Abstract
Allogeneic mesenchymal stem cells (allo-MSCs) have potent regenerative and immunosuppressive potential and are being investigated as a therapy for osteoarthritis; however, little is known about the immunological changes that occur in allo-MSCs after ex vivo induced or in vivo differentiation. Three-dimensional chondrogenic differentiation was induced in an alginate matrix, which served to immobilize and potentially protect MSCs at the site of implantation. We show that allogeneic differentiated MSCs lost the ability to inhibit T-cell proliferation in vitro, in association with reduced nitric oxide and prostaglandin E2 secretion. Differentiation altered immunogenicity as evidenced by induced proliferation of allogeneic T cells and increased susceptibility to cytotoxic lysis by allo-specific T cells. Undifferentiated or differentiated allo-MSCs were implanted subcutaneously, with and without alginate encapsulation. Increased CD3+ and CD68+ infiltration was evident in differentiated and splenocyte encapsulated implants only. Without encapsulation, increased local memory T-cell responses were detectable in recipients of undifferentiated and differentiated MSCs; however, only differentiated MSCs induced systemic memory T-cell responses. In recipients of encapsulated allogeneic cells, only differentiated allo-MSCs induced memory T-cell responses locally and systemically. Systemic alloimmune responses to differentiated MSCs indicate immunogenicity regardless of alginate encapsulation and may require immunosuppressive therapy for therapeutic use.
Introduction
Mesenchymal stem (stromal) cells (MSCs) are a nonhematopoietic adult stem cell population that can be isolated from various tissues, including bone marrow, adipose tissue, and umbilical cord.1 Their potential to differentiate to mesenchymal cell types, including osteoblasts, adipocytes, and chondrocytes, make these remarkable cells particularly attractive for use in regeneration and/or replacement of damaged or diseased tissue.2 Both preclinical studies3,4 and clinical trials5 have shown efficacy of MSC administration in cartilage repair for the treatment of degenerative cartilage disease6,7, including osteoarthritis (OA). Another distinctive characteristic of MSCs is their ability to interact with many immune cell populations to modulate host immune responses in vivo.8,9 MSC administration is, therefore, attractive for the treatment of graft-versus-host disease, as well as an aid to enable allogeneic cell engraftment.10 In this context, both autologous and allogeneic MSC (allo-MSC) administration has been successfully used to treat various autoimmune diseases in experimental models and clinical trials11,12,13,14,15. One limitation for the use of autologous MSC is the reported possibility that those isolated from patients with autoimmune disorders are less efficacious than those from healthy donors15,16. In this respect, allo-MSCs from healthy donors are an attractive source of regenerative cells for the treatment of OA. The fact that allo-MSCs can be readily isolated and expanded in vitro to sufficient numbers to treat several patients17 is a further indication of the potential of these cells in regenerative medicine.
OA is characterized by articular cartilage loss and synovial inflammation.18 The use of allo-MSCs to treat OA is envisioned based on their (i) impressive ability to differentiate to cartilage19,20; (ii) their inherent ability to release anti-inflammatory factors,21 including prostaglandin E2 (PGE2), TSG-6, and nitric oxide (NO)22,23,24; and (iii) their low levels of major histocompatibility complex (MHC) and costimulatory proteins.25 However, despite these unique characteristics, growing evidence indicates that allo-MSCs are not fully immunoprivileged in an immunocompetent host.26 Encapsulating these cells in matrices such as calcium alginate, either differentiated ex vivo or undifferentiated, may be necessary to retain the cells at the site of injury or to shield them from an allogeneic immune response in vivo.27 Recent reports have highlighted changes in the immunological response to allogeneic differentiated MSCs following administration in vivo.28,29,30,31 However, currently no unequivocal functional data exist that characterize the immunological consequences of chondrogenic differentiation in vivo in a fully allogeneic model.15,32,33 In fact, studies addressing immunological changes in chondrogenically differentiated cells have produced conflicting results.15,32,33 Consequently, a comprehensive understanding of the allogeneic immune response to chondrogenically differentiated MSCs is crucial for elucidating the success of stem cell–based cartilage repair in vivo. The focus of this study was to analyze changes in immunosuppressive potential of, and the recipient immune response to, chondrogenically differentiated MSCs using a fully MHC-mismatched (allogeneic) immunocompetent rat model. We demonstrate that allogeneic differentiated MSCs have decreased immunomodulatory potential and increased levels of surface MHCs compared with undifferentiated MSCs. These changes were associated with loss of immunosuppressive capacity and increased tendency to stimulate proinflammatory alloimmune responses in vitro. In vivo, chondrogenically differentiated, encapsulated allo-MSCs induced memory T-cell responses both locally and systemically unlike their undifferentiated counterparts. Therefore, in the absence of immunosuppressive therapy, the allogeneic host response may limit the effective engraftment of allogeneic differentiated MSCs in vivo, regardless of whether the cells were encapsulated.
Results
MSCs lose their immunosuppressive properties on chondrogenic differentiation in vitro
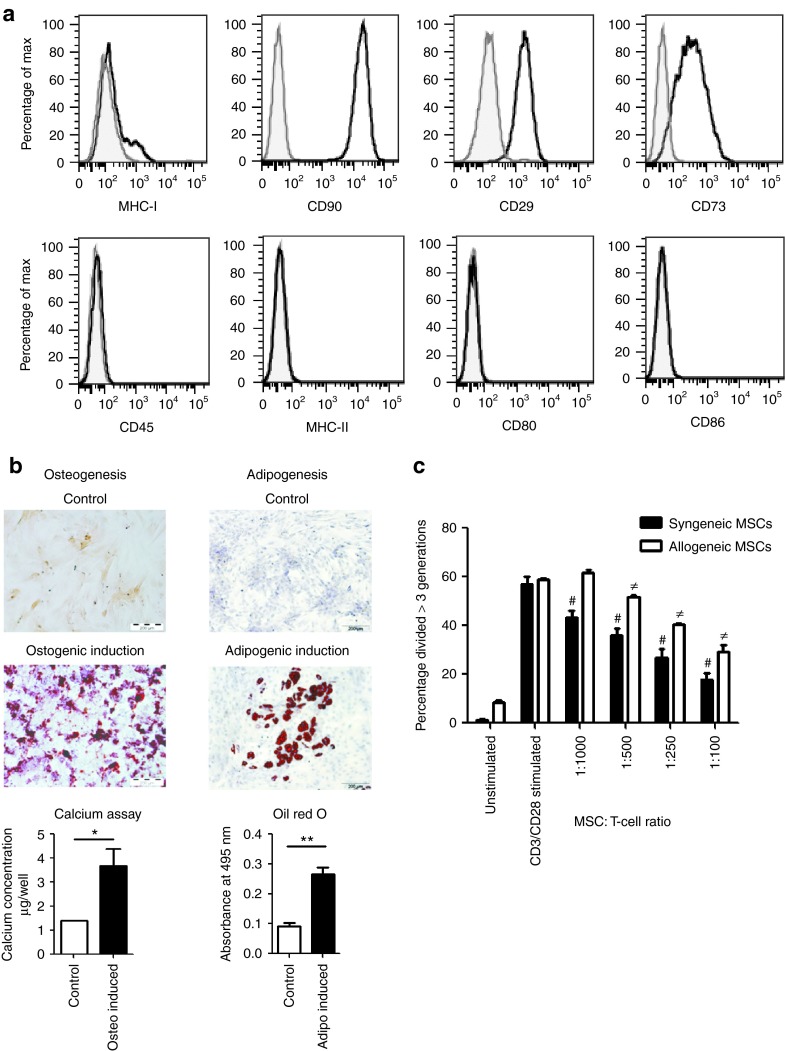
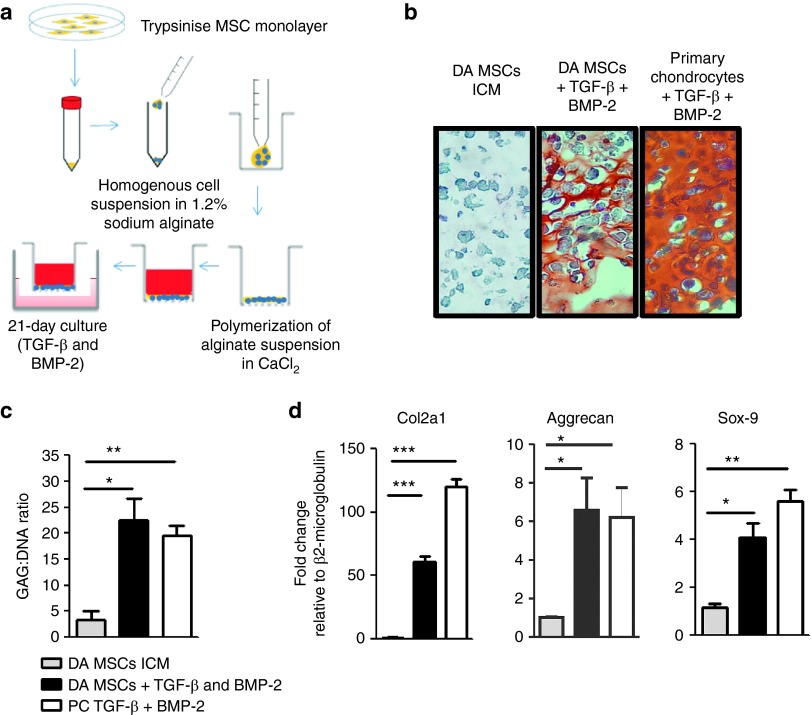
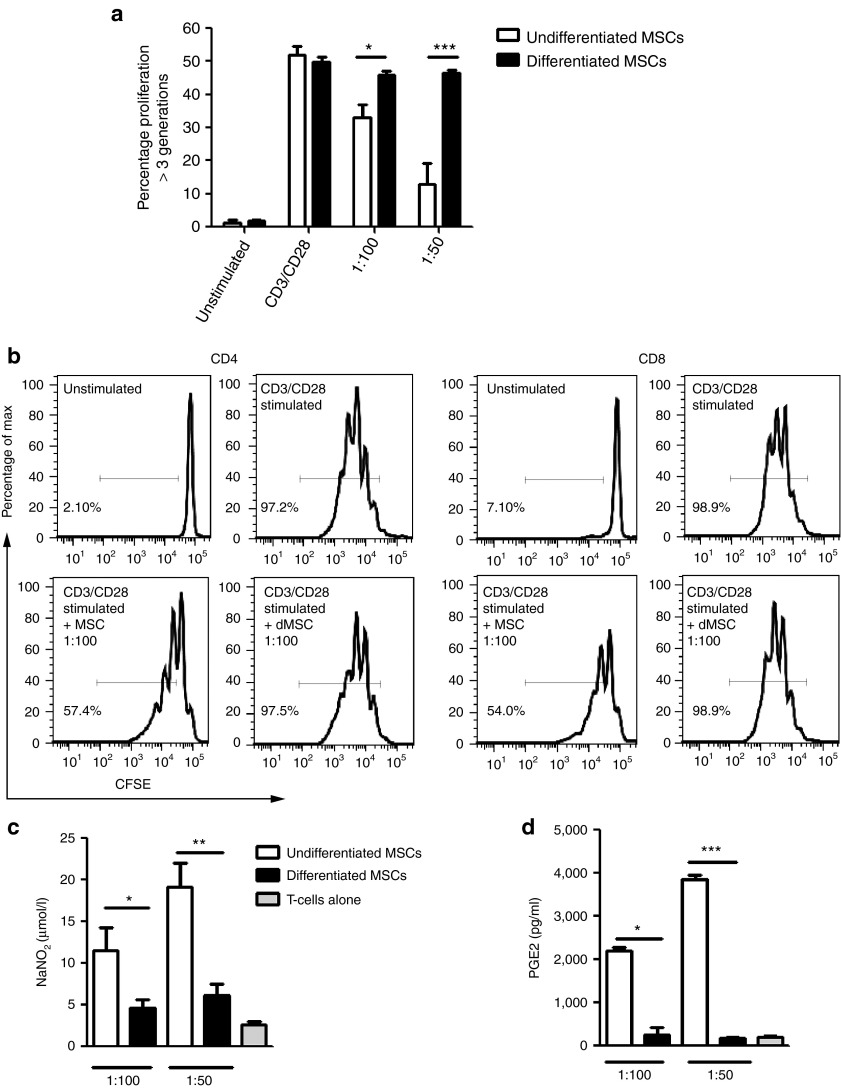
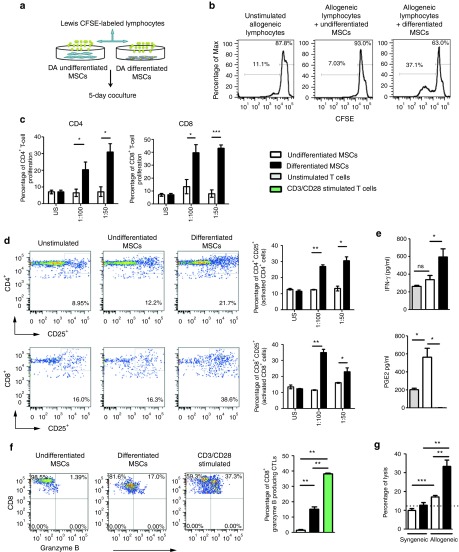
MSCs were isolated from Lewis (LEW) and Dark Agouti (DA) rats, expanded in culture, and were shown to express low levels of MHC-I, uniformly high levels of CD90, CD29, and CD73, and lacked expression of CD45, MHC-II, CD80, and CD86 (Figure 1a). Cell surface expression of DA MSCs is shown, which was comparable with LEW MSC expression profiles (data not shown). Rat MSCs could be induced to differentiate into osteocytes and adipocytes (Figure 1b). In anti-CD3/CD28 stimulated cocultures, DA MSCs suppressed LEW lymphocyte proliferation, albeit at lower efficiency than syngeneic LEW MSCs (Figure 1c). Following 3-week culture, with TGF-β3 and BMP-2 in alginate layers (Figure 2a and Supplementary Figure S1), MSCs developed morphological features and showed safranin O staining that was comparable with similarly cultured primary chondrocytes (Figure 2b). Positive safranin O staining indicates sulfated glycosaminoglycan in the alginate layers of chondrogenically primed MSCs and primary chondrocytes, in contrast to the absence of staining in undifferentiated MSCs. This was confirmed by 1,9-dimethyl-methylene blue assay (Figure 2c), which indicates the formation of glycosaminoglycan-rich tissue in differentiated DA MSCs after alginate culture, a characteristic of articular cartilage (Figure 2c). Transcripts encoding Col2a1, aggrecan, and sox-9 were upregulated in chondrogenically primed MSCs, to similar levels as that of primary chondrocytes, providing further evidence of MSC differentiation to a chondrogenic phenotype (Figure 2d). Undifferentiated MSCs and chondrogenically differentiated DA MSCs were cocultured with polyclonal stimulated allogeneic and syngeneic mixed lymphocytes (Supplementary Figure S2a). Allogeneic undifferentiated MSCs significantly inhibited total T-cell proliferation, in a dose-dependent manner; however, chondrogenically differentiated MSCs did not (Figure 3a). These results were confirmed using undifferentiated and differentiated LEW MSCs in coculture with DA T cells (Supplementary Figure S2c). Allogeneic undifferentiated MSCs suppressed both CD4+ and CD8+ T-cell proliferation as indicated by carboxyfluorescein succinimidyl ester (CFSE) dilution at 1:100 MSC: T-cell ratio; however, this effect was lost following chondrogenic differentiation (Figure 3b). The loss of immunosuppressive effects following chondrogenic differentiation of allo-MSCs was also observed in human peripheral blood mononucleated cell (PBMC): MSC cocultures. Using three MSC donors, we observed significant suppression of proliferation in CD4+ and CD4− T-cell populations following culture with allogeneic undifferentiated MSCs; however, similar to our observations in the allogeneic rat model, this suppressive effect was lost following chondrogenic differentiation (Supplementary Figure S3a,b). Analysis of supernatants confirmed significant dose-dependent reduction of NO (Figure 3c) and PGE2 (Figure 3d) secretion in coculture containing rat allogeneic differentiated MSCs as compared with undifferentiated MSCs.
Figure 1.
Characterization of rat mesenchymal stem cells (MSCs). (a) Rat MSCs are CD45, MHC-I, MHC-II, CD80, and CD86 low or negative and have surface expression of CD90, CD29, and CD73. Histograms of flow cytometry data from DA MSC (passage 3) are shown. Antibody-specific staining is indicated by the heavy black line, with appropriate isotype controls indicated by the gray line. (b) Osteogenic and adipogenic differentiations were induced in rat MSCs as previously described.49 Alizarin Red (osteogenesis) and Oil red O (adipogenesis) staining are shown. Quantification of calcium and Oil red O are shown in panel c. (c) CFSE-labeled T cells were stimulated with anti-CD3/CD28 beads in the presence of undifferentiated allogeneic and syngeneic rat MSCs at indicated ratios. Representative results of three independent experiments are shown ± SEM. #P < 0.001 syngeneic MSCs compared with CD3/CD28 stimulated T cells. ≠P < 0.001 allogeneic MSCs compared with CD3/CD28 stimulated T cells. DA, Dark Agouti; CFSE, carboxyfluorescein succinimidyl ester; MHC, major histocompatibility complex. *P < 0.05; **P < 0.01
Figure 2.
Confirmation of chondrogenic differentiation in three-dimensional alginate layer culture. (a) Chondrogenic differentiation of mesenchymal stem cells (MSCs) was induced using TGF-β3 and BMP-2 for 3 weeks in alginate layers. (b) Safranin O staining indicates the presence of glycosaminoglycan (GAG)-producing cells in differentiated alginate cultures and primary chondrocyte controls but not in undifferentiated alginate layers. (c) Cells were released from alginate layers and the GAG: DNA ratios were determined. (d) Differentiated MSCs in alginate layers upregulated Collagen IIa, type 1 (Col2a1), aggrecan and sox-9 gene transcripts as determined by reverse transcription polymerase chain reaction. Data from three independent experiments are shown ± SEM. *P < 0.05. **P < 0.01. ***P < 0.001. DA, Dark Agouti; CFSE, carboxyfluorescein succinimidyl ester; ICM, incomplete chondrogenic medium; MHC, major histocompatibility complex; PC, primary chondrocyte.
Figure 3.
Chondrogenic differentiated mesenchymal stem cells (MSCs) do not suppress allogeneic T-cell proliferation. (a) CFSE-labeled allogeneic T cells were stimulated with anti-CD3/CD28 beads in the presence of undifferentiated or differentiated MSCs at indicated ratios for 4 days. (b) The corresponding CFSE dilution histograms are shown to indicate the percentage of CD4+ and CD8+ lymphocyte proliferation. CD4+ and CD8+ proliferation was determined as outlined in the gating strategy in Supplementary Figure S2b. As above, allogeneic stimulated Lewis T cells were cultured in the presence of undifferentiated and differentiated DA MSCs. Coculture supernatants were harvested and analyzed by (c) Griess assay and (d) PGE2 ELISA. Data from three independent experiments are shown, except in the case of PGE2 for which two independent experiments are shown. *P < 0.05. **P < 0.01. ***P < 0.001. DA, Dark Agouti; CFSE, carboxyfluorescein succinimidyl ester; dMSC, differentiated MSC; MHC, major histocompatibility complex.
Chondrogenic differentiation enables the induction of allogeneic lymphocyte proliferation and activation and increased allo-MSC susceptibility to cytotoxic T-cell lysis in vitro
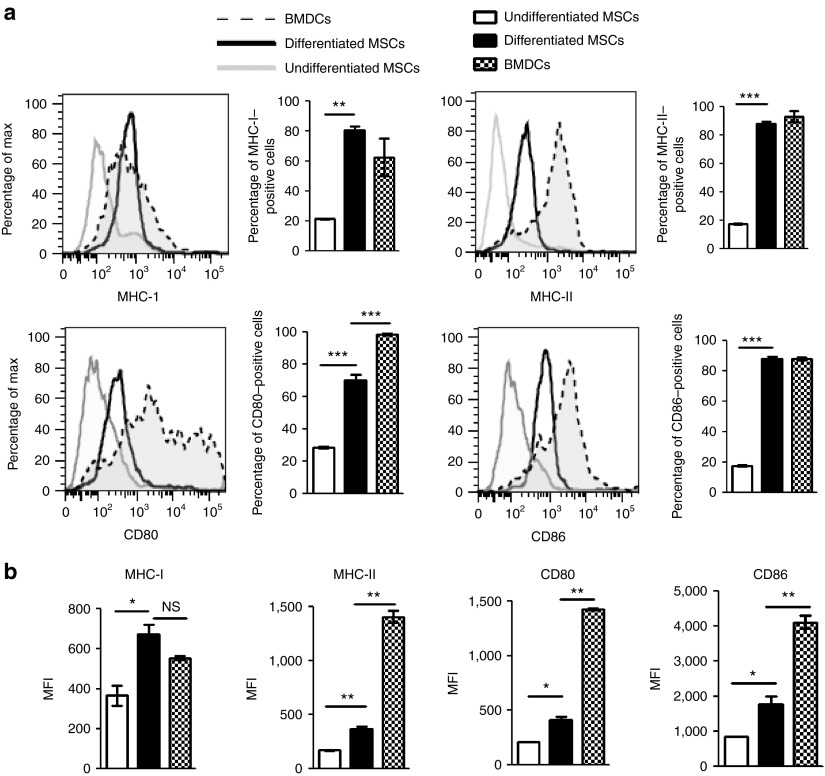
Chondrogenically differentiated MSCs demonstrated surface expression levels of MHC-I, MHC-II, CD80, and CD86 that were clearly higher than those of undifferentiated MSCs but lower than the levels expressed by bone marrow–derived dendritic cells, which were profiled as positive controls (Figure 4a,b). The mean fluorescence intensities of MHC-II-, CD80-, and CD86-positive cells were significantly lower than that of bone marrow–derived dendritic cells (Figure 4b), despite the fact that the frequency of differentiated cells expressing each of these antigens was similar to that of bone marrow–derived dendritic cells. To functionally confirm the induction of an immunogenic phenotype, one way–mixed lymphocyte reaction assays were performed to assess the ability of differentiated MSCs to induce the proliferation of allogeneic lymphocytes (Figure 5a). Chondrogenically differentiated DA MSCs induced readily detectable proliferation of LEW lymphocytes, whereas undifferentiated DA MSCs did not (Figure 5b). More specifically, lymphocyte proliferation was shown to include both CD4+ and CD8+ T-cell subsets (Supplementary Figure S2b) in a cell dose-dependent manner (Figure 5c). The interleukin-2 (IL-2) receptor subunit (IL-2R), or CD25, is upregulated on the surface of activated T cells and B cells. The percentages of CD4+CD25+ and CD8+CD25+ cells in MSC-containing mixed lymphocyte reactions are shown in Figure 5d. We observed significant activation of CD4+ and CD8+ lymphocytes in cocultures with chondrogenically differentiated cells in contrast to undifferentiated cells (Figure 5d). Allogeneic T cells cocultured with chondrogenically differentiated MSCs secreted higher levels of interferon-γ (IFN-γ) and significantly less PGE2 (Figure 5e). Similar results were observed in cocultures using a LEW MSC and DA allogeneic lymphocytes strain combination (data not shown), indicating that these observations are not a DA MSC–specific effect. Because cytotoxic lymphocytes use the granule exocytosis pathway to kill target cells, we used flow cytometry to analyze the expression of granzyme B in proliferating CD8+ allospecific T cells. The frequency of CD8+-expressing granzyme B cells was significantly increased in cocultures with chondrogenically differentiated cells in contrast to undifferentiated cells (Figure 5f), indicating the induction of cytotoxic CD8+ T cells by allogeneic differentiated MSCs, although the frequency was significantly lower than that observed in CD3/CD28 stimulated T cells. Recognition of MHC-I expression on allogeneic cells is essential to elicit cytotoxic CD8+ T-cell (CTL) effector functions.34 Because MHC-I expression was significantly increased following chondrogenic differentiation, we hypothesized that chondrogenically differentiated allo-MSCs may be more susceptible than their undifferentiated counterparts to lysis by donor-specific cytotoxic T cells (CTLs). To test this possibility in vitro, we performed cytotoxicity assays with Calcein-labeled allogeneic undifferentiated and differentiated cells (Supplementary Figure S4). Allogeneic undifferentiated MSCs, which express low levels of MHC-I, were effectively lysed by CTLs added at a ratio of 100:1 when compared with syngeneic MSCs. However, this effect was significantly more evident in allogeneic differentiated cells, which showed a marked upregulation in MHC-I expression in vitro. In this case, differentiation resulted in a twofold increase of specific lysis (Figure 5g). These results suggest that chondrogenic differentiation induces the proliferation of CTLs, which correlated with an increased susceptibility to lysis by donor-specific CTLs.
Figure 4.
Chondrogenic differentiation induces the expression of immune antigens in vitro. (a) Undifferentiated and differentiated mesenchymal stem cells (MSCs) were characterized for the expression of MHC-I, MHC-II, and costimulatory molecules CD80 and CD86 by flow cytometry using fluorochrome-matched isotype control antibodies. Bar graphs indicate the percentage of positive cells. Bone marrow–derived dendritic cells (BMDCs) were used as positive controls. (b) The mean fluorescence intensity (MFI) of MHC-I, MHC-II, CD80, and CD86 was calculated for undifferentiated MSCs, differentiated MSCs, and BMDCs. The results of three independent experiments are shown ± SEM. Statistical significance was determined using ANOVA. *P < 0.05. **P < 0.01. ***P < 0.001. MHC, major histocompatibility complex; NS, not significant.
Figure 5.
Chondrogenically differentiated mesenchymal stem cells (MSCs) induce proliferation of allogeneic T cells in vitro and have increased susceptibility to lysis by antigen-specific T cells. (a) CFSE-labeled lymphocytes were cultured in the presence or absence of undifferentiated MSCs or differentiated MSCs at varying ratios. (b) On day 5, cells were harvested and proliferation was analyzed. Representative plots of three independent experiments are shown. (c) Percentages of CD4+ and CD8+ lymphocyte proliferation are indicated at ratios of 1:100 and 1:50 (MSC: lymphocyte). (d) Lymphocytes were cocultured as in a. Representative dot plots indicating percentages of CD4+CD25+ and CD8+CD25+ lymphocytes are shown. (e) Cell culture supernatants were analyzed by enzyme-linked immunosorbent assay to determine the levels of interferon-γ (IFN-γ) and prostaglandin E2 (PGE2). (f) Representative flow plots of the frequency of granzyme B expression in CD8+ T cells are shown (left) and a summary graph of this data is shown (right). DA-specific allogeneic cytotoxic T cells (CTLs) were generated in a one-way mixed lymphocyte culture of Lewis and DA T cells as outlined in Supplementary Figure S4. (g) The percentage lysis is shown following incubation of syngeneic Lewis or allogeneic DA rat MSCs, either undifferentiated or differentiated with alloantigen-specific CTLs in an effector to target ratio of 100:1. The results of three independent experiments are shown ± SEM, n = 3. Statistical significance was determined by two-tailed, unpaired t-tests and ANOVA. *P < 0.05. **P < 0.01. ***P < 0.001. DA, Dark Agouti; CFSE, carboxyfluorescein succinimidyl ester; MHC, major histocompatibility complex.
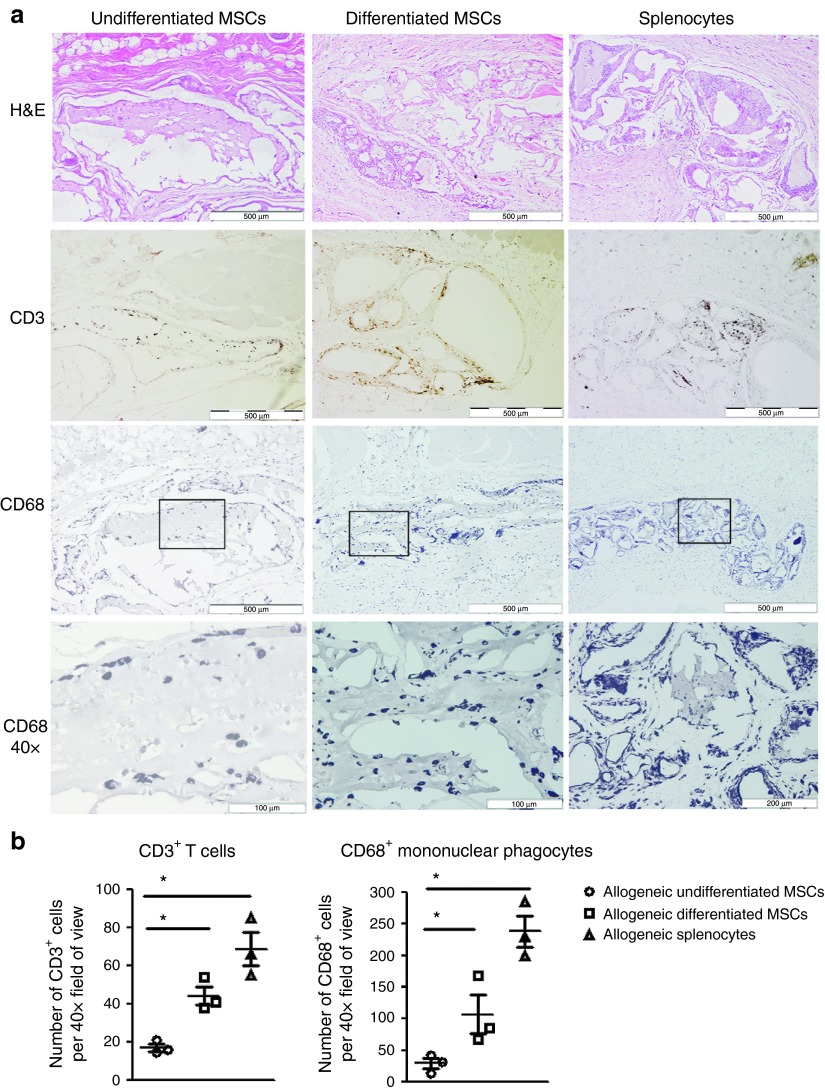
Chondrogenically differentiated MSCs induce local innate and adaptive immune infiltration, which is evident up to 6 weeks after implantation
Immunological rejection of allogeneic organs and tissues is characterized by a cellular immune response comprising of T cells and mononuclear phagocytes within the implant area. To determine the immunological consequences of subcutaneous implantation of allogeneic undifferentiated and chondrogenically differentiated MSCs in vivo, we analyzed the innate and adaptive immune cell infiltrate by hemotoxylin and eosin (H&E) and immunohistochemical staining for mononuclear phagocytes (CD68+) and T-cell (CD3+) markers (Supplementary Figure S5a). In these experiments, MSC-containing implants were compared with implants containing allogeneic splenocytes, which would be expected to induce robust antidonor immune responses. Allogeneic undifferentiated MSC alginate implants were clearly visible and contained low numbers of infiltrating mononuclear cells (Figure 6a and Supplementary Figure S5b). In contrast, the implants retrieved from allogeneic differentiated MSC- and splenocyte-containing implants exhibited structural damage and were heavily infiltrated by mononuclear immune cells. Furthermore, immunohistochemical analysis demonstrated increased numbers of T cells and mononuclear phagocytes within implants containing differentiated allo-MSCs and splenocytes compared with those containing undifferentiated allo-MSCs (Figure 6a,b). The number of CD3+ T cells increased from 17.22 ± 1.928 in undifferentiated MSC implants to 44.33 ± 4.910 and 68.67 ± 8.762 in allogeneic differentiated MSC- and splenocyte-implanted recipients, respectively. Similarly, the number of CD68+ mononuclear phagocytes increased from 29.58 ± 8.387 in allogeneic undifferentiated MSC implants to 107.3 ± 20.78 and 238.3 ± 24.89 in allogeneic differentiated MSC and splenocyte implants, respectively. Syngeneic differentiated MSC implants displayed very low levels of CD68 staining (data not shown). These results indicate that a local immune response, characterized by T-cell and mononuclear phagocyte infiltrates, is mounted against implanted chondrogenically differentiated allo-MSC that is largely absent in undifferentiated allo-MSC implants.
Figure 6.
Chondrogenically differentiated mesenchymal stem cells (MSCs) induce local innate and adaptive immune infiltration, which are evident up to 6 weeks after implantation. Lewis rats were subcutaneously implanted with 106 allogeneic undifferentiated, differentiated MSCs or allogeneic splenocytes encapsulated in alginate as outlined in Supplementary Figure S5a. Six weeks after implantation, the area surrounding the implant was removed for histological analysis. (a) Representative histological sections illustrating hemotoxylin and eosin (H&E) staining (10×, upper row), anti-CD3+ staining (10×, second row), and anti-CD68+ (10×, third row and 40×, fourth row) staining in subcutaneous sections of allogeneic undifferentiated, differentiated MSCs or allogeneic splenocytes. 40× magnification indicate the morphological characteristics of infiltrating mononuclear phagocytes (lower panel). (b) The number of positive CD3+ and CD68+ cells counted per 40× field of view are indicated on the histograms (n = 3). The results of three independent experiments are shown ± SEM, n = 4. *P < 0.05.
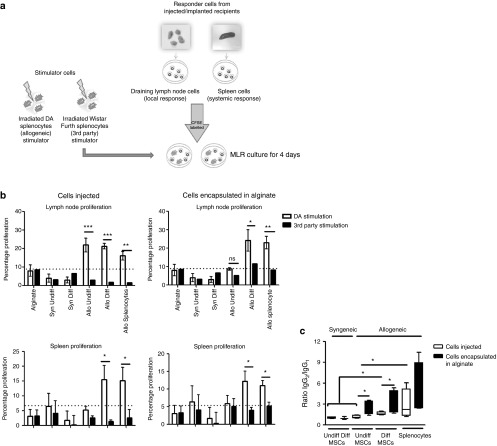
Chondrogenically differentiated allogeneic cells induce a systemic allogeneic lymphocyte response, which is not prevented by encapsulation in alginate
Donor (DA)- and third party (Wistar Furth, WF)-specific T-cell responses were next analyzed using CFSE-labeled lymphocyte preparations from draining lymph nodes and spleens from groups of LEW rats 6 weeks following various unencapsulated and alginate encapsulated subcutaneous cell implants (Supplementary Figure S6). Lymphocyte proliferation following 4-day culture with purified DA or WF OX62+ dendritic cells (Figure 7a) was quantified based on the percent proliferation as indicated by CFSE dilution (Figure 7b). In the case of unencapsulated cells, enhanced donor-specific T-cell proliferation was observed for the draining lymph node cells of animals that had received undifferentiated allo-MSCs, differentiated allo-MSCs, and allo-splenocytes. However, splenocytes from the same animals demonstrated increased donor-specific proliferation only in recipients of chondrogenically differentiated allo-MSCs and splenocytes (Figure 7b, left). Similar results were obtained for groups of animals that received alginate-encapsulated cells with the exception that there was a minimal increase in donor-specific T-cell proliferation in draining lymph nodes of the recipients of undifferentiated allo-MSCs (Figure 7b, right). The results were interpreted as indicating that (i) subcutaneously implanted undifferentiated allo-MSCs may induce an antidonor T-cell memory response that remains localized to draining lymphoid tissue and is abrogated by alginate encapsulation and (ii) similarly implanted chondrogenically differentiated allo-MSCs induced an antidonor T-cell memory that was detectable in both draining lymphoid tissue and spleen and is not preventable by alginate encapsulation. Finally, the nature of the antidonor antibody responses elicited was analyzed by flow cytometry of donor strain (DA) cells incubated with recipient sera from the various groups described above. Compared with recipients of syngeneic MSCs, anti-DA antibody was detectable in sera from all recipients of allo-MSCs whether undifferentiated, chondrogenically differentiated, unencapsulated, or alginate-encapsulated as well as in sera from recipients of allogeneic splenocytes (data not shown). However, when isotype-specific staining was applied, it was observed that implantation of alginate-encapsulated cells was associated with higher IgG2/IgG1 ratios (indicative of a predominant T-helper 1 type immune response)35 when compared with implantation of unencapsulated cells (Figure 7c). For recipients of both unencapsulated and encapsulated cells, the IgG2/IgG1 ratios among the groups followed a rank order of allogeneic splenocytes > differentiated allo-MSCs > undifferentiated allo-MSCs (Figure 7c). Thus, in similar fashion to the T-cell responses, donor-specific B-cell (antibody) response with potential to mediate allograft rejection was shown to be enhanced by chondrogenic differentiation of MSCs. These results also indicated that alginate encapsulation, while providing some barrier to the induction of antidonor T-cell responses against weakly immunogenic undifferentiated MSCs, enhances the generation of antidonor IgG2 antibodies.
Figure 7.
Chondrogenically differentiated allogeneic cells induce a systemic allogeneic memory lymphocyte and alloantibody immune response, which is not prevented by encapsulation in alginate. Lewis (LEW) rats were subcutaneously implanted with 106 allogeneic undifferentiated, differentiated mesenchymal stem cells (MSCs) or allogeneic splenocytes with and without alginate encapsulation as outlined in Supplementary Figure S6. Six weeks after implantation, the draining lymph nodes, spleen, and serum were isolated for further analysis. (a) Experimental outline for ex vivo restimulation of draining lymph node lymphocytes and splenocytes using irradiated DA and Wistar Furth stimulator cells is shown. (b) Proliferation of CFSE-labeled total lymphocytes isolated from draining lymph nodes (upper graphs) and spleen (lower graphs) was measured following 5-day stimulation with DA (allospecific) and Wistar Furth splenocytes (third party). Increased proliferation in response to DA-specific stimulation indicated presensitization to DA antigen in vivo. (c) LEW rats were implanted or injected with 106 allogeneic undifferentiated, differentiated MSCs or allogeneic splenocytes. Serum was harvested 6 weeks after implant or injection and incubated with thymocytes of allogeneic DA rats. Isotype-specific binding was determined using antirat IgG1 and antirat IgG2 antibodies. The ratio of IgG2:IgG1 was calculated and is indicated for recipients of injected allogeneic cells and implanted allogeneic cells. Results are presented from n = 4 animals per group ± SEM, n = 4. *P < 0.05. **P < 0.01. ***P < 0.001. Allo, allogeneic; DA, Dark Agouti; Diff, differentiated; CFSE, carboxyfluorescein succinimidyl ester; MHC, major histocompatibility complex; Syn, syngeneic; Undiff, undifferentiated.
Discussion
Therapeutic applications of allo-MSCs that involve direct regeneration of mesenchymal tissues, such as cartilage and bone, require that MSC differentiation along a single lineage be achieved and maintained in vivo. In the context of OA, understanding the immunogenicity of bone marrow–derived allo-MSCs following either induced or spontaneous differentiation is fundamentally important if these cells are to be used successfully to treat patients with OA.3 To date, limited studies have addressed this clinically relevant question, with most relying solely on interpretation of in vitro data to indicate immunological changes that occur in these cells following chondrogenic differentiation33. Consequently, knowledge of the immunological consequences of chondrogenically differentiated allo-MSC transplantation is essential before their therapeutic application, and is the focus of this study. We determine the immunological functions of, and donor-specific immune responses to, allogeneic chondrogenically differentiated MSCs. Chondrogenic differentiation was induced in an alginate gel system, allowing for simple single cell isolation, balanced distribution of cells, and diffusion of factors throughout the hydrogel and facilitates the three-dimensional environment required for chondrogenesis20
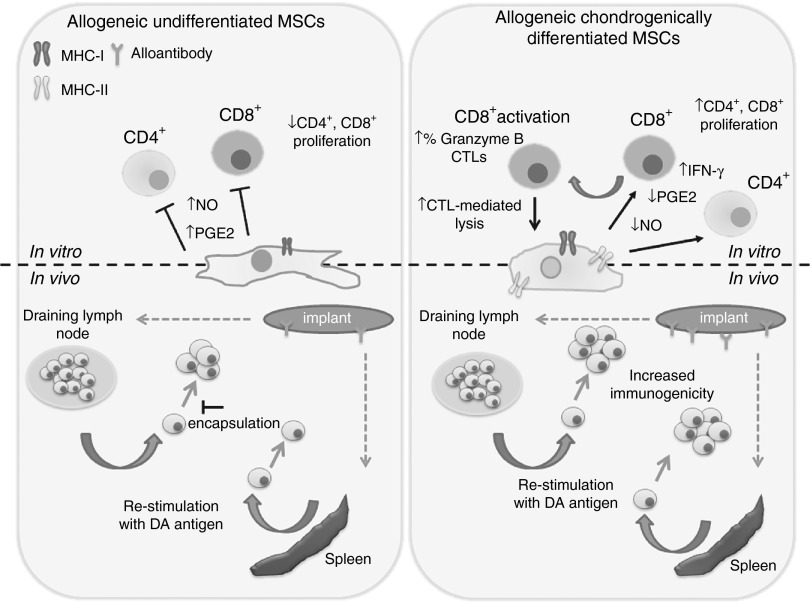
The proposition that allo-MSCs are immunoprivileged and can be transplanted across MHC barriers without adverse immune effects has developed over the last decade. The concept of the potent immunosuppressive and immune privileged nature of MSCs has resulted in increasing numbers of studies with allo-MSCs. Despite this, it has been noted that these studies have not generally been accompanied by robust investigation of possible antidonor immune responses. The use of MSCs for the treatment of OA is relevant from two perspectives: first, with their well-described immunomodulatory properties they could attenuate the inflammatory environment36 of the joint and second, by way of their regenerative potential, MSCs could differentiate to replace damaged cartilage.37 However, studies addressing the use of MSC therapy for the treatment of OA have produced conflicting results.14,38,39,40 Here, we demonstrate that induced chondrogenic differentiation of rat MSCs resulted in loss of MSC immunosuppressive properties in vitro. Many studies have shown that MSCs suppress the proliferation and activation of various immune cells through the release of soluble mediators, including PGE2 and NO.41,42,43 Both PGE2 and NO were significantly reduced following differentiation, suggesting that differentiated MSCs lack the ability to inhibit an inflammatory response. Not only did we observe inhibition of immunosuppressive effects after differentiation into chondrocyte-like cells, we could also show the ability of these differentiated MSCs to induce proliferation of proinflammatory, cytotoxic allogeneic lymphocytes in vitro, which secrete higher levels of IFN-γ. Because T cells are believed to play a critical role in orchestrating the inflammatory response in OA,44 these data would suggest an inability of differentiated allo-MSCs to modulate inflammation in the context of OA. Instead, these observations suggest the possibility that allogeneic differentiated MSC transplantation may actually exacerbate the inflammatory responses by their ability to promote proliferation and activation of allogeneic CD4+ and cytotoxic CD8+ T cells (Figure 8). Therefore, consideration must be given to the loss of the immunosuppressive properties in vitro and the emergence of an immunogenic phenotype after differentiation before transplantation, as these properties are likely to affect their therapeutic benefit in vivo.
Figure 8.
Chondrogenic differentiation increases antidonor immune response to allogeneic mesenchymal stem cells (MSCs) in vitro and in vivo. Loss of MSC immunosuppressive potential following chondrogenic differentiation was associated with decreased prostaglandin E2 (PGE2) and NO. Chondrogenic differentiation induced the expression of cell surface immune antigens in vitro, including MHC-I and MHC-II. Differentiated MSCs induced proliferation of allogeneic CD4+ T cells and CD8+ cytotoxic T cells (CTLs) cells in vitro and displayed increased susceptibility to lysis by antigen-specific CD8+ T cells. Chondrogenically differentiated implants induced local innate and adaptive mononuclear cell infiltration. Chondrogenically differentiated cells induced a systemic allogeneic memory lymphocyte and alloantibody immune response which, unlike allogeneic undifferentiated MSCs, was not prevented by encapsulation in alginate. MHC, major histocompatibility complex.
The cell surface expression of MHC-I and MHC-II molecules contribute to different types of allogeneic rejection. MHC-I molecules are mainly associated with acute rejection, which is thought to be mediated by CD8+ CTLs, whereas MHC-II molecules are suggested to be involved in a chronic rejection dependent on CD4+ T cells and local lymph node sensitization45. Undifferentiated allo-MSCs have been shown to express low levels of MHC-I, contributing to their immunoprivileged status25. Induced MHC-I expression on allogeneic differentiated MSCs enables lysis of these cells in vitro by presensitized allo-activated CTLs, when compared with undifferentiated allo-MSCs. This would imply that differentiated allo-MSCs could directly present antigen to CD8+ T cells, enabling effective lysis and rejection in vivo. The induced expression of MHC-II on allo-MSC following differentiation could lead to further enhancement of the CTL response by stimulating CD4+ T-helper cells.46 Furthermore, although T-cell activation is central to allogeneic rejection, mononuclear phagocytes are increasingly acknowledged as key inflammatory mediators that enhance T-cell–driven organ rejection47. CD68+ mononuclear phagocytes were significantly more abundant in implants containing allogeneic differentiated MSCs and splenocytes. Since mononuclear phagocyte activity and protease expression have been shown to participate in tissue damage and graft rejection48, these observations suggest that rejection and subsequent destruction of the allogeneic differentiated MSC and splenocyte implants may be due to both direct T-cell–mediated lysis of implanted cells and T-cell activation of mononuclear phagocytes48.
In addition, ex vivo restimulation of lymphocytes from recipient animals confirmed increased memory T-cell responses in draining lymph nodes of animals injected with undifferentiated and differentiated MSCs; however, only differentiated MSCs induced systemic memory T-cell responses. Implantation of undifferentiated MSCs in alginate reduced local T-cell responses by 75%, indicating the possibility that undifferentiated allo-MSCs following encapsulation in alginate may modulate the local immune response through their ability to secrete PGE2 and NO locally. Alginate-encapsulated differentiated allogeneic T cells induced detectable memory T-cell responses locally and systemically, possibly reflecting the observations of our in vitro study that indicated the lack of secretion of immunosuppressive factors, in addition to increased immunogenicity and secretion of IFN-γ on differentiation of allo-MSCs (Figure 8). Systemic alloimmune responses to differentiated MSCs indicate that they become immunogenic and may require immunosuppressive therapy for therapeutic use. For the purpose of regeneration, MSC-based therapy for the treatment of OA presumably would require the efficient engraftment of these cells in the inflamed joint. The fact that allogeneic undifferentiated MSCs, differentiated MSCs, and splenocytes all provoked a significantly enhanced adaptive alloantibody response when implanted in alginate provides evidence that retention of allogeneic differentiated cells over a prolonged period of time can evoke a stronger adaptive alloimmune response in vivo. Our observation that alloantibody levels were significantly elevated in animals that received an allogeneic implant (either undifferentiated or differentiated) as compared with a subcutaneous injection (unshielded) is of relevance from the perspective of tissue engineering strategies that propose to retain allogeneic cells at a location for a prolonged period of time30. Therapeutic strategies to limit postimplant alloantibody production may be required to improve long-term differentiated MSC survival by reducing the incidence of chronic allograft rejection.
Our findings are also relevant for the related issue of the immunological consequences of spontaneous differentiation of allo-undifferentiated MSCs following therapeutic delivery in vivo and may explain the discrepancies between studies using undifferentiated MSCs therapeutically in models of OA.14,40 A summary of our findings are outlined in Figure 8. While our observations question the use of these cells therapeutically, further investigation of their use in therapeutic models of OA will provide further information on strategies to limit the immune response to these cells following transplantation. Future investigation and manipulation of allogeneic chondrogenically differentiated MSC–associated immune responses, possibly using genetic engineering approaches, holds a relevance to the future success of cellular therapeutics that could be used for the treatment of OA.
Materials and Methods
Animals. All procedures performed were conducted in a fully accredited animal housing facility under a license granted by the Department of Health, Ireland and were approved by the Animals Ethics Committee of the National University of Ireland, Galway, Ireland. Male LEW and DA rats were obtained from Harlan Laboratories (Derby, UK). All animals were aged between 6 and 12 weeks.
Media and reagents. Unless otherwise stated, all media and reagents were purchased from Sigma-Aldrich (Dorset, UK). All labware consumables, including all culture flasks, centrifuge tubes, 96-well round bottom plates, and enzyme-linked immunosorbent assay plates were purchased from Sarstedt (Wexford, Ireland). Cell culture 24-well 0.4 µm inserts were purchased from Grenier Bio-one (Dublin, Ireland). Recombinant cytokines were purchased from Peprotech (Hamburg, Germany).
Isolation and characterization of MSCs. MSCs were isolated from the femur and tibia of 6- to 12-week-old DA and LEW rats as previously described.49 MSCs were maintained in standard cell culture conditions in α-minimum essential media, F-12 supplement (1:1 ratio), 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin (rat MSC medium). Tri-lineage differentiation assays were carried out to confirm the osteogenic and adipogenic capacity of the cells, as previously described.50 In addition, cell surface characterization for established markers such as MHC-I, CD29, CD90, CD73, CD45, CD80, CD86, and MHC-II was carried out by flow cytometry, as previously described.25 Antibodies were purchased from BD Biosciences (Oxford, UK), Biolegend (London, UK), and Serotec (Kidlington, UK), and samples were analyzed using FACS Canto (BD Biosciences). Primary chondrocytes were isolated from articular cartilage of the rat knee as positive controls for chondrogenesis. The articular cartilage was removed from the condyle of the joint in chips and incubated for 1 hour in 2 mg/ml protease. Extracellular matrix was digested with 1.5 mg/ml collagenase D (Roche, West Sussex, UK) at 37 °C overnight, and chondrocytes were plated at 5,000 cells/cm.17
Preparation of alginate layers. Chondrogenesis was induced in a three-dimensional alginate matrix system.20 Rat and human MSCs cultured in monolayer were trypsinized and resuspended to a concentration of 50 × 106 cells/ml in 0.15 mol/l NaCl–25 mmol/l 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES). An equal volume of 2.4% sterile filtered sodium alginate was then gently added until a homogenous mixture was achieved. Forty microliters of the cell/alginate mix was carefully pipetted onto the surface of a sterile 24-well transwell insert. Alginate polymerization was induced by incubation in 0.1 mol/l CaCl2–25 mmol/l HEPES. Layers were incubated in incomplete chondrogenic medium (94% high-glucose Dulbecco's modified Eagle medium, 1% fetal bovine serum, 1% insulin-transferrin-sodium selenite supplements solution, 50 µg/ml ascorbic acid-2-phosphate, 40 µg/ml proline, 1 mmol/l sodium pyruvate, 100 U/ml penicillin, and 100 µg/ml streptomycin), supplemented with 10 ng/ml TGF-β3 and 100 ng/ml BMP-2 (Rat MSC differentiation only) (Peprotech) to induce chondrogenesis. Medium was changed daily for the course of the experiment. Cells were isolated into a single cell suspension from alginate layers by dissociation in sodium citrate solution (150 mmol/l NaCl, 25 nmol/l sodium citrate, and 20 mmol/l ethylenediaminetetraacetic acid) and collagenase D. Glycosaminoglycan production was assayed by the 1,9-dimethyl-methylene blue assay (Invitrogen, Paisley, UK). DNA quantities were determined by the PicoGreen using a commercial kit (Molecular Probes, Paisley, UK). The glycosaminoglycan/DNA ratio was determined for each layer. Histological and reverse transcription polymerase chain reaction (PCR) analysis and confirmation of chondrogenic differentiation was confirmed for each experiment. All layers were free of contamination and were confirmed to be mycoplasma negative. Pico-green analysis was also carried out to confirm similar number of encapsulated cells in each layer.
Quantitative real-time reverse transcription PCR. Cells were isolated from alginate layers as outlined above, and RNA was isolated using the TriZol method (Invitrogen). cDNA was synthesized from 1 µg of isolated RNA and used for real-time PCR. Intron-spanning RNA primers for rat transcripts Sry-related HMG box (SOX)-9, β-2 microglobulin and collagen, type II, alpha 1 (Col2a1) were sourced from Qiagen, Manchester, UK (product codes: QT00427602, QT00176295, QT02380840, respectively), aggrecan primer set was custom designed and sourced from Sigma. Sybr-green real-time reverse transcription PCR was performed using the Applied Biosystems, Dublin, Ireland StepOnePlus real-time PCR machine.
Isolation of human PBMCs. Human PBMCs were isolated from whole blood using Ficoll-Hypaque (Invitrogen) and harvested by careful pipetting of the corresponding density band (buffy coat) and then washed in phosphate-buffered saline twice (10 minutes at 400g) and subjected to low-speed centrifugations to remove platelets. PBMCs were resuspended in culture medium (RPMI 1640, Gibco, Dublin, Ireland) containing 10% fetal calf serum, 50 µmol/l β mercaptoethanol, nonessential amino acids, and l-glutamine in RPMI.
T-cell proliferation assays and mixed lymphocyte reaction cultures. Lymphocytes were obtained from the spleen and lymph nodes of LEW and DA rats. T cells were washed with 0.1% bovine serum albumin/phosphate-buffered saline and stained in prewarmed (37 °C) 10 µmol/l Vybrant CFDA SE (CFSE)/phosphate-buffered saline staining solution (Invitrogen, Carlsbad, CA) as per manufacturer's instructions. 2 × 105 CFSE-stained T cells were stimulated at a 1:1 ratio with anti-rCD3/anti-rCD28-labeled beads in T-cell media (RPMI 1640 supplemented with 10% fetal calf serum, 50 µmol/l β-mercaptoethanol, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1 mmol/l sodium pyruvate, and 2 mmol/l l-glutamine). For human MSC:PBMC assays, 2 × 105 CFSE stained PBMCs were stimulated in 96-well round-bottomed plates with anti-hCD3/anti-hCD28-soluble antibodies (BD Biosciences) in MLC medium. Assays were incubated at various MSC:T-cell ratios in a humidified incubator for 4 days at 37 °C following which rat and human T-cell proliferation and CD4 and CD8 expression were assayed by flow cytometry (CD4-APC and CD8α-PE; Biolegend. For rat mixed lymphocyte reactions; syngeneic MSCs, allo-MSCs, or chondrogenically differentiated MSCs were plated in 96-well round bottom plates. CFSE-labeled untreated lymphocytes were used as responders. A total of either 2 × 103 or 1 × 103 stimulating cells were cocultured with 1 × 105 CFSE-labeled responding lymphocytes for 5 days. The activation state and expression of granzyme B in T-cell subsets was assessed by surface expression of CD4-APC, CD8-PeCy7, CD25-FITC (Biolegend, BD Biosciences), and intracellular granzyme B (Biolegend), respectively. For intracellular staining, cells were fixed and permeabilized overnight at 4 °C using fixation/permeabilization concentrate and diluents (eBioscience, Hatfield, UK). Next day, cells were incubated with anti-granzyme B-APC antibody in permeabilization buffer for 1 hour, followed by three washes. T-cell proliferation, activation, and differentiation was analyzed on a FACS Canto.
Cytotoxicity assay. Alloantigen-specific CTLs were generated in one-way mixed lymphocyte cultures with LEW lymphocytes and γ-irradiated DA lymphocytes (ratio 2:1) in MLC medium (2% heat-inactivated rat serum, 10% fetal calf serum, and 50 µmol/l β-mercaptoethanol in RPMI). After 5 days, T cells were harvested, washed, and resuspended in MLC medium. MSCs were stained in 10 µmol/l Calcein AM (1 × 106 cells/ml). 1 × 104 MSCs/well were cocultured with 1 × 106/well (1:100) or 5 × 106/well (1:50) T cells, or treated with 0.9% Triton-X (for maximum lysis), or treated with medium (spontaneous release) in five replicates each. LEW MSCs (syngeneic) were used as a control for these experiments. After 4 hours, supernatant was harvested and calcein fluorescences (F) measured using an enzyme-linked immunosorbent assay plate reader. Specific lysis was calculated from mean fluorescence of replicates as follows: (F[sample]−F[spontaneous release]) / (F[maximum lysis]−F[spontaneous release]) × 100 = Percentage specific lysis.
IFN-γ, NO, and PGE2 assays. IFN-γ cytokine determination in allogeneic mixed lymphocyte supernatants, were quantified using enzyme-linked immunosorbent assay (R&D Systems, Abingdon, UK), using the manufacturers protocols. NO levels in coculture supernatants were analyzed by the Griess assay. Briefly, 100 μl of the medium were placed in a 96-well plate and an equal amount of Griess reagent (1% sulfanilamide and 0.1% N-1-(naphthyl) ethylenediamine-diHCl in 2.5% H3PO4) was added. The plate was incubated for additional 5 minutes at room temperature, and then the absorbance was measured at 540 nm with a microplate reader. The amount of NO was calculated using sodium nitrite standard curve. In addition, supernatants from cocultures were analyzed by a Parameter Assay Kit for PGE2 (R&D Systems).
Subcutaneous implantation. LEW rats were anesthetized (xylazine and ketamine), and an incision was made in the skin at the upper right quadrant of the back. Alginate layers (containing differentiated allo-MSCs, differentiated syngeneic MSCs, undifferentiated allo-MSCs, undifferentiated syngeneic MSCs, allogeneic splenocytes, or without cells) were implanted as a 1- to 2-mm thick layer containing 1 × 106 cells in naïve LEW rats. Incisions were sutured using 3-0 Vicryl Sutures. In addition, 1 × 106 dissociated cells from each group were injected subcutaneously. Animals were sacrificed 6 weeks after cell implantation, and blood, spleen, draining lymph nodes, and excised skin from implant site in recipient animals were removed for analysis.
Histology and immunohistochemical staining. Histological analysis was performed on cultured alginate layers and excised skin from implanted/injected animals. Alginate layers were preserved overnight in 10% neutral buffered formalin (Sigma-Aldrich) supplemented with 100 mmol/l CaCl2. Layers were then paraffin embedded, and 7-µm sections were stained with Safranin O to quantify the production of extracellular matrix. For subcutaneous implants, identified sections of injected and/or implanted skin were excised from each animal at sacrifice. Tissue was fixed in formalin and paraffin embedded. Sections were stained by hemotoxylin and eosin as previously described.50 For immunohistochemistry, sections were rehydrated; endogenous peroxidase activity was blocked with H2O2. Sections were incubated with mouse antirat CD68 and rabbit antirat CD3 antibodies for 1 hour, followed by incubation with appropriate secondary antibodies for 30 minutes at room temperature. Positive antibody binding was detected by use of ABC reagent and DAB. The number of positively stained CD3 and CD68 cells was counted per 40× field of view (4–7 random fields chosen/implant).
Allogeneic restimulation assays. Animals were sacrificed 6 weeks following implantation or injection. Single cell suspensions were obtained from the spleen and draining lymph nodes of each animal as previously described50. 1 × 105 CFSE-labeled lymphocytes from each implanted or injected animal (responder cells) were added to each well of a 96-well plate. Donor derived DA or fully MHC mismatched (third party; Wistar Furth) splenocytes were isolated as stimulator cells and irradiated (dose of 20 Gy). Responder cells were incubated as described for T-cell assays for 4 days at a ratio of 1:10; stimulator: responder. After 4 days, proliferation and activation of CD4+ and CD8+ cells from the responder animal were determined as described in the T-cell proliferation assay section.
Alloantibody production. The presence of antidonor-specific (DA) antibodies in the recipient (LEW) animal serum was determined by incubation of isolated serum (25 µl; 1:2 dilution of neat serum) with donor strain derived thymocytes (5 × 105) in vitro. Fc receptors were first blocked (anti-CD32 antibody, BD Biosciences) for 5 minutes, then serum was incubated with thymocytes at 4 °C for 40 minutes. Anti-DA-specific antibody binding was detected using antirat IgG1 and IgG2 antibodies (Acris, Herford, Germany) for 40 minutes at 4 °C. Bound antibody was detected by flow cytometry using a FACS Canto.
Statistical analysis. All data were analyzed with Graphpad software (Graphpad Software, CA) and are expressed as mean ± SEM unless otherwise indicated. For in vitro data, comparisons between two groups were made with a two-tailed unpaired t-test (Mann–Whitney; one-tailed nonparametric analysis was performed on in vivo subcutaneous implantation data). Comparisons among multiple groups were made with one-way ANOVAs. Differences were considered statistically significant when P value was <0.05.
SUPPLEMENTARY MATERIAL Figure S1. Experimental strategy for 3D alginate culture for induction of chondrogenesis of rat MSCs. Figure S2. Lewis chondrogenic differentiated MSCs do not suppress allogeneic T-cell proliferation. Figure S3. Chondrogenic differentiated human MSCs do not suppress allogeneic T-cell proliferation. Figure S4. Experimental strategy for the generation of cytotoxic T cells. Figure S5. Subcutaneous implantation experimental strategy and morphological evidence for chondrogenic differentiation in syngeneic differentiated and allogeneic differentiated alginate layers. Figure S6. Experimental strategy for the in vivo analysis of injection of unencapsulated and implantation of alginate-encapsulated differentiated and undifferentiated syngeneic and allogeneic MSCs.
Acknowledgments
The authors thank Bairbre McNicholas (REMEDI, NUI Galway) for expert assistance in immunohistochemical staining and analysis. The authors declare no conflict of interest. This research was supported by funding from Science Foundation Ireland (SFI); Grant number: 09/SRC/B1794, European Union Regional Development Funds, Irish Cancer Society Research Fellowship (Aideen Ryan (CRF12RYA)), and the Programme for Research in Third level Institutions (PRTLI) Cycle 5 for the structured PhD programme in Biomedical Engineering and Regenerative Medicine (BMERM) to Mr. Paul Lohan.
Supplementary Material
References
- Sensebé L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87 suppl. 9:S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-? and TNF-a. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Ma A, Song L, Hu Y, Dun H, Daloze P, et al. 2013Cartilage regeneration by selected chondrogenic clonal mesenchymal stem cells in the collagenase-induced monkey osteoarthritis model. J Tissue Eng Regen Med. doi: 10.1002/term.1676. [DOI] [PubMed]
- Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21:1717–1729. doi: 10.1007/s00167-012-2329-3. [DOI] [PubMed] [Google Scholar]
- Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Wehner R, Bornhäuser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation. Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- Ball LM, Bernardo ME, Roelofs H, van Tol MJ, Contoli B, Zwaginga JJ, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- Zheng ZH, Li XY, Ding J, Jia JF, Zhu P. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:22–30. doi: 10.1093/rheumatology/kem284. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Goronzy JJ. Stem cell aging and autoimmunity in rheumatoid arthritis. Trends Mol Med. 2004;10:426–433. doi: 10.1016/j.molmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Curtin C, Barry FP, O'Flatharta C, Murphy JM. Mesenchymal stem cells and osteoarthritis: remedy or accomplice. Hum Gene Ther. 2010;21:1239–1250. doi: 10.1089/hum.2010.138. [DOI] [PubMed] [Google Scholar]
- Jorgensen C, Noël D. Mesenchymal stem cells in osteoarticular diseases. Regen Med. 2011;6 suppl. 6:44–51. doi: 10.2217/rme.11.80. [DOI] [PubMed] [Google Scholar]
- Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38:457–466. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr Cartil. 2012;20:1186–1196. doi: 10.1016/j.joca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41:2840–2851. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Schu S, Nosov M, O'Flynn L, Shaw G, Treacy O, Barry F, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094–2103. doi: 10.1111/j.1582-4934.2011.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far. Immunol Cell Biol. 2013;91:40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- Kotobuki N, Katsube Y, Katou Y, Tadokoro M, Hirose M, Ohgushi H. In vivo survival and osteogenic differentiation of allogeneic rat bone marrow mesenchymal stem cells (MSCs). Cell Transplant. 2008;17:705–712. doi: 10.3727/096368908786092793. [DOI] [PubMed] [Google Scholar]
- Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- Sullivan C, Murphy JM, Griffin MD, Porter RM, Evans CH, O'Flatharta C, et al. Genetic mismatch affects the immunosuppressive properties of mesenchymal stem cells in vitro and their ability to influence the course of collagen-induced arthritis. Arthritis Res Ther. 2012;14:R167. doi: 10.1186/ar3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, McClurg A, Zhou GQ, McCaigue M, Armstrong MA, Li G. Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells, and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells. 2007;25:364–370. doi: 10.1634/stemcells.2006-0268. [DOI] [PubMed] [Google Scholar]
- Technau A, Froelich K, Hagen R, Kleinsasser N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310–317. doi: 10.3109/14653249.2010.504769. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Suberbielle E, Monnet C, Duplan V, Martin-Blondel G, Farrugia F, et al. Neurons are MHC class I-dependent targets for CD8 T cells upon neurotropic viral infection. PLoS Pathog. 2011;7:e1002393. doi: 10.1371/journal.ppat.1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes DS, Heidler KM, Niemeier M, Schwenk GR, Mathur PN, Breite WM, et al. Increased bronchoalveolar IgG2/IgG1 ratio is a marker for human lung allograft rejection. J Investig Med. 1994;42:652–659. [PubMed] [Google Scholar]
- Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol. 2011;33:593–602. doi: 10.1007/s00281-011-0267-7. [DOI] [PubMed] [Google Scholar]
- Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, Rico L, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010;12:R31. doi: 10.1186/ar2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- Sakkas LI, Platsoucas CD. The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:409–424. doi: 10.1002/art.22369. [DOI] [PubMed] [Google Scholar]
- Modo M, Rezaie P, Heuschling P, Patel S, Male DK, Hodges H. Transplantation of neural stem cells in a rat model of stroke: assessment of short-term graft survival and acute host immunological response. Brain Res. 2002;958:70–82. doi: 10.1016/s0006-8993(02)03463-7. [DOI] [PubMed] [Google Scholar]
- Benichou G, Yamada Y, Yun SH, Lin C, Fray M, Tocco G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy. 2011;3:757–770. doi: 10.2217/imt.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyburn KR, Jose MD, Wu H, Atkins RC, Chadban SJ. The role of macrophages in allograft rejection. Transplantation. 2005;80:1641–1647. doi: 10.1097/01.tp.0000173903.26886.20. [DOI] [PubMed] [Google Scholar]
- Christen T, Nahrendorf M, Wildgruber M, Swirski FK, Aikawa E, Waterman P, et al. Molecular imaging of innate immune cell function in transplant rejection. Circulation. 2009;119:1925–1932. doi: 10.1161/CIRCULATIONAHA.108.796888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney GE, Howard L, O'Brien T, Windebank AJ, Barry FP. Elevation of cAMP in mesenchymal stem cells transiently upregulates neural markers rather than inducing neural differentiation. Stem Cells Dev. 2009;18:387–398. doi: 10.1089/scd.2008.0080. [DOI] [PubMed] [Google Scholar]
- Treacy O, Ryan AE, Heinzl T, O'Flynn L, Cregg M, Wilk M, et al. Adenoviral transduction of mesenchymal stem cells: in vitro responses and in vivo immune responses after cell transplantation. PLoS ONE. 2012;7:e42662. doi: 10.1371/journal.pone.0042662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.