Abstract
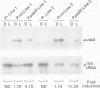
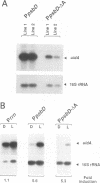
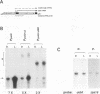
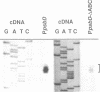
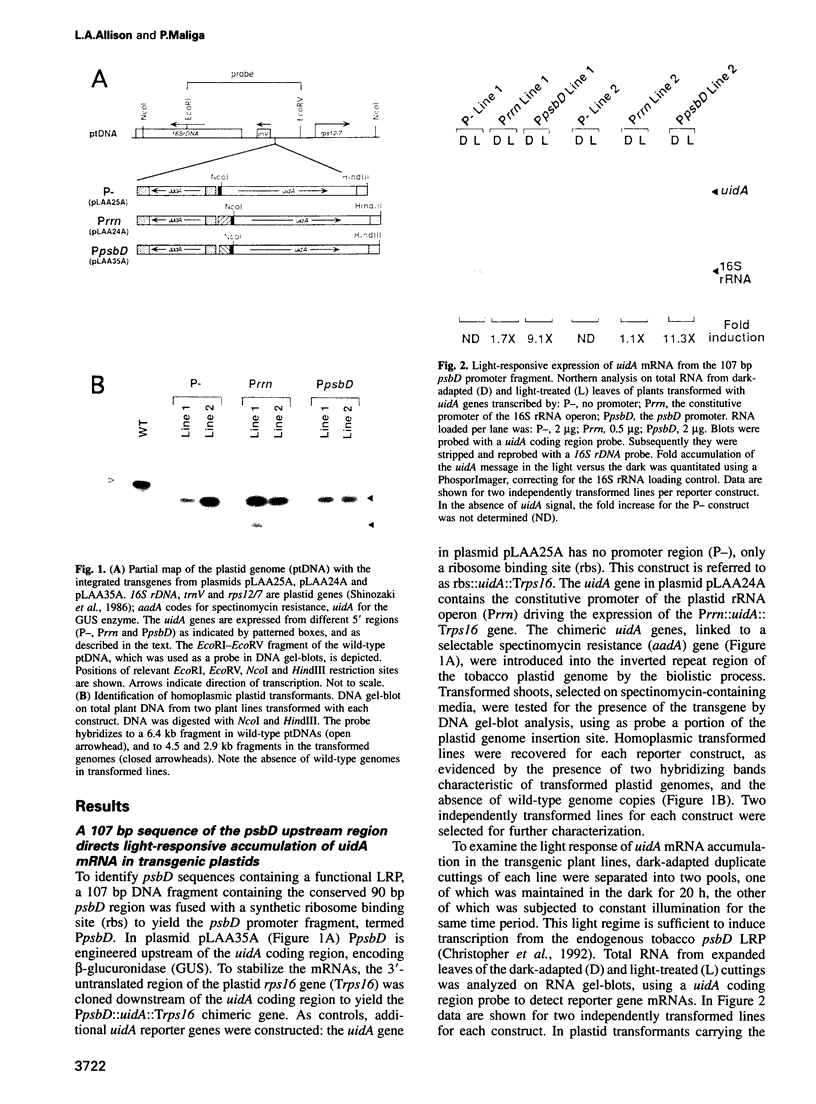
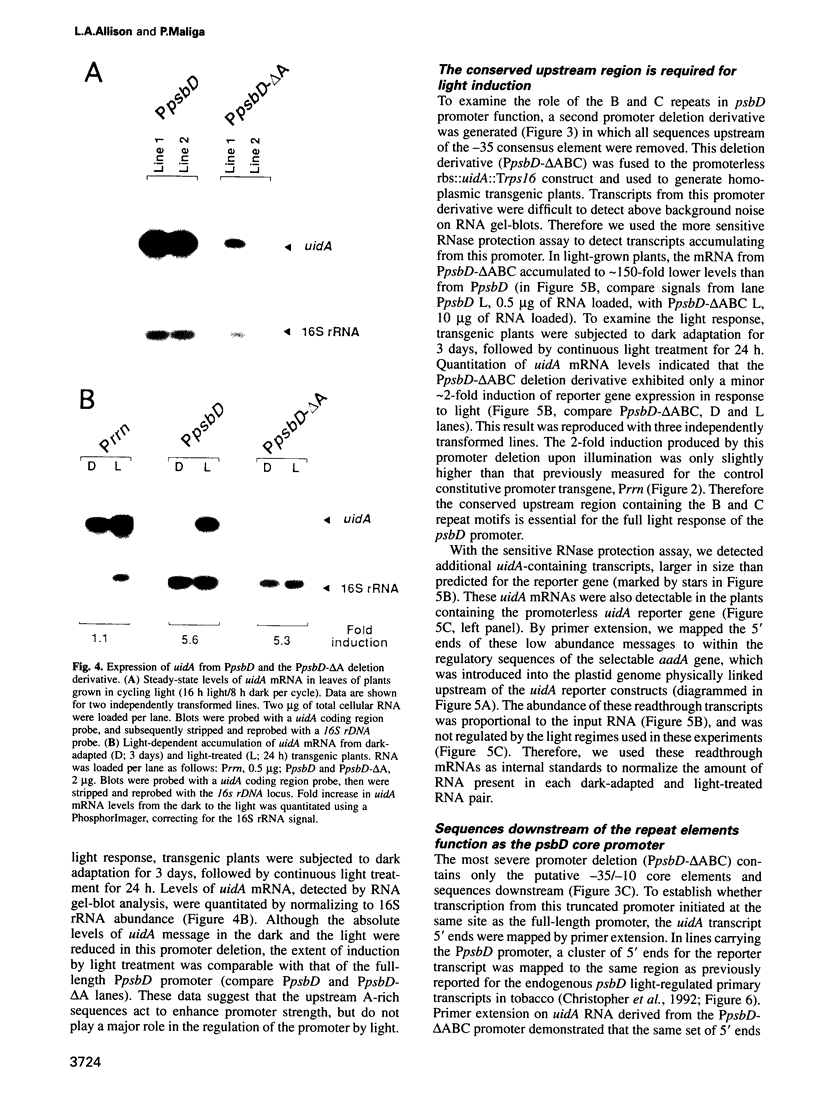
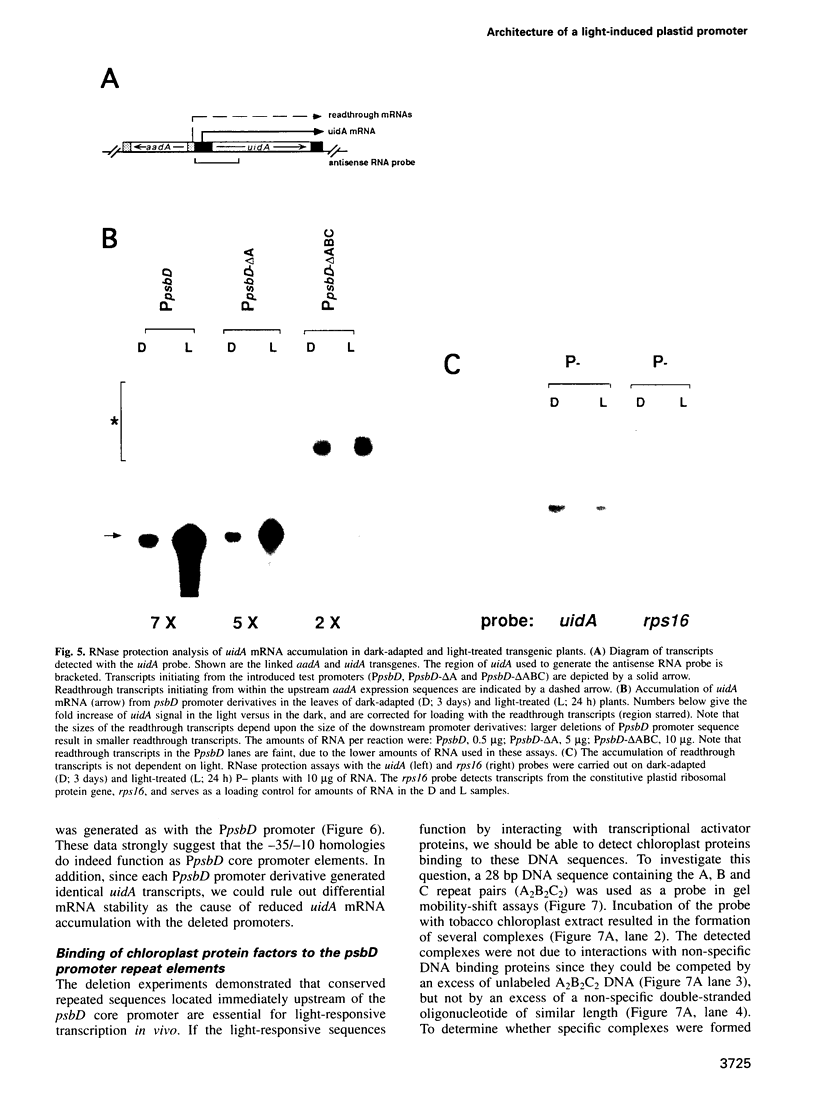
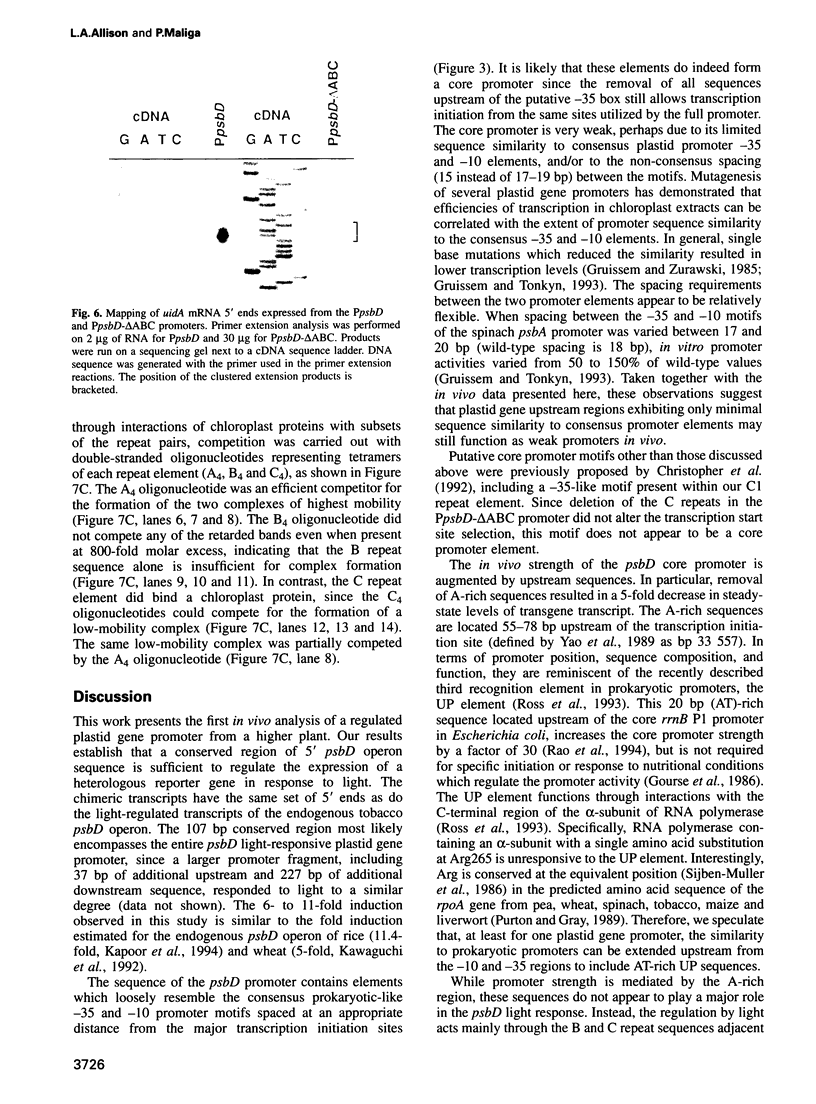
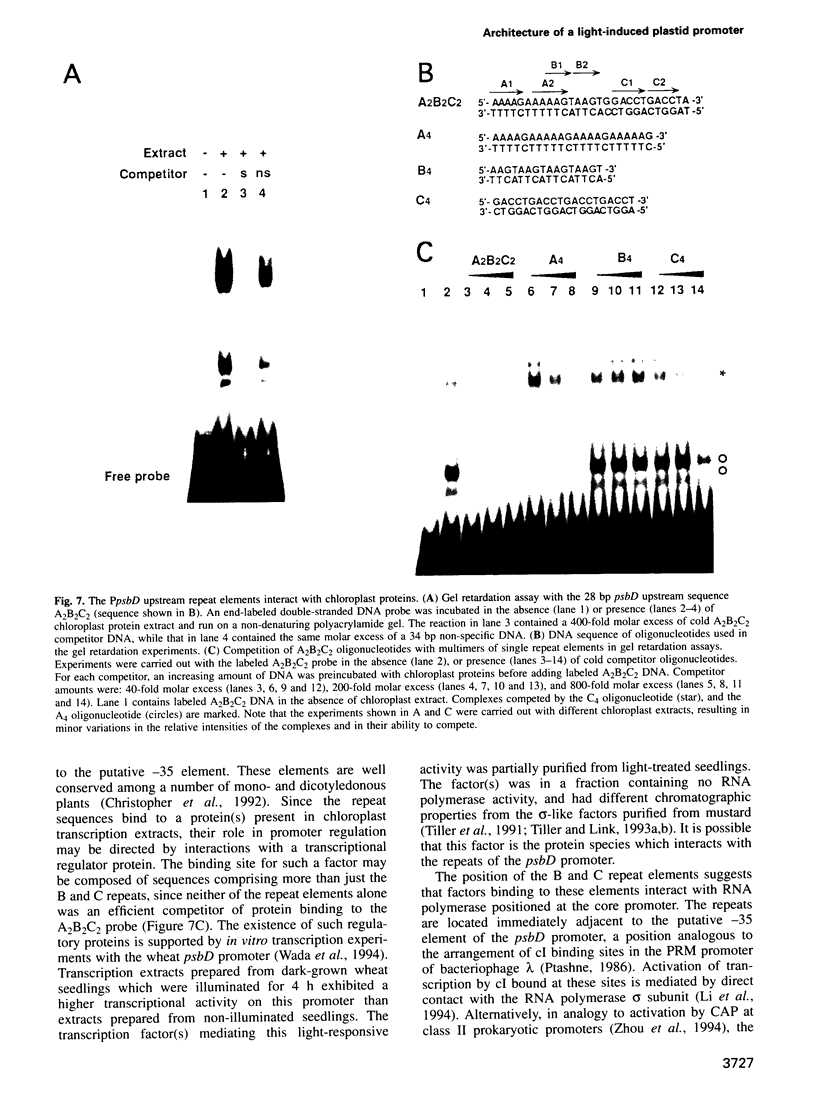
The psbD operon of higher plant plastids is regulated transcriptionally through the activity of an upstream light-responsive promoter. To identify promoter elements important for the regulation, portions of the tobacco psbD 5' region were fused to the reporter gene, uidA, and were introduced into the tobacco plastid genome by targeted gene insertion. Examination of uidA mRNA accumulation in dark-adapted and light-treated transplastomic plants revealed that a 107 bp segment of psbD 5' sequence was sufficient to promote light-responsive expression of the reporter gene in vivo. The 107 bp promoter region contains three pairs of short, repeated sequences upstream of the core promoter -10/-35 elements. Deletion of the upstream-most A-rich sequences resulted in a 5-fold decrease in reporter gene mRNA accumulation, but did not affect the light response. Additional removal of the second and third repeated elements further reduced the promoter strength approximately 30-fold and almost eliminated the light-dependent accumulation of uidA transcripts. These data indicate that the architecture of chloroplast promoters is more complex than previously assumed, and may comprise general enhancer and regulatory elements in addition to the core promoter motifs. Transcriptional regulation of psbD may be mediated by the chloroplast proteins which were shown to interact with the repeated sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeza L., Bertrand A., Mache R., Lerbs-Mache S. Characterization of a protein binding sequence in the promoter region of the 16S rRNA gene of the spinach chloroplast genome. Nucleic Acids Res. 1991 Jul 11;19(13):3577–3581. doi: 10.1093/nar/19.13.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner B. J., Rapp J. C., Mullet J. E. Plastid Genes Encoding the Transcription/Translation Apparatus Are Differentially Transcribed Early in Barley (Hordeum vulgare) Chloroplast Development (Evidence for Selective Stabilization of psbA mRNA). Plant Physiol. 1993 Mar;101(3):781–791. doi: 10.1104/pp.101.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D., Gatenby A. A. Mutational analysis of the maize chloroplast ATPase-beta subunit gene promoter: the isolation of promoter mutants in E. coli and their characterization in a chloroplast in vitro transcription system. EMBO J. 1985 Dec 30;4(13B):3641–3648. doi: 10.1002/j.1460-2075.1985.tb04129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher D. A., Kim M., Mullet J. E. A novel light-regulated promoter is conserved in cereal and dicot chloroplasts. Plant Cell. 1992 Jul;4(7):785–798. doi: 10.1105/tpc.4.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher D. A., Mullet J. E. Separate photosensory pathways co-regulate blue light/ultraviolet-A-activated psbD-psbC transcription and light-induced D2 and CP43 degradation in barley (Hordeum vulgare) chloroplasts. Plant Physiol. 1994 Apr;104(4):1119–1129. doi: 10.1104/pp.104.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Mullet J. E. Blue light regulates the accumulation of two psbD-psbC transcripts in barley chloroplasts. EMBO J. 1989 Oct;8(10):2785–2794. doi: 10.1002/j.1460-2075.1989.tb08424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Sexton T. B., Mullet J. E. Light-dependent changes in psbD and psbC transcripts of barley chloroplasts: accumulation of two transcripts maintains psbD and psbC translation capability in mature chloroplasts. EMBO J. 1988 May;7(5):1289–1297. doi: 10.1002/j.1460-2075.1988.tb02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Greenberg B. M., Zurawski G., Hallick R. B. Chloroplast gene expression and promoter identification in chloroplast extracts. Methods Enzymol. 1986;118:253–270. doi: 10.1016/0076-6879(86)18077-3. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Zurawski G. Analysis of promoter regions for the spinach chloroplast rbcL, atpB and psbA genes. EMBO J. 1985 Dec 16;4(13A):3375–3383. doi: 10.1002/j.1460-2075.1985.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iratni R., Baeza L., Andreeva A., Mache R., Lerbs-Mache S. Regulation of rDNA transcription in chloroplasts: promoter exclusion by constitutive repression. Genes Dev. 1994 Dec 1;8(23):2928–2938. doi: 10.1101/gad.8.23.2928. [DOI] [PubMed] [Google Scholar]
- Kapoor S., Maheshwari S. C., Tyagi A. K. Developmental and light-dependent cues interact to establish steady-state levels of transcripts for photosynthesis-related genes (psbA, psbD, psaA and rbcL) in rice (Oryza sativa L.). Curr Genet. 1994 Apr;25(4):362–366. doi: 10.1007/BF00351491. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H., Fukuda I., Shiina T., Toyoshima Y. Dynamical behavior of psb gene transcripts in greening wheat seedlings. I. Time course of accumulation of the pshA through psbN gene transcripts during light-induced greening. Plant Mol Biol. 1992 Nov;20(4):695–704. doi: 10.1007/BF00046454. [DOI] [PubMed] [Google Scholar]
- Klein U., De Camp J. D., Bogorad L. Two types of chloroplast gene promoters in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3453–3457. doi: 10.1073/pnas.89.8.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Salvador M. L., Bogorad L. Activity of the Chlamydomonas chloroplast rbcL gene promoter is enhanced by a remote sequence element. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10819–10823. doi: 10.1073/pnas.91.23.10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Moyle H., Susskind M. M. Target of the transcriptional activation function of phage lambda cI protein. Science. 1994 Jan 7;263(5143):75–77. doi: 10.1126/science.8272867. [DOI] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Mullet J. E., Hanley-Bowdoin L., Chua N. H. In vitro transcription of chloroplast protein genes. Methods Enzymol. 1986;118:232–253. doi: 10.1016/0076-6879(86)18076-1. [DOI] [PubMed] [Google Scholar]
- Purton S., Gray J. C. The plastid rpoA gene encoding a protein homologous to the bacterial RNA polymerase alpha subunit is expressed in pea chloroplasts. Mol Gen Genet. 1989 May;217(1):77–84. doi: 10.1007/BF00330945. [DOI] [PubMed] [Google Scholar]
- Rao L., Ross W., Appleman J. A., Gaal T., Leirmo S., Schlax P. J., Record M. T., Jr, Gourse R. L. Factor independent activation of rrnB P1. An "extended" promoter with an upstream element that dramatically increases promoter strength. J Mol Biol. 1994 Feb 4;235(5):1421–1435. doi: 10.1006/jmbi.1994.1098. [DOI] [PubMed] [Google Scholar]
- Ross W., Gosink K. K., Salomon J., Igarashi K., Zou C., Ishihama A., Severinov K., Gourse R. L. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science. 1993 Nov 26;262(5138):1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- Sexton T. B., Christopher D. A., Mullet J. E. Light-induced switch in barley psbD-psbC promoter utilization: a novel mechanism regulating chloroplast gene expression. EMBO J. 1990 Dec;9(13):4485–4494. doi: 10.1002/j.1460-2075.1990.tb07899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijben-Müller G., Hallick R. B., Alt J., Westhoff P., Herrmann R. G. Spinach plastid genes coding for initiation factor IF-1, ribosomal protein S11 and RNA polymerase alpha-subunit. Nucleic Acids Res. 1986 Jan 24;14(2):1029–1044. doi: 10.1093/nar/14.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub J. M., Maliga P. Translation of psbA mRNA is regulated by light via the 5'-untranslated region in tobacco plastids. Plant J. 1994 Oct;6(4):547–553. doi: 10.1046/j.1365-313x.1994.6040547.x. [DOI] [PubMed] [Google Scholar]
- Sun E., Wu B. W., Tewari K. K. In vitro analysis of the pea chloroplast 16S rRNA gene promoter. Mol Cell Biol. 1989 Dec;9(12):5650–5659. doi: 10.1128/mcb.9.12.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Hajdukiewicz P., Maliga P. Stable transformation of plastids in higher plants. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8526–8530. doi: 10.1073/pnas.87.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller K., Eisermann A., Link G. The chloroplast transcription apparatus from mustard (Sinapis alba L.). Evidence for three different transcription factors which resemble bacterial sigma factors. Eur J Biochem. 1991 May 23;198(1):93–99. doi: 10.1111/j.1432-1033.1991.tb15990.x. [DOI] [PubMed] [Google Scholar]
- Tiller K., Link G. Phosphorylation and dephosphorylation affect functional characteristics of chloroplast and etioplast transcription systems from mustard (Sinapis alba L.). EMBO J. 1993 May;12(5):1745–1753. doi: 10.1002/j.1460-2075.1993.tb05822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller K., Link G. Sigma-like transcription factors from mustard (Sinapis alba L.) etioplast are similar in size to, but functionally distinct from, their chloroplast counterparts. Plant Mol Biol. 1993 Feb;21(3):503–513. doi: 10.1007/BF00028807. [DOI] [PubMed] [Google Scholar]
- Wada T., Tunoyama Y., Shiina T., Toyoshima Y. In Vitro Analysis of Light-Induced Transcription in the Wheat psbD/C Gene Cluster Using Plastid Extracts from Dark-Grown and Short-Term-Illuminated Seedlings. Plant Physiol. 1994 Apr;104(4):1259–1267. doi: 10.1104/pp.104.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W. B., Meng B. Y., Tanaka M., Sugiura M. An additional promoter within the protein-coding region of the psbD-psbC gene cluster in tobacco chloroplast DNA. Nucleic Acids Res. 1989 Dec 11;17(23):9583–9591. doi: 10.1093/nar/17.23.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Pendergrast P. S., Bell A., Williams R., Busby S., Ebright R. H. The functional subunit of a dimeric transcription activator protein depends on promoter architecture. EMBO J. 1994 Oct 3;13(19):4549–4557. doi: 10.1002/j.1460-2075.1994.tb06776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubenko O. V., Allison L. A., Svab Z., Maliga P. Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res. 1994 Sep 25;22(19):3819–3824. doi: 10.1093/nar/22.19.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]