Abstract
Background
Seasonal fluxes in 25-hydroxyvitamin D [25(OH)D] in children can impact bone turnover, and in turn potentially affect bone accrual and peak bone mass.
Objective
To examine the effect of seasonal flux on the association among 25(OH)D and parathyroid hormone (PTH) on markers of bone turnover in pre- and early pubertal black and white children.
Design
Data were collected during summer (June –September) and winter (December – March) in 6- to 12-yr-old children. Measurements included serum 25(OH)D, PTH, osteocalcin (OC), collagen type 1 cross-linked C-telopeptide (CTx), dietary intake of vitamin D and calcium, skin color, sunlight exposure, and body-mass-index (BMI).
Results
A total of 138 children (mean [±SD] age: 9.1±1.7 year, black: 94, male: 81) were studied. 25(OH)D (41.2±13 vs 34.5±11.1 ng/mL, p<0.001) were higher and CTx were lower (0.8±0.3 vs 0.9±0.5 ng/mL, p<0.001) in all participants during summer when compared to winter. Furthermore, seasonal differences in CTx were more pronounced in blacks (summer: 0.7±0.3 vs winter: 1.0±0.5 ng/mL, p<0.001). PTH was a significant predictor of serum CTx and OC after adjusting for race, season, Tanner stage, dietary calcium, skin color and BMI.
Conclusion
25(OH)D declined significantly in both black and whites during winter. CTx significantly increased during winter in blacks than whites suggesting increased rates of resorption in blacks during winter. Benefits of enhancement of wintertime vitamin D status on bone health need further exploration.
Keywords: Season, vitamin D, children, 25-hydroxyvitamin D, parathyroid hormone, bone turnover, biomarkers, osteocalcin, collagen type 1 cross-linked C-telopeptide
Introduction
Vitamin D is essential for calcium homeostasis.1 Serum 25-hydroxyvitamin D [25(OH)D] is the recognized biomarker of vitamin D status.2 Circulating concentrations of 25(OH)D is reflective of inputs of vitamin D from diet, supplements, and sunlight exposure. Casual exposure to sunlight is the major determinant of human vitamin D status as very few foods contain vitamin D.3–5 For this reason, residents of latitudes above 35° are vulnerable for seasonal hypovitaminosis D during winter,6 as their wintertime sunlight lack ultraviolet-B photons that are essential for vitamin D-photoproduction.7–8
Vitamin D deficiency causes calcium malabsorption, and the resulting calcium deprivation leads to compensatory increase in parathyroid hormone (PTH).1 The consequences of secondary hyperparathyroidism include increased bone turnover, bone loss, and susceptibility to fragility fractures in adults, and impaired acquisition of peak bone mass and histological rickets in children.9–10
Seasonal fluxes in serum 25(OH)D and calcitropic hormones have been associated with seasonal variations in bone mass,11 rates of fractures and falls in adults,12 and bone mineral density in pubertal girls.13 It is unknown, if seasonal fluxes in 25(OH)D impact accrual of peak bone mass during childhood and adolescence. Understanding the impact of seasonal flux on vitamin D status (25[OH]D, PTH) and bone turnover in children is critical for developing strategies to promote enhancement of peak bone mass, since majority of bone acquisition happens during adolescence.14 Therefore, we have examined the effect of seasonal flux on the associations among serum 25(OH)D and PTH on markers of bone turnover in pre- and early pubertal black and white youth, adjusting for the influence of dietary vitamin D and calcium, skin color, sun light exposure and body-mass-index (BMI).
Subjects and Methods
Subjects
We studied 138 healthy community-dwelling 6- to12-year-old pre- and early pubertal black and white youth living in Pittsburgh, PA (latitude: 40.4° North). Enrolled subjects were free of metabolic rickets, hepatic, renal or malabsorptive disorders, and were not on treatment with anticonvulsant or systemic glucocorticoids. Subjects were recruited from the Primary Care Center of Children’s Hospital of Pittsburgh and from the Greater Pittsburgh area. The Primary Care Center (PCC) is a busy academic pediatric practice that provides care for a diverse population of children in an urban setting representative of surrounding communities. Majority of children seen in the PCC are from a lower socio economic stratum as nearly 75% of them are Medicaid eligible, and approximately 70% of children seen in the clinic are African American. The study was approved by the University of Pittsburgh’s Institutional Review Board. Participant assent and signed informed parental consent were obtained prior to research participation.
Study Design
Subjects were enrolled between August 2006 and September 2008, and were assessed during summer (June through September) and winter (December through March). Subjects could be entered in the study at summer or winter (baseline) and then followed up in the subsequent season (winter/summer). The effects of season, diet and race on the vitamin D status of this cohort has been reported.15 This manuscript addresses the relationship between biomarkers of bone turnover and seasonal fluxes in 25(OH)D and PTH in this cohort - to help advance our understanding of the role vitamin D nutrition for skeletal health in children. Subjects’ race was identified by the parents and pubertal status was ascertained by physical examination using Tanner criteria.16–17 The study procedures were completed at the Children’s Hospital of Pittsburgh NIH-funded Pediatric Clinical and Translational Research Center (PCTRC). Subjects’ heights and weights were obtained and BMI was calculated at study entry and exit.
Diet calcium and vitamin D
Dietary intake of vitamin D and calcium were assessed at study entry and exit using a vitamin D and calcium focused food frequency questionnaire.18 The questionnaire estimated the ingestion of multivitamins, calcium supplements, cod liver oil, and vitamin D supplements; average daily intake of vitamin D-fortified milk, other dairy, orange juice, breakfast bars and cereal; and monthly intake of fish and dried mushrooms. The reported dietary data were analyzed using the Food Processor SQL, version 10.4.0 (2008) ESHA Research, Salem, OR, and intake of vitamin D and calcium were quantified in IU/day and mg/day, respectively.
Sunlight exposure
Summertime sunlight exposure was assessed by a questionnaire that addressed the following: (a) average daily duration of summertime sunlight exposure (≤2 hours or >2 hours); (b) parts of the body that are typically exposed to sunlight (face, hands, arms, and legs); (c) frequency of sunscreen use; and (d) travel to a sunny location.
Skin color
Fitzpatrick sun-reactive skin typing was estimated by the parents.19–20 Individuals with skin types I to III are typically light-skinned and exhibit varying degree of sun sensitivity – type I (easy burn, no tan); type II (easy burn, slight tan); and type III (burn, then tan). Individuals with skin types IV and V are dark-skinned and exhibit significant tanning with sunlight exposure – type IV (no burn, good tan) and type V (never burn, marked tan).
Biochemical measurements
Serum 25(OH)D, PTH, calcium, phosphorus, albumin, and markers of bone turnover (Osteocalcin [OC]: marker of formation; collagen type 1 cross-linked C-telopeptide [CTx]: marker of resorption) were assessed at study entry and exit. Blood samples for biochemical testing were collected by venipuncture in a non-fasting state throughout the day. Serum 25(OH)D was measured by liquid chromatography tandem mass spectrophotometry (LC-MS/MS) assay as described before.21 LC-MS/MS assay measures the contributions of 25(OH)D2 and 25(OH)D3 and is equally specific in distinguishing both. The inter-assay and intra-assay coefficient of variation (CV) were 8.1% and 9.4%, respectively. The lowest limit of detection was 2 ng/mL. Serum PTH was measured using Immutopics Human Bioactive PTH 1–84 Elisa kit from Immutopics, Inc, San Clemente, CA. The inter-assay and intra-assay CV were 5% to 8% and 3% to 5%, respectively.
Serum OC was measured by MicroVue osteocalcin enzyme immunoassay kit from Quidel Corporation, San Diego, CA. The inter-assay and intra-assay CV were 4.8% to 9.8% and 8% to 10%, respectively. Serum CTx was measured using Serum CrossLaps ELISA (enzyme-linked immunosorbent assay) kit, from Immunodiagnostic Systems Limited, Boldon, Tyne & Wear, UK. The inter-assay and intra-assay CV were 2.5% to 10.9% and 1.8% to 3%, respectively.
Statistical Analysis
We compared the demographic and clinical characteristics at study entry and the reported summertime sun exposure measures between black and white subjects using 2-sample t-tests and χ2 or Fisher’s exact tests. Seasonal differences in dietary and biochemical data were examined by paired t-test in all participants and separately in black and white participants.
We assessed the crude unadjusted correlations between our two primary exposure variables (serum 25(OH)D and PTH) with the bone turnover markers (OC and CTx) by season and by race. PTH was log transformed for correlation and regression analyses. We then used linear mixed models with random subject effects to control for two seasonal measures for each participant adjusting for the child’s Tanner stage and race, and season. We tested for moderating influences of race and season on the associations between each of the serum measures and bone turnover markers by using interaction terms between race (or season) and each serum measure. Once the significance of these interactions was determined (α=0.05), we assessed the confounding effects of dietary vitamin D and calcium, sunlight exposure, skin color, and BMI on the relationships between bone turnover markers and serum 25(OH)D and PTH. Potential confounders were included in the full models only if they were associated with the predictor and/or the outcome. We defined confounders a priori as having an impact on the associations of the primary independent variables and outcomes by showing a 10% difference in the crude measure of association after controlling for the potential confounder. All inference was based on two-sided α=0.05.
Results
One hundred forty children were originally studied during summer and/or winter. Two were dropped for not having measurements of OC at either time point. This left 138 children (black 94, white 44, and male 81) with a mean (± SD) age of 9.1±1.7 year. Majority of the children had paired data with assessments completed during summer and winter (N=122). Blacks were more likely to be obese or overweight, and have a parent-reported Fitzpatrick skin type of IV or V, indicative of darker skin pigmentation (Table 1).
Table 1.
Subject Characteristics at Study Entry
| All Participants (N=138) | Black (N=94) | White (N=44) | |
|---|---|---|---|
| Age (years) | 9.1 (1.7) | 8.9 (1.7) | 9.4 (1.7) |
| Sex | |||
| Male | 81 (58.7%) | 55 (58.5%) | 26 (59.1%) |
| Female | 57 (41.3%) | 39 (41.5%) | 18 (40.9%) |
| Tanner Stage (%) | |||
| Stage I | 100 (72.5%) | 68 (72.3%) | 32 (72.7%) |
| Stage II | 38 (27.5%) | 26 (27.7%) | 12 (27.3%) |
| Weight (kg) | 34.3 (10.4) | 34.5 (9.8) | 34.0 (11.8) |
| Height (cm) | 133.9 (10.6) | 133.2 (9.8) | 135.5 (12.2) |
| BMI (kg/m2) | 18.8 (3.7) | 19.2 (3.7) | 18.0 (3.5) |
| Weight Category (%) | |||
| Normal (BMI <85th %ile) | 83 (60.1%)* | 50 (53.2%) | 33 (75.0%) |
| Overweight (BMI 85th – 95th %ile) | 28 (20.3%) | 21 (22.3%) | 7 (15.9%) |
| Obese (BMI > 95th %ile) | 27 (19.6%) | 23 (24.5%) | 4 (9.1%) |
| Skin Type (%) | |||
| Skin Type I–III | 43 (31.2%)*** | 7 (7.4%) | 36 (81.8%) |
| Skin Type IV–V | 95 (68.8%) | 87 (92.6%) | 8 (18.2%) |
| Summertime Sun Exposure Data | |||
| Duration | |||
| 2 hours or less | 17 (13.6%) | 9 (10.6%) | 8 (20.0%) |
| More than 2 hours | 108 (86.4%) | 76 (89.4%) | 32 (80.0%) |
| Sunscreen Use | |||
| No | 72 (56.3%)*** | 65 (74.7%) | 7 (17.1%) |
| Yes | 56 (43.8%) | 22 (25.3%) | 34 (82.9%) |
| Sunscreen Frequency | |||
| Often/Sometimes | 17 (30.4%)** | 2 (8.7%) | 15 (45.5%) |
| Seldom | 39 (69.6%) | 21 (91.3%) | 18 (54.5%) |
| Holiday | |||
| No | 87 (68.0%)** | 67 (77.0%) | 20 (48.8%) |
| Yes | 41 (32.0%) | 20 (23.0%) | 21 (51.2%) |
black vs. white:
p<0.05,
p<0.01,
p<0.001
Dietary calcium and vitamin D (Table 2)
Table 2.
Dietary and biochemical data by season
| All Participants (N=121) | Black (N=82) | White (N=39) | ||||
|---|---|---|---|---|---|---|
| Summer | Winter | Summer | Winter | Summer | Winter | |
| Dietary Data | ||||||
| Combined Milk (servings/day) | 2.4 (1.7)* | 2.7 (1.9) | 2.3 (1.6)* | 2.7 (1.8) | 2.5 (2.0) | 2.5 (2.0) |
| Combined Milk + OJ (servings/day) | 2.8 (1.8)* | 3.2 (2.1) | 2.9 (1.8)* | 3.3 (2.2) | 2.7 (1.9) | 2.9 (1.8) |
| Dietary Calcium (mg/day) | 1114 (592)* | 1239 (789) | 1150 (577)* | 1295 (894) | 1037 (622) | 1120 (488) |
| Dietary Vitamin D (IU/day) | 430 (216) | 484 (307) | 421 (208)* | 507 (331) | 449 (232) | 437 (244) |
| Calcium intake <1000 mg/day | 48% | 47% | 47% | 41% | 50%* | 32% |
| Vitamin D intake <400 IU/day | 51% | 50% | 54% | 49% | 43% | 51% |
| Biochemical Data | ||||||
| Calcium (mg/dL) | 9.6 (0.3)*** | 9.8 (0.3) | 9.6 (0.3)*** | 9.8 (0.3) | 9.6 (0.3) | 9.7 (0.3) |
| Phosphorus (mg/dL) | 5.0 (0.6) | 4.9 (0.5) | 5.0 (0.5) | 5.0 (0.5) | 4.8 (0.7) | 4.8 (0.5) |
| Albumin (g/dL) | 4.28 (0.3)* | 4.32 (0.3) | 4.2 (0.3) | 4.3 (0.3) | 4.4 (0.3) | 4.4 (0.3) |
| 25(OH)D (ng/mL) | 41.2 (13.0)*** | 34.5 (11.1) | 39.1 (11.5)** | 33.8 (11.3) | 45.5 (14.9)** | 36.2 (10.7) |
| PTH (pg/mL) | 37.0 (25.3) | 38.6 (23.7) | 38.7 (22.5) | 41.9 (23.5) | 33.0 (30.7) | 31.8 (23.1) |
| OC (ng/mL) | 17.1 (10.4) | 18.0 (11.2) | 17.2 (11.3) | 19.0 (11.3) | 16.9 (8.3) | 15.8 (10.9) |
| CTx (ng/mL) | 0.8 (0.3)*** | 0.9 (0.5) | 0.7 (0.3)*** | 1.0 (0.5) | 0.8 (0.4) | 0.8 (0.4) |
| Vitamin D Status | ||||||
| Deficient [25(OH)D <20 ng/mL] | 2%** | 6% | 2%* | 8% | 0% | 0% |
| Insufficient [25(OH)D 20 – <30 ng/mL] | 16% | 33% | 17% | 34% | 15% | 31% |
| Sufficient [25(OH)D ≥30 ng/mL] | 82% | 61% | 81% | 58% | 82% | 69% |
summer vs. winter:
p<0.05,
p<0.01,
p<0.001;
OJ: vitamin D-fortified orange juice
The mean daily intake of vitamin D and calcium were above 400 IU and 1000 mg during summer and winter in all 3 groups. Percentage of children reporting a daily intake of vitamin D and calcium less than 400 IU and 1000 mg, respectively, are shown in table 2. Blacks reported a lower intake of vitamin D-fortified beverages and dietary vitamin D and calcium during summer compared to winter.
Sunlight exposure (Table 1)
Blacks reported less sunscreen use and less travel to more sunny locations during holidays compared to whites.
Seasonal variations in biochemical parameters (Table 2)
In all participants serum calcium and albumin were lower during the summer. This difference in serum calcium remained significant in black but not white children. Serum 25(OH)D concentrations were consistently higher during summer compared to winter in all participants and in blacks and whites when examined separately. CTx levels were lower during summer in all participants and in blacks. PTH and OC did not differ between summer and winter in all 3 groups. Seasonal differences in vitamin D status in all participants and by race are shown in table 2. None of the white children were vitamin D-deficient (serum 25[OH]D <20 ng/mL) either during summer or winter, and only a few black children were classified as vitamin D-deficient (summer: 2%, winter: 8%). However, vitamin D insufficiency (serum 25[OH]D 20 – <30 ng/mL) was not uncommon in either racial group during summer and winter and the proportion of children classified as vitamin D-insufficient increased by 2-fold between summer and winter.
Influence of 25(OH)D, PTH, and dietary intake on bone turnover (Table 3)
Table 3.
Pearson correlation coefficients between independent variables (25(OH)D, PTH, dietary intake) and bone turnover markers stratified by season and race. Significant correlations are shown in bold.
| All Subjects | Black | White | ||||
|---|---|---|---|---|---|---|
| Summer | Winter | Summer | Winter | Summer | Winter | |
| 25(OH)D & PTH (log) | −.17 (0.069) | −.17 (0.060) | −.12 (0.293) | −.2 (0.068) | −.16 (0.375) | −.047 (0.778) |
| 25(OH)D & OC | −.052 (0.559) | −.0011 (0.990) | .0011 (0.992) | −.052 (0.636) | −.19 (0.240) | .17 (0.315) |
| 25(OH)D & CTx | .11 (0.226) | −.11 (0.231) | .2 (0.067) | −.12 (0.269) | −.087 (0.584) | −.045 (0.782) |
| PTH (log) & OC | .059 (0.534) | .18 (0.047) | .039 (0.732) | .11 (0.339) | .1 (0.554) | .27 (0.096) |
| PTH (log) & CTx | .096 (0.308) | .32 (0.000) | .1 (0.378) | .19 (0.088) | .17 (0.343) | .57 (0.000) |
| Diet vitamin D & OC | −.07 (0.435) | .07 (0.441) | −.067 (0.542) | −.028 (0.799) | −.078 (0.622) | .32 (0.044) |
| Diet vitamin D & CTx | .012 (0.891) | .03 (0.743) | .019 (0.863) | .011 (0.923) | −.023 (0.884) | .032 (0.839) |
| Diet calcium & OC | −.013 (0.886) | .099 (0.275) | −.089 (0.413) | .082 (0.463) | .18 (0.244) | .12 (0.464) |
| Diet calcium & CTx | .074 (0.408) | .14 (0.116) | −.0025 (0.982) | .15 (0.169) | .23 (0.133) | .042 (0.792) |
Pearson correlations [r (p value)] reported
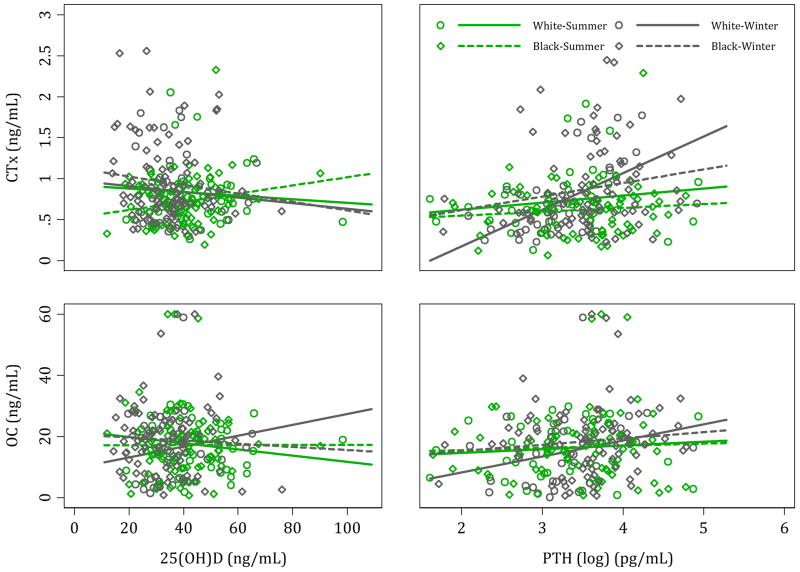
25(OH)D was not significantly correlated with either OC or CTx in the combined sample or when stratified by race. PTH had a weak positive correlation with OC in all participants during winter (r=0.18, p=0.047). Also, PTH had a moderate positive correlation with CTx during winter, in all participants (r=0.32, p=<0.001) and in whites (r=0.57, p=<0.001). Scatter plots of the relationship between bone turnover markers and 25(OH)D and PTH by race and season is shown in figure 1. Dietary vitamin D was positively associated with OC during winter in whites (r=0.32, p=0.044) and no other associations were detected between dietary intake of calcium and vitamin D and the two bone turnover markers.
Figure 1.
Scatter plots of the relationship between bone turnover markers and 25(OH)D and PTH by race and season.
Multivariable modeling (Table 4)
Table 4.
Linear mixed model results for modeling each independent variable on bone turnover markers. Model coefficients and standard errors are presented. For the race and season interactions, if the interaction was not significant, the race or season adjusted coefficient of the independent variable is presented with its corresponding standard error. If the interaction was significant, coefficients for the independent variable are presented stratified by corresponding interacting variable.
| Outcome | Predictor | Adjusted
Model beta(SE) |
Full Model beta (SE) |
|---|---|---|---|
|
| |||
| CTx | 25(OH)D | −.00077 (.0021) | −.00078 (.0021) |
| Diet vitamin D | -- | ||
| Diet calcium | .00007 (.000037) | ||
| Sun exposure (>2 hrs) | -- | ||
| Skin type (IV, V) | .066 (.083) | ||
| BMI (inverse) | -- | ||
|
| |||
| PTH (log) | Summer: .087(.052) | Summer: .077 (.053) | |
| Winter: .27(.06)*** | Winter: .27 (.061)*** | ||
| Diet vitamin D | -- | ||
| Diet calcium | . | .000046 (.00004) | |
| Sun exposure (>2 hrs) | -- | ||
| Skin type (IV, V) | .098 (.096) | ||
| BMI (inverse) | −4.3 (3.5) | ||
|
| |||
| OC | 25(OH)D | −.036(.054) | −.037 (.054) |
| Diet vitamin D | -- | ||
| Diet calcium | -- | ||
| Sun exposure (>2 hrs) | -- | ||
| Skin type (IV, V) | −.56 (2.6) | ||
| BMI (inverse) | -- | ||
|
| |||
| PTH (log) | 2.6(1.1)* | 2.5 (1.1)* | |
| Diet vitamin D | -- | ||
| Diet calcium | .00098 (.0011) | ||
| Sun exposure (>2 hrs) | -- | ||
| Skin type (IV, V) | −.072 (2.7) | ||
| BMI (inverse) | 47 (97) | ||
p<0.05,
p<0.01,
p<0.001
No association was detected between 25(OH)D and bone turnover markers as indicated by the non-significant coefficients from the linear mixed models (CTx β̂25(OH)D = −0.00077, OC β̂25(OH)D = −0.036). Neither race nor season was detected as a modifier for the associations between 25(OH)D and each of the bone turnover markers [25(OH)D interactions with race and season were not significant]. Diet calcium and skin type were added into final models due to their associations with the outcomes and 25(OH)D, but controlling for these did not change the parameter estimates for 25(OH)D. PTH and serum CTx were positively associated in the winter compared to not associated in the summer (PTH*season interaction p=0.016, CTx β̂PTH,summer = 0.087, β̂PTH,winter = 0.27). Adjusting for important potential confounders (such as dietary intake of calcium, skin type, and BMI) did not attenuate the strength of the associations. PTH showed a strong positive association with OC that did not vary by race or season and was not attenuated when adjusted for dietary calcium, skin color, and BMI.
Discussion
Our data suggest that 6- to 12-yr-old pre- and early pubertal black and white youth residing at a higher latitude (40.4° N) are vulnerable for seasonal hypovitaminosis D during winter. Furthermore, the rates of bone resorption (indicated by the concentrations of CTx) were reciprocally higher during winter compared to summer in all participants and in blacks when examined separately. These findings suggest that seasonal hypovitaminosis D in children could potentially impact their bone mineral accrual through increased rates of bone resorption during winter and the effects of seasonal hypovitaminosis D on bone health may vary across racial groups.
Although the mean daily intake of vitamin D of our cohort was above the current American Academy of Pediatrics recommended daily intake22 and the Institute of Medicine’s (IOM) estimated average daily requirement for this age group (400 IU/day),2 50% of the children failed to meet this cutoff. We have previously shown that race/season is the dominant of determinant of circulating 25(OH)D concentration in this cohort: 25(OH)D in whites during summer are higher than those found in blacks during summer or winter and in whites during winter.15 Also, in this cohort, dietary intake of vitamin D was a significant predictor of 25(OH)D concentrations only in whites during winter.15 These findings emphasize the relevance of casual sunlight exposure as the major determinant of vitamin D status.
In our bivariate correlation analyses, we found a positive association between PTH and CTx during winter in all subjects. The physiologic compensatory increase in PTH associated with the seasonal hypovitaminosis D could be one of the possible explanations for the higher rates of bone resorption (higher CTx concentrations) in all participants during winter. However, the seasonal differences in CTx concentrations and PTH-CTx relationships between the 2 racial groups lacked congruence. In whites, CTx concentration did not vary between summer and winter and there was a positive association between PTH and CTx during winter. In blacks CTx concentrations were significantly higher during winter compared to summer, and their PTH-CTx relationship during winter was weak and insignificant. We are unable to explain the race-related differences in the seasonal variations in CTx concentrations and PTH-CTx relationships. Racial differences in bone turnover associated with seasonal flux need further exploration.
In our multivariate modeling analyses - PTH was a significant predictor of CTx and OC after adjusting for race, season, Tanner stage, skin color, BMI and dietary intake of calcium. The relationship between PTH and CTx was influenced by season, and was stronger during winter than summer. Adjustment for race, Tanner stage, BMI, skin color, and dietary intake of calcium did not attenuate this relationship. Also, race was not a modifying factor in this relationship. The positive association between PTH and OC did not vary by race or season and was not attenuated with confounder adjustment.
We found no significant difference in PTH concentrations between summer and winter in all participants and in blacks and whites when examined separately. However, the association between PTH and 25(OH)D during winter trended towards significance in all participants and in blacks but not in whites. Our cohort’s adequacy of dietary calcium intake during winter might explain these findings. The mean calcium intake during winter (all participants: 1239 mg/day; blacks: 1295 mg/day; whites: 1120 mg/day) was above or close to the IOM recommended dietary allowance for calcium intake for the age range of our subjects (4–8 yr: 1000 mg/day; 9–13 yr: 1300 mg/day).2 Furthermore, the concentration of 25(OH)D in our cohort during winter may not have met the “vitamin D deficiency” threshold level for causing a significant increase in PTH. Also, our observed seasonal fluxes in serum calcium and albumin were clinically non-relevant.
Hypovitaminosis D in children and adolescents has been shown to be associated with reduced bone mineral density (BMD).23–26 In 10- to 12-yr-old pre- and early pubertal Finnish girls (N=193, latitude: 62°), examined during winter, those who were vitamin D-deficient (25[OH]D ≤10 ng/mL) had higher PTH and rates of bone resorption, and lower cortical bone mineral density compared to vitamin D-insufficient (10.1 – 16 ng/mL) and –sufficient (>16 ng/mL) children.24 Higher rates of profound vitamin D deficiency (32%) and lower dietary intake of calcium (733 mg/day) are the explanatory factors for the secondary hyperparathyroidism seen in this study, and the consequent higher rate of bone resorption and reduced BMD.
In a cross-sectional study of early- and mid-pubertal Finnish girls with adequate dietary calcium intake (N=196, mean age 11.4 yr, latitude: 60° N) sampled during September to March, concentrations of 25(OH)D varied across the sampling points (September, October, November, February, and March).13 In concordance with our findings, 25(OH)D concentrations were highest in those sampled during September and lowest in those sampled in February (24 vs. 15 ng/mL). PTH concentrations did not vary across the sampling time-points but was inversely correlated with 25(OH)D (r=−0.325, p <0.001). OC (marker of bone formation) and BMD varied across the sampling time-points in early pubertal girls. BMD was 7.6% higher in those sampled during October vs. those studied in March, and OC (marker of bone formation) was highest in those studied in September vs. those studied during March. In our study, CTx (marker of bone resorption) varied with the seasonal flux in 25(OH)D and was higher during winter than summer in all participants and blacks. Although OC trended higher in the winter in all participants and blacks, the seasonal differences were not statistically significant. The contrast between their findings and ours could be attributed to the differences in the study design.
Inference of our findings of increased rates of bone resorption corresponding with seasonal hypovitaminosis D for having a detrimental effect on bone health is limited by lack of assessment of temporal changes in bone mineral status. However, changes in bone turnover often can reflect changes in bone density. Our data support the relevance of PTH as a mediator for changes in bone turnover associated with seasonal flux in 25(OH)D. Optimal circulating concentrations of 25(OH)D is essential for bone health and calcium homeostasis. Compensatory hyperparathyroidism, a physiological consequence of hypovitaminosis D,9, 26–31 helps to maintain ionic calcium in the physiologic range by directly increasing bone resorption and renal reabsorption of calcium.1 Also, through activation of renal 1α-hydroxylase,32 PTH converts 25(OH)D to 1,25-dihydroxyvitamin D, and thereby promotes intestinal calcium absorption.1 Dietary calcium intake can modify the effects of PTH on bone health: higher intakes can ameliorate the detrimental effects of PTH on bone health.9, 33–34 Increased rates of bone resorption and bone turnover mediated by the elevated levels of PTH associated with hypovitaminosis D negatively impact bone mineralization and can potentially affect peak bone mass.
Defining the threshold levels of 25(OH)D for optimal skeletal health outcomes remains contentious. IOM has suggested that maintaining 25(OH)D levels ≥20 ng/mL is adequate for ensuring skeletal health in North American residents throughout the year provided their dietary calcium intakes are adequate.2 The Endocrine Society practice guidelines and experts in the field have argued that the optimal levels of 25(OH)D for skeletal health outcomes should be ≥30 ng/mL.35–36 25(OH)D concentrations in children and adolescents above which PTH levels plateau have ranged between 16 to 36 ng/mL.26, 37 However, some data sets have failed to find such a threshold level.38 Furthermore, the vitamin D effects on bone formation are influenced by the prevailing calcium intake.34
Small sample, short duration of study, inclusion of obese subjects, limited number of whites, and lack of direct measures of bone mineral status (dual energy X-ray absorptiometry and/or peripheral quantitative computerized tomography) and assessments of temporal changes in BMD with seasonal changes are some of the limitations of our study. PTH, CTx, and OC exhibit circadian rhythms in their circulating concentrations.38–40 Furthermore, concentrations of bone turnover markers can be affected by food intake.41 Our measurements of theses assays in blood samples collected throughout the day in a non-fasting state can have wider variations and could also potentially influence our findings. Information on the explanatory variables of vitamin D status (sunlight exposure, skin color type, and dietary calcium and vitamin D intake) and longitudinal study design are strengths of our study. We acknowledge that parent-reported sunreactive skin typing and sun exposure should be validated by objective assessments of melanin index with reflectometer and sun exposure with ultraviolet dosimeter, respectively.
In conclusion, we have shown that pre- and early pubertal children living at higher latitudes are at risk for seasonal hypovitaminosis D during winter. Furthermore, despite adequate daily calcium intakes, such children had higher rates of bone resorption during winter than summer. These findings suggest that seasonal hypovitaminosis D in children could potentially impact their bone mineral accrual through increased rates of bone resorption during winter and the effects of vitamin D optimization during winter on bone health parameters needs further exploration. Well-controlled longitudinal studies, conducted over a longer duration throughout the pubertal years, with biannual assessments of 25(OH)D, PTH, markers of bone turnover, and bone mineral assessments are warranted for examining the effects of seasonal hypovitaminosis D on bone mineralization and peak bone mass.
Acknowledgments
Grant support: This work was supported by the following National Institutes of Health grants: R03HD053479 (KR), K23HD052550 (KR), K24DK062895 (SLG), UL1 RR024153-01 (PCTRC) and 5UL1 RR024153-04
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- PTH
Parathyroid hormone
- OC
Osteocalcin
- CTx
Collagen type 1 cross-linked C-telopeptide
- BMI
body-mass-index
- PCTRC
Pediatric Clinical and Translational Research Center
- LC-MS/MS
liquid chromatography tandem mass spectrophotometry
- ELISA
enzyme-linked immunosorbent assay
- BMD
bone mineral density
- IOM
Institute of Medicine
Footnotes
Disclosure summary: Authors have no relevant financial relationships or conflicts of interest to disclose.
Author Contributions
KR was responsible for study design, securing funding, data acquisition and interpretation, and manuscript drafting and its revisions. MFH and JB conducted the 25(OH)D, PTH, and bone turnover marker assays. MFH also critically reviewed and revised the manuscript. CGM and EC were responsible for data analysis, interpretation and manuscript drafting. FO and MAH were responsible for data acquisition. AN analyzed the vitamin D and calcium focused food frequency questionnaires and critically reviewed and revised the manuscript. SLG was responsible for study design, concept, and drafting and critical revisions of the manuscript.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 3.Haddad JG, Jr, Hahn TJ. Natural and synthetic sources of circulating 25-hydroxyvitamin D in man. Nature. 1973;244(5417):515–517. doi: 10.1038/244515a0. [DOI] [PubMed] [Google Scholar]
- 4.Lawson DE, Paul AA, Black AE, Cole TJ, Mandal AR, Davie M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br Med J. 1979;2(6185):303–305. doi: 10.1136/bmj.2.6185.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poskitt EM, Cole TJ, Lawson DE. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. Br Med J. 1979;1(6158):221–223. doi: 10.1136/bmj.1.6158.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 8.Webb AR. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92(1):17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.McKenna MJ, Freaney R. Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos Int. 1998;8 (Suppl 2):S3–6. doi: 10.1007/pl00022725. [DOI] [PubMed] [Google Scholar]
- 10.Slemenda CW, Peacock M, Hui S, Zhou L, Johnston CC. Reduced rates of skeletal remodeling are associated with increased bone mineral density during the development of peak skeletal mass. J Bone Miner Res. 1997;12(4):676–682. doi: 10.1359/jbmr.1997.12.4.676. [DOI] [PubMed] [Google Scholar]
- 11.Rapuri PB, Kinyamu HK, Gallagher JC, Haynatzka V. Seasonal changes in calciotropic hormones, bone markers, and bone mineral density in elderly women. J Clin Endocrinol Metab. 2002;87(5):2024–2032. doi: 10.1210/jcem.87.5.8475. [DOI] [PubMed] [Google Scholar]
- 12.Pasco JA, Henry MJ, Kotowicz MA, Sanders KM, Seeman E, Pasco JR, et al. Seasonal periodicity of serum vitamin D and parathyroid hormone, bone resorption, and fractures: the Geelong Osteoporosis Study. J Bone Miner Res. 2004;19(5):752–758. doi: 10.1359/JBMR.040125. [DOI] [PubMed] [Google Scholar]
- 13.Viljakainen HT, Palssa A, Karkkainen M, Jakobsen J, Cashman KD, Molgaard C, et al. A seasonal variation of calcitropic hormones, bone turnover and bone mineral density in early and mid-puberty girls - a cross-sectional study. Br J Nutr. 2006;96(1):124–130. doi: 10.1079/bjn20061719. [DOI] [PubMed] [Google Scholar]
- 14.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 15.Rajakumar K, Holick MF, Jeong K, Moore CG, Chen TC, Olabopo F, et al. Impact of season and diet on vitamin D status of African American and Caucasian children. Clin Pediatr (Phila) 2011;50(6):493–502. doi: 10.1177/0009922810397334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nucci AM, Russell C, Luo R, Ganji V, Olabopo F, Hopkins B, Holick MF, Rajakumar K. The effectiveness of a short food frequency questionnaire in determining vitamin D intake in children. Dermato-Endocrinology. 2013;5:205–210. doi: 10.4161/derm.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pathak MA, Jimbow K, Szabo G, et al. Sunlight and melanin pigmentation. In: Smith K, editor. Photochemical and Photobiological Reviews. New York: Plenum Press; 1976. pp. 211–239. [Google Scholar]
- 20.Jimbow K, Fitzpatrick TB, Wick WM. Biochemistry and physiology of melanin pigmentation. In: Goldsmith L, editor. Physiology, Biochemistry, and Molecular Biology of the Skin. New York: Oxford University Press; 1991. pp. 873–909. [Google Scholar]
- 21.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 22.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 23.Cashman KD, Hill TR, Cotter AA, Boreham CA, Dubitzky W, Murray L, et al. Low vitamin D status adversely affects bone health parameters in adolescents. Am J Clin Nutr. 2008;87(4):1039–1044. doi: 10.1093/ajcn/87.4.1039. [DOI] [PubMed] [Google Scholar]
- 24.Cheng S, Tylavsky F, Kroger H, Karkkainen M, Lyytikainen A, Koistinen A, et al. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr. 2003;78(3):485–492. doi: 10.1093/ajcn/78.3.485. [DOI] [PubMed] [Google Scholar]
- 25.Lehtonen-Veromaa MK, Mottonen TT, Nuotio IO, Irjala KM, Leino AE, Viikari JS. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study. Am J Clin Nutr. 2002;76(6):1446–1453. doi: 10.1093/ajcn/76.6.1446. [DOI] [PubMed] [Google Scholar]
- 26.Outila TA, Karkkainen MU, Lamberg-Allardt CJ. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr. 2001;74(2):206–210. doi: 10.1093/ajcn/74.2.206. [DOI] [PubMed] [Google Scholar]
- 27.Docio S, Riancho JA, Perez A, Olmos JM, Amado JA, Gonzalez-Macias J. Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res. 1998;13(4):544–548. doi: 10.1359/jbmr.1998.13.4.544. [DOI] [PubMed] [Google Scholar]
- 28.El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001;107(4):E53. doi: 10.1542/peds.107.4.e53. [DOI] [PubMed] [Google Scholar]
- 29.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 30.Guillemant J, Cabrol S, Allemandou A, Peres G, Guillemant S. Vitamin D-dependent seasonal variation of PTH in growing male adolescents. Bone. 1995;17(6):513–516. doi: 10.1016/8756-3282(95)00401-7. [DOI] [PubMed] [Google Scholar]
- 31.Guillemant J, Le HT, Maria A, Allemandou A, Peres G, Guillemant S. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos Int. 2001;12(10):875–879. doi: 10.1007/s001980170040. [DOI] [PubMed] [Google Scholar]
- 32.Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, et al. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci U S A. 1998;95(4):1387–1391. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonofiglio D, Maggiolini M, Catalano S, Marsico S, Aquila S, Giorno A, et al. Parathyroid hormone is elevated but bone markers and density are normal in young female subjects who consume inadequate dietary calcium. Br J Nutr. 2000;84(1):111–116. [PubMed] [Google Scholar]
- 34.Tylavsky FA. Seasonal variation in calcitropic hormones and bone accrual in puberty. Br J Nutr. 2006;96(1):4–6. doi: 10.1079/bjn20061786. [DOI] [PubMed] [Google Scholar]
- 35.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26(3):455–457. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 36.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 37.Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int. 2005;16(1):109–113. doi: 10.1007/s00198-004-1656-8. [DOI] [PubMed] [Google Scholar]
- 38.Hill KM, McCabe GP, McCabe LD, Gordon CM, Abrams SA, Weaver CM. An inflection point of serum 25-hydroxyvitamin D for maximal suppression of parathyroid hormone is not evident from multi-site pooled data in children and adolescents. J Nutr. 2010;140(11):1983–1988. doi: 10.3945/jn.110.124966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser WD, Ahmad AM, Vora JP. The physiology of the circadian rhythm of parathyroid hormone and its potential as a treatment for osteoporosis. Curr Opin Nephrol Hypertens. 2004;13(4):437–444. doi: 10.1097/01.mnh.0000133985.29880.34. [DOI] [PubMed] [Google Scholar]
- 40.Heuck C, Skjaerbaek C, Wolthers OD. Diurnal rhythm of serum osteocalcin in normal children. Acta Paediatr. 1998;87(9):930–932. doi: 10.1080/080352598750031563. [DOI] [PubMed] [Google Scholar]
- 41.Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002;30(6):886–890. doi: 10.1016/s8756-3282(02)00728-7. [DOI] [PubMed] [Google Scholar]