI] [PubMed] [Google Scholar]
- 35.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moser M, Bauer M, Schmid S, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 37.Carloni V, Romanelli RG, Mercurio AM, et al. Knockout of alpha6 beta1-integrin expression reverses the transformed phenotype of hepatocarcinoma cells. Gastroenterology. 1998;115:433–442. doi: 10.1016/s0016-5085(98)70210-0. [DOI] [PubMed] [Google Scholar]
- 38.Cariati M, Naderi A, Brown JP, et al. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int J Cancer. 2008;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- 39.Chung J, Kim TH. Integrin-dependent translational control: Implication in cancer progression. Microsc Res Tech. 2008;71:380–386. doi: 10.1002/jemt.20566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: HemSC proliferation (A,B) and adhesion (C,D)
A- Proliferation of HemSC cultured in EBM-2/20% over 4 days. Cellular phosphatase activity was the same when the cells were plated on fibronectin or laminin.
B- Proliferation of HemSC cultured in EBM-2/20% over 4 days evaluated by measuring cellular phosphatase activity. Proliferation on fibronectin-coated wells not affected by blocking mAb against α6-integrin.
C- Adhesion assay: cells allowed to adhere to fibronectin or laminin for 20 minutes. Number of adherent cells determined by p-NPP assay.
D- Adhesion assay: cells allowed to adhere to fibronectin for 20 minutes. Number of adherent cells determined by p-NPP assay. In contrast to laminin (Figure 2C), adhesion on fibronectin was not affected by blocking mAb against α6-integrin.
Supplemental Figure 2: Assessment of siRNA efficiency and specificity by qRT-PCR (A) and flow cytometry (B-I)
A -HemSC cultured and transfected as described in Materials and Methods. At various times after transfection, quantity of α6-integrin subunit mRNA was determined by RT-qPCR.
B - α6-integrin protein expression at cell surface measured by flow cytometry from 2-to-7 days. Results for HemSC transfected siRNA α6 integrin normalized to control siRNA-transfected HemSC. Results reported as means ± S.E.M. of three different experiments. α6-Integrin subunit protein expression reduced on HemSC transfected with siRNA α6 (about 10% of control value from day 2 to day 5 and 20% after 5 days).
C-H - At day 3 after siRNA transfection, quantity of α6-integrin subunit measured by flow cytometry (H) and compared to cell surface expression of integrins α1 (C), α2 (D), α3 (E), α4 (F), α5 (G), αvβ3 (I) to verify the specificity of siRNA α6 integrin transfection. No reduction in cell surface expression of other integrins observed. Isotype-matched control IgG2a binding to control siRNA-transfected shown as a red line and to α6-siRNA transfected HemSC as a green line. Integrin mAb binding to control siRNA transfected HemSC shown as a black line and to α6-siRNA transfected HemSC as blue line.