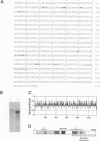
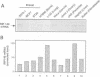
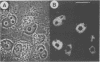
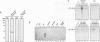Abstract
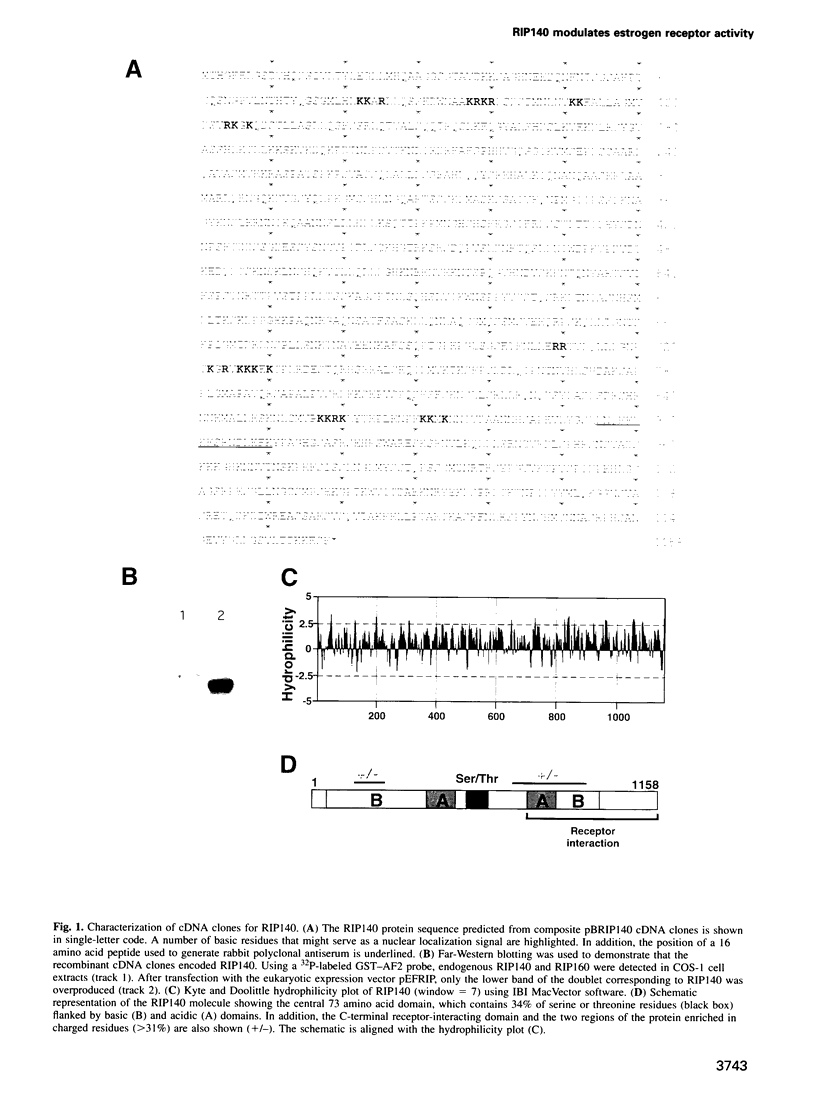
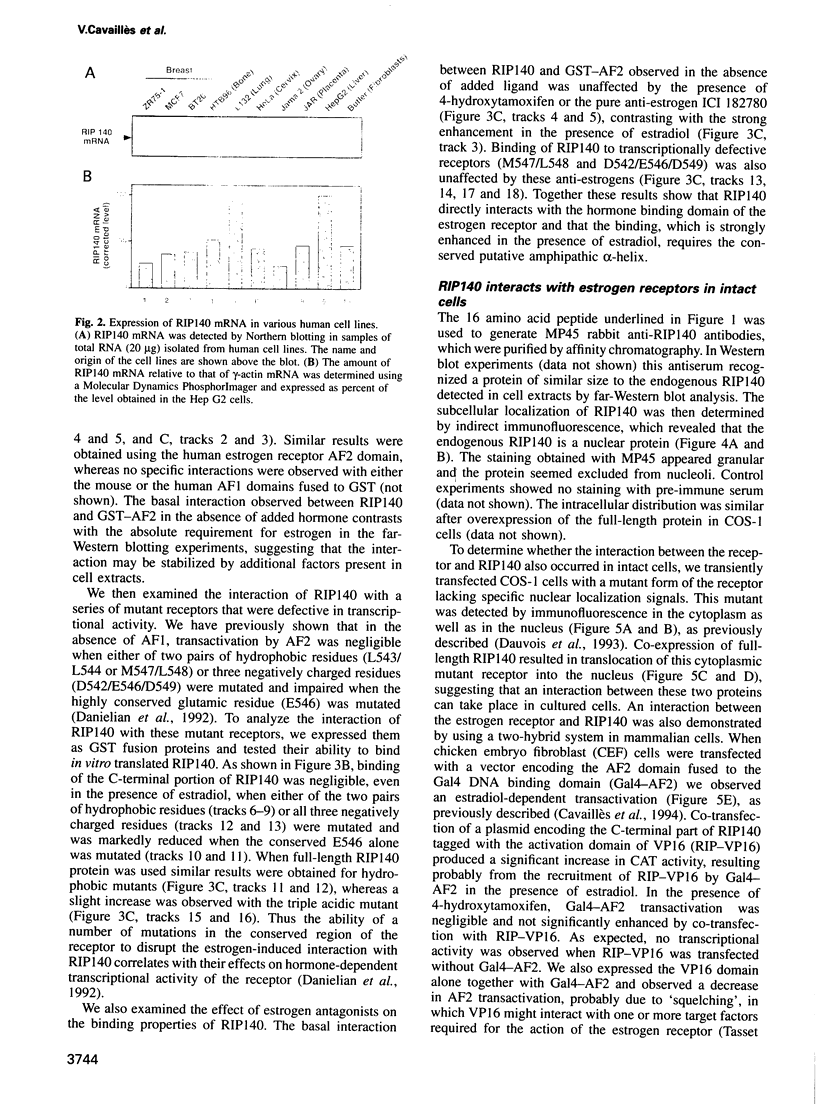
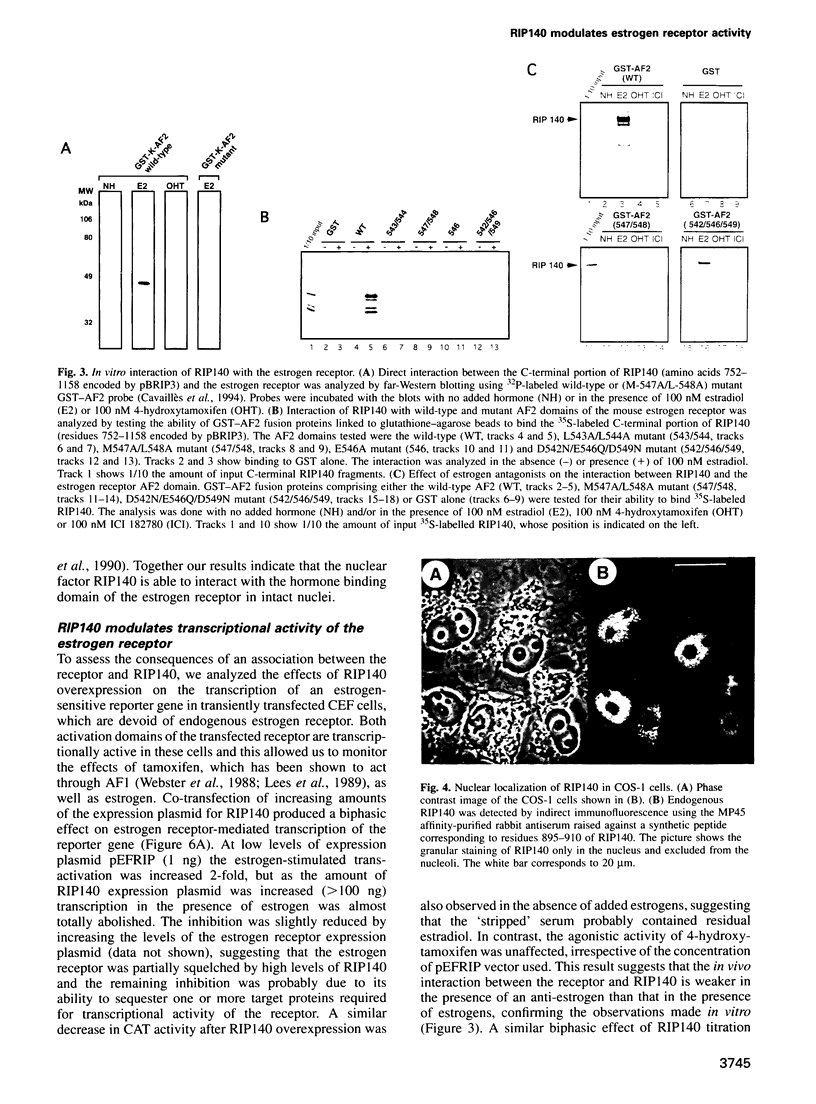
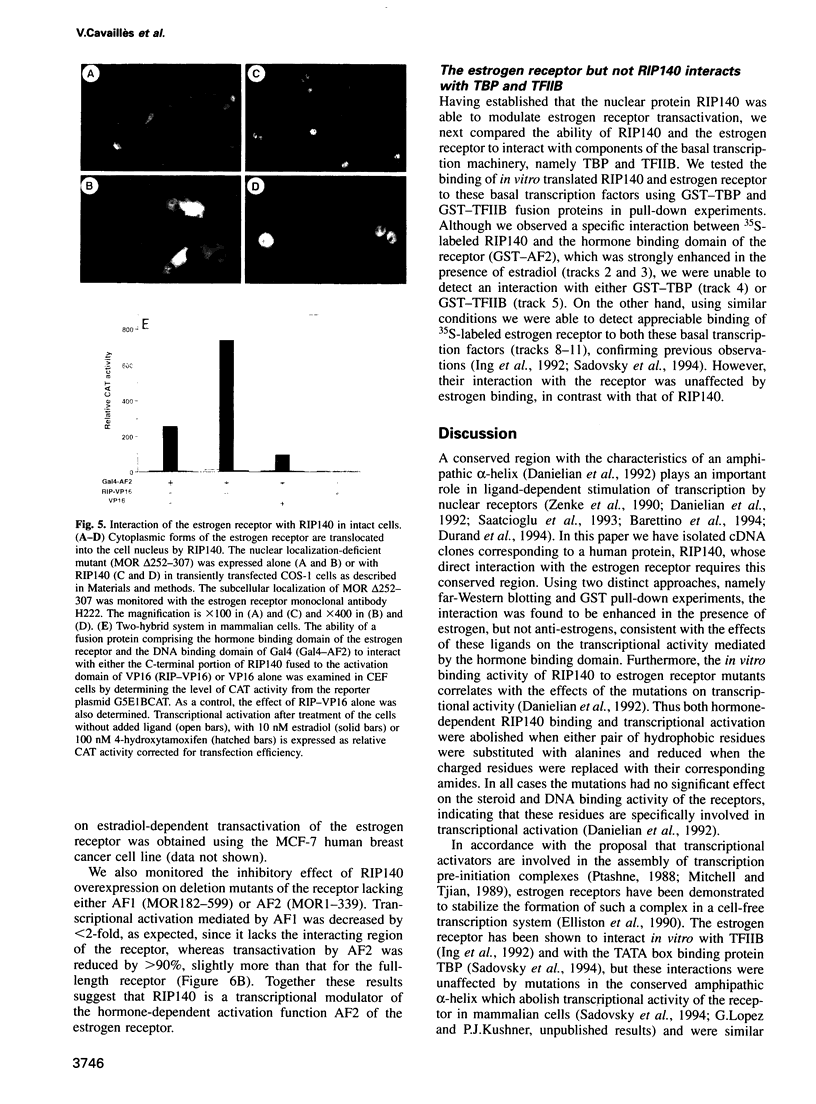
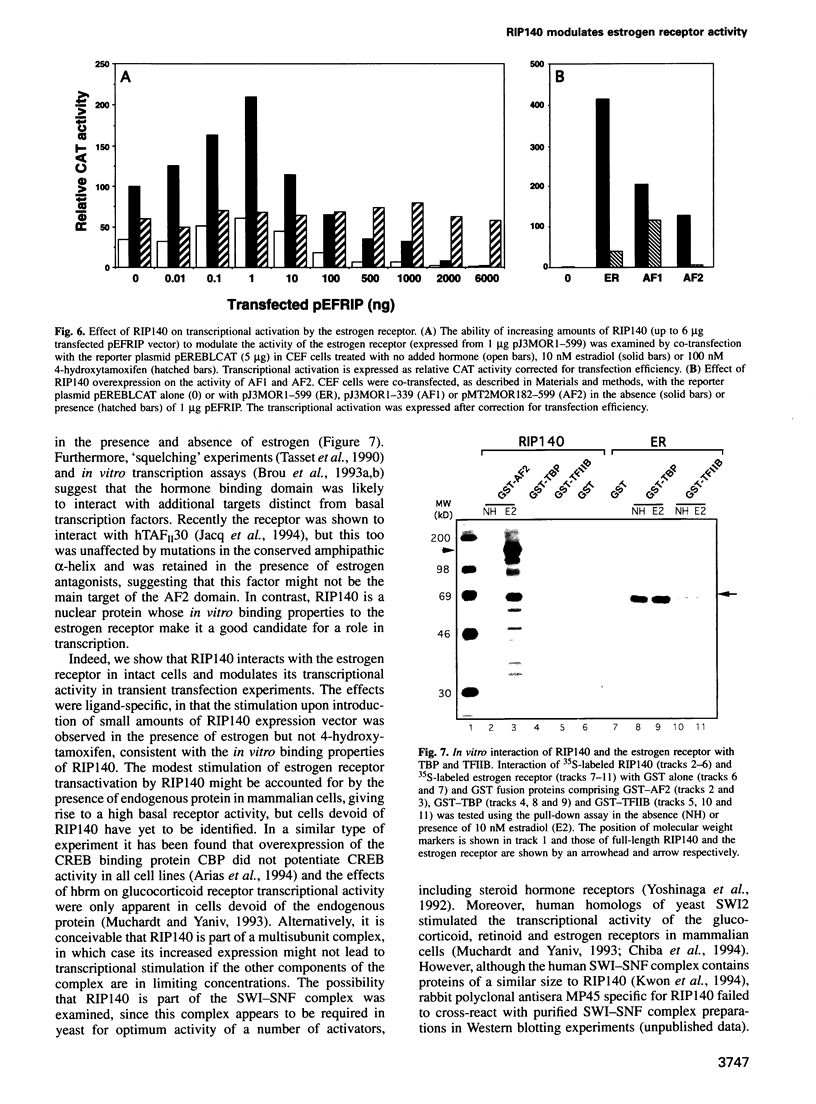
A conserved region in the hormone-dependent activation domain AF2 of nuclear receptors plays an important role in transcriptional activation. We have characterized a novel nuclear protein, RIP140, that specifically interacts in vitro with this domain of the estrogen receptor. This interaction was increased by estrogen, but not by anti-estrogens and the in vitro binding capacity of mutant receptors correlates with their ability to stimulate transcription. RIP140 also interacts with estrogen receptor in intact cells and modulates its transcriptional activity in the presence of estrogen, but not the anti-estrogen 4-hydroxytamoxifen. In view of its widespread expression in mammalian cells, RIP140 may interact with other members of the superfamily of nuclear receptors and thereby act as a potential co-activator of hormone-regulated gene transcription.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Kerlavage A. R., Fields C., Venter J. C. 3,400 new expressed sequence tags identify diversity of transcripts in human brain. Nat Genet. 1993 Jul;4(3):256–267. doi: 10.1038/ng0793-256. [DOI] [PubMed] [Google Scholar]
- Arias J., Alberts A. S., Brindle P., Claret F. X., Smeal T., Karin M., Feramisco J., Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994 Jul 21;370(6486):226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Ha I., Reinberg D., Tsai S., Tsai M. J., O'Malley B. W. Interaction of human thyroid hormone receptor beta with transcription factor TFIIB may mediate target gene derepression and activation by thyroid hormone. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8832–8836. doi: 10.1073/pnas.90.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barettino D., Vivanco Ruiz M. M., Stunnenberg H. G. Characterization of the ligand-dependent transactivation domain of thyroid hormone receptor. EMBO J. 1994 Jul 1;13(13):3039–3049. doi: 10.1002/j.1460-2075.1994.tb06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Brou C., Chaudhary S., Davidson I., Lutz Y., Wu J., Egly J. M., Tora L., Chambon P. Distinct TFIID complexes mediate the effect of different transcriptional activators. EMBO J. 1993 Feb;12(2):489–499. doi: 10.1002/j.1460-2075.1993.tb05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C., Wu J., Ali S., Scheer E., Lang C., Davidson I., Chambon P., Tora L. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993 Jan 11;21(1):5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillès V., Dauvois S., Danielian P. S., Parker M. G. Interaction of proteins with transcriptionally active estrogen receptors. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10009–10013. doi: 10.1073/pnas.91.21.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Attardi L. D., Verrijzer C. P., Yokomori K., Tjian R. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell. 1994 Oct 7;79(1):93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- Chiba H., Muramatsu M., Nomoto A., Kato H. Two human homologues of Saccharomyces cerevisiae SWI2/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoic acid receptor. Nucleic Acids Res. 1994 May 25;22(10):1815–1820. doi: 10.1093/nar/22.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy B., Green M. R. Eukaryotic activators function during multiple steps of preinitiation complex assembly. Nature. 1993 Dec 9;366(6455):531–536. doi: 10.1038/366531a0. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S., Danielian P. S., White R., Parker M. G. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S., White R., Parker M. G. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993 Dec;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- Durand B., Saunders M., Gaudon C., Roy B., Losson R., Chambon P. Activation function 2 (AF-2) of retinoic acid receptor and 9-cis retinoic acid receptor: presence of a conserved autonomous constitutive activating domain and influence of the nature of the response element on AF-2 activity. EMBO J. 1994 Nov 15;13(22):5370–5382. doi: 10.1002/j.1460-2075.1994.tb06872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht B. D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991 Aug 9;66(3):563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Elliston J. F., Fawell S. E., Klein-Hitpass L., Tsai S. Y., Tsai M. J., Parker M. G., O'Malley B. W. Mechanism of estrogen receptor-dependent transcription in a cell-free system. Mol Cell Biol. 1990 Dec;10(12):6607–6612. doi: 10.1128/mcb.10.12.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Zinn K., Maniatis T. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol Cell Biol. 1986 Mar;6(3):801–810. doi: 10.1128/mcb.6.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Roeder R. G. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell. 1994 Aug 12;78(3):513–523. doi: 10.1016/0092-8674(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Gill G., Pascal E., Tseng Z. H., Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Tjian R. Eukaryotic coactivators associated with the TATA box binding protein. Curr Opin Genet Dev. 1992 Apr;2(2):236–242. doi: 10.1016/s0959-437x(05)80279-5. [DOI] [PubMed] [Google Scholar]
- Goodrich J. A., Hoey T., Thut C. J., Admon A., Tjian R. Drosophila TAFII40 interacts with both a VP16 activation domain and the basal transcription factor TFIIB. Cell. 1993 Nov 5;75(3):519–530. doi: 10.1016/0092-8674(93)90386-5. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Sobel N. B., King W. J., Jensen E. V. Immunochemical studies of estrogen receptors. J Steroid Biochem. 1984 Jan;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- Halachmi S., Marden E., Martin G., MacKay H., Abbondanza C., Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994 Jun 3;264(5164):1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- Ham J., Thomson A., Needham M., Webb P., Parker M. Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Res. 1988 Jun 24;16(12):5263–5276. doi: 10.1093/nar/16.12.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey T., Weinzierl R. O., Gill G., Chen J. L., Dynlacht B. D., Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993 Jan 29;72(2):247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- Ing N. H., Beekman J. M., Tsai S. Y., Tsai M. J., O'Malley B. W. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J Biol Chem. 1992 Sep 5;267(25):17617–17623. [PubMed] [Google Scholar]
- Ingles C. J., Shales M., Cress W. D., Triezenberg S. J., Greenblatt J. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature. 1991 Jun 13;351(6327):588–590. doi: 10.1038/351588a0. [DOI] [PubMed] [Google Scholar]
- Jacq X., Brou C., Lutz Y., Davidson I., Chambon P., Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994 Oct 7;79(1):107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Pallas D. C., DeCaprio J. A., Kaye F. J., Livingston D. M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991 Feb 8;64(3):521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Kwon H., Imbalzano A. N., Khavari P. A., Kingston R. E., Green M. R. Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature. 1994 Aug 11;370(6489):477–481. doi: 10.1038/370477a0. [DOI] [PubMed] [Google Scholar]
- Lahooti H., White R., Danielian P. S., Parker M. G. Characterization of ligand-dependent phosphorylation of the estrogen receptor. Mol Endocrinol. 1994 Feb;8(2):182–188. doi: 10.1210/mend.8.2.8170474. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991 Mar 8;64(5):971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Ha I., Maldonado E., Reinberg D., Green M. R. Binding of general transcription factor TFIIB to an acidic activating region. Nature. 1991 Oct 10;353(6344):569–571. doi: 10.1038/353569a0. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchardt C., Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J. 1993 Nov;12(11):4279–4290. doi: 10.1002/j.1460-2075.1993.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakawa K., Matsubara K., Fukushima A., Yoshii J., Okubo K. Chromosomal assignments of 3'-directed partial cDNA sequences representing novel genes expressed in granulocytoid cells. Genomics. 1994 Sep 15;23(2):379–389. doi: 10.1006/geno.1994.1514. [DOI] [PubMed] [Google Scholar]
- Nagpal S., Saunders M., Kastner P., Durand B., Nakshatri H., Chambon P. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell. 1992 Sep 18;70(6):1007–1019. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- O'Hara P. J., Horowitz H., Eichinger G., Young E. T. The yeast ADR6 gene encodes homopolymeric amino acid sequences and a potential metal-binding domain. Nucleic Acids Res. 1988 Nov 11;16(21):10153–10169. doi: 10.1093/nar/16.21.10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. G. Steroid and related receptors. Curr Opin Cell Biol. 1993 Jun;5(3):499–504. doi: 10.1016/0955-0674(93)90016-j. [DOI] [PubMed] [Google Scholar]
- Pierrat B., Heery D. M., Chambon P., Losson R. A highly conserved region in the hormone-binding domain of the human estrogen receptor functions as an efficient transactivation domain in yeast. Gene. 1994 Jun 10;143(2):193–200. doi: 10.1016/0378-1119(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Regier J. L., Shen F., Triezenberg S. J. Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcriptional activator. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):883–887. doi: 10.1073/pnas.90.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatcioglu F., Bartunek P., Deng T., Zenke M., Karin M. A conserved C-terminal sequence that is deleted in v-ErbA is essential for the biological activities of c-ErbA (the thyroid hormone receptor). Mol Cell Biol. 1993 Jun;13(6):3675–3685. doi: 10.1128/mcb.13.6.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovsky Y., Webb P., Lopez G., Baxter J. D., Fitzpatrick P. M., Gizang-Ginsberg E., Cavailles V., Parker M. G., Kushner P. J. Transcriptional activators differ in their responses to overexpression of TATA-box-binding protein. Mol Cell Biol. 1995 Mar;15(3):1554–1563. doi: 10.1128/mcb.15.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Ciesiolka T., Gruss P. A nexus between Oct-4 and E1A: implications for gene regulation in embryonic stem cells. Cell. 1991 Jul 26;66(2):291–304. doi: 10.1016/0092-8674(91)90619-a. [DOI] [PubMed] [Google Scholar]
- Senger G., Ragoussis J., Trowsdale J., Sheer D. Fine mapping of the human MHC class II region within chromosome band 6p21 and evaluation of probe ordering using interphase fluorescence in situ hybridization. Cytogenet Cell Genet. 1993;64(1):49–53. doi: 10.1159/000133559. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Tansey W. P., Ruppert S., Tjian R., Herr W. Multiple regions of TBP participate in the response to transcriptional activators in vivo. Genes Dev. 1994 Nov 15;8(22):2756–2769. doi: 10.1101/gad.8.22.2756. [DOI] [PubMed] [Google Scholar]
- Tasset D., Tora L., Fromental C., Scheer E., Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990 Sep 21;62(6):1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S. K., Peterson C. L., Herskowitz I., Yamamoto K. R. Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science. 1992 Dec 4;258(5088):1598–1604. doi: 10.1126/science.1360703. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]