Abstract
Objective
-
-
To assess the impact of histotripsy treatment parameters (pulse number and pulse-repetition frequency [PRF]) on the efficiency of histotripsy induced homogenization of the prostatic urethra.
Material and Methods
-
-
A total of 34 transabdominal prostate histotripsy treatments were applied along a perpendicular plane traversing the prostatic urethra of 21 canine subjects.
-
-
Prostate histotripsy was applied with 1) escalating pulse number with fixed PRF or 2) at fixed pulse number with varying PRFs.
-
-
The development of urethral homognization within 14 days of histotripsy was evaluated endoscopically and confirmed histologically.
Results
-
-
Within 14 days of histotripsy 50%, 83%, 83%, and 100% of subjects receiving 12.5k, 25k, 50k, and 100k pulses per mm of treatment path (delivered at 500Hz PRF), respectively developed prostatic urethral disintegration.
-
-
Delivery of 100k pulses per mm was required to achieve urethral disintegration in all subjects within 24 hours of histotripsy treatment.
-
-
Increasing histotripsy PRF from 50Hz to 500Hz to 2,000Hz while applying a constant dose of 25k pulses per mm treatment was associated with increased rate of urethral disintegration (50% vs 75% vs 100% at 14 days, respectively).
Conclusions
-
-
Increasing the number of histotripsy pulses and/or increasing the PRF of histotripsy treatment applied to the urethra may improve the rate and efficiency of prostatic urethral disintegration in the canine model.
-
-
This understanding will aid in the development of treatment strategies for prostate histotripsy for BPH in human trials.
Keywords: Benign Prostatic Hyperplasia (BPH), Focused Ultrasound, Prostate
Introduction
Histotripsy is a focused ultrasound technology that delivers short (<50 µs), intense pulses of acoustic energy at low duty cycle to induce microbubble formation within a defined geometric focal volume while minimizing thermal effects.(1–5) The oscillation and collapse of these bubbles mechanically homogenizes targeted tissues into a fine slurry of acellular debris.(1, 6, 7) Analysis of the homogenate has revealed that >99.9% of particles are less than 6 µm in size.(8) Volumetric ablation greater than the size of a single focal volume is accomplished by moving the bubble cloud throughout the targeted region. Histotripsy therapy can be guided and monitored in real-time with conventional ultrasound because the bubble cloud appears as a distinct hyperechoic (bright) focus and targeted tissue echogenicity decreases (becomes darker) corresponding to progressive tissue disintegration.(9, 10)
The overarching objective of this research is to develop a histotripsy therapy for benign prostatic hyperplasia (BPH) that replicates the debulking capability of transurethral resection of the prostate (TURP) yet is less invasive and hence more widely applicable to patients with significant co-morbidity and/or suitable for use outside the operating room. Towards this goal, histotripsy has been evaluated in an in vivo canine model, demonstrating the ability to homogenize prostate tissue.(11) In this model, transabdominal histotripsy of the prostate appears safe and effective(12) producing dose dependent tissue debulking of targeted prostatic tissue(13) with minimal hematuria, even in anticoagulated subjects.(14) The strategy for effective histotripsy treatment of BPH is to produce a TURP-like defect by homogenizing parenchymal tissue as well as the adjacent prostatic urethra in order to facilitate drainage of the resultant homogenate with voiding.(12) However, the prostatic urethra is more resilient to mechanical forces than the prostate parenchyma and requires a greater number of acoustic pulses to achieve tissue disintegration.(13) In vitro studies have also demonstrated that substrate stiffness is inversely correlated with histotripsy susceptibility and lesion size(15) and that altering the pulse repetition frequency (PRF) can affect the efficiency of cavitation induced tissue homogenization.(16) As a result, the purpose of this study was to explore the efficiency of prostatic urethra homogenization in the in vivo canine model when varying the number and rate (PRF) of applied histotripsy pulses in order to better optimize treatment parameters in anticipation of future clinical applications.
Material and Methods
Experimental Setup and Procedure
After receiving approval from the university committee for animal use and care 21 intact male canines weighing 25.0 to 33.6 kg were obtained. Intramuscular Penicillin G benzathine (40,000 IU/kg) was administered prior to the procedure and on post procedure days (POD) 3 and 7 for prophylaxis. Carprofen (2.2 mg/kg/day) was administered orally prior to and for 24 hours post-procedure for analgesia. Subjects were anesthetized with subcutaneous acepromazine (0.1 mg/kg) and intravenous propofol (2–8 mg/kg) and intubated. After intubation each subject underwent digital disimpaction and tap water enema, was positioned supine, and the abdomen and suprapubic region were shaved. Inhalational anesthesia (isoflurane 1–2%) was maintained throughout treatment. Flexible endoscopy was performed with an 8.2 French flexible ureteroscope (Dur-8, Gyrus ACMI) prior to histotripsy treatment to document a normal lower urinary tract and to serve as an intrasubject control.
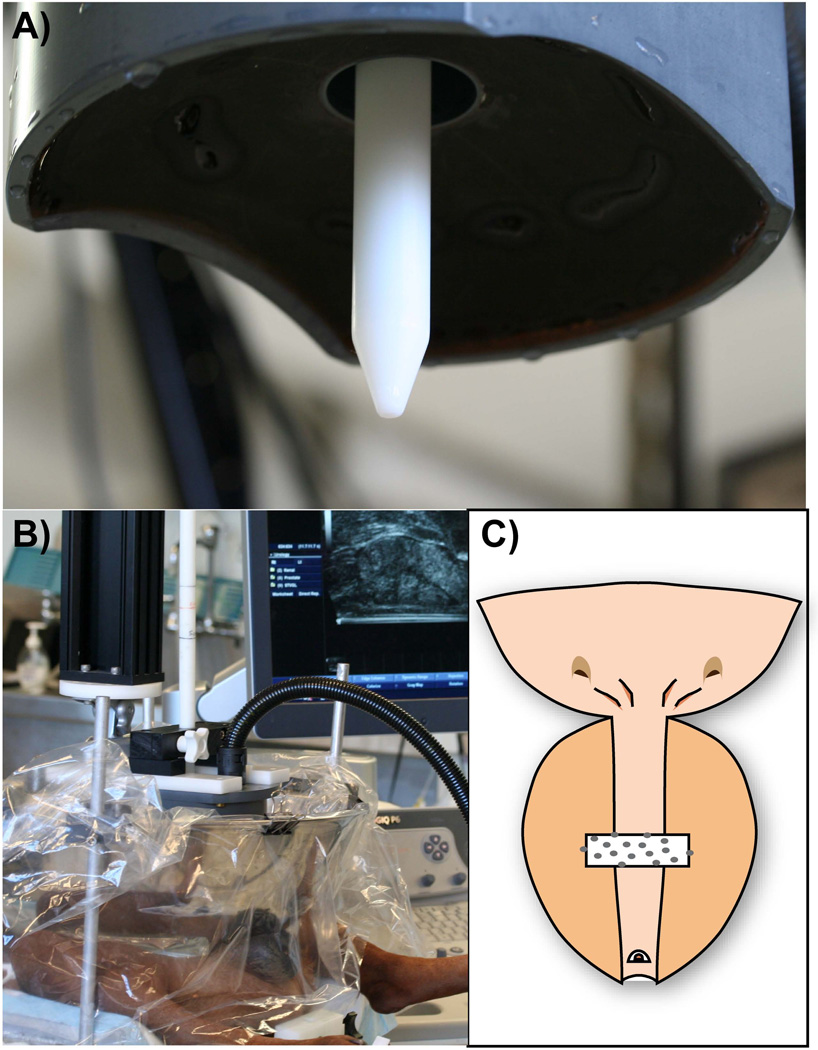
Transrectal ultrasound (TRUS) imaging was performed using a Logiq 6 ultrasound scanner (GE Healthcare, Waukesha, WI, USA) with a model ERB probe positioned in a custom holder. Prostatic volume was computed using a stepper volume technique by contouring the prostate margin on transverse slices at 2.5 mm intervals. The therapeutic histotripsy system consists of a 16-element piezoceramic composite array (750 kHz, 11 × 14-cm diameter oval shape, focal length 10 cm, focal volume 3 × 3× 8 mm (Figure 1A); Imasonic, Voray sur l’Ognon, France) on a 3-axis computer-controlled positioning system (MATLAB, MathWorks, Natick, MA, USA). Coupling was achieved by placing the therapy transducer in a bath of degassed water contained in a plastic membrane in direct contact with the shaved abdomen (FIGURE 1B). Twenty-one subjects underwent treatment with histotripsy pulses consisting of 5 cycle (6.7 microseconds) bursts of acoustic energy delivered at a prescribed PRF for a specified number of pulses. During each treatment, the histotripsy bubble cloud was translated along a single transverse plane that intersected the urethra and periurethral tissues as previously described(17) (FIGURE 1C). In subjects with sufficiently large prostates two treatments were applied at separate locations at least 1 cm apart. In the initial phase of the study, treatment consisted of 12.5k, 25k, 50k, or 100k pulses applied per mm of path length at a PRF of 500 Hz to evaluate the effect of number of histotripsy pulses on prostatic urethral disintegration. In the second phase of the study, treatment consisted of applying 25k pulses per mm of path length at PRF of 50, 500, 2000 Hz in order to evaluate the effect of altering PRF on prostatic urethral disintegration. A urinary catheter was not placed following treatment.
Figure 1.
Histotripsy experimental setup: During treatment the transducer (A) is lowered into a bath of degassed water in direct contact with abdomen (B). Treatment was targeted along a plane transecting the urethra (C).
Post-Histotripsy Care and Urethral Evaluation
After completing histotripsy treatment, cystoscopy was performed to evaluate for acute changes in urethral appearance. All subjects recovered from anesthesia and were monitored for treatment-related adverse events. Cystoscopy was repeated on POD 1, 3, 7, and then at euthanasia on POD14. TRUS was repeated on POD7 under anesthesia and at the time of euthanasia with intravenous pentobarbital sodium 140–160 mg/kg. A suprapubic incision was then made and a cystotomy created through which antegrade cystourethroscopy was performed using a 16 Fr flexible cystoscope (Cy2, Olympus). The prostate, bladder and adjacent rectum were surgically removed en-bloc and inspected grossly for injury. Tissues were fixed in formalin for 1 week, cut into 5-mm thick slabs, dehydrated in 25% ethanol, paraffin embedded, cut using a microtome in 5µm sections at 1 mm increments, mounted, and stained with hematoxylin and eosin for histologic assessment.
Statistical Analysis
Cumulative rates of urethral disintegration stratified by treatment parameters (number of pulses and PRF) were plotted graphically for interpretation.
Results
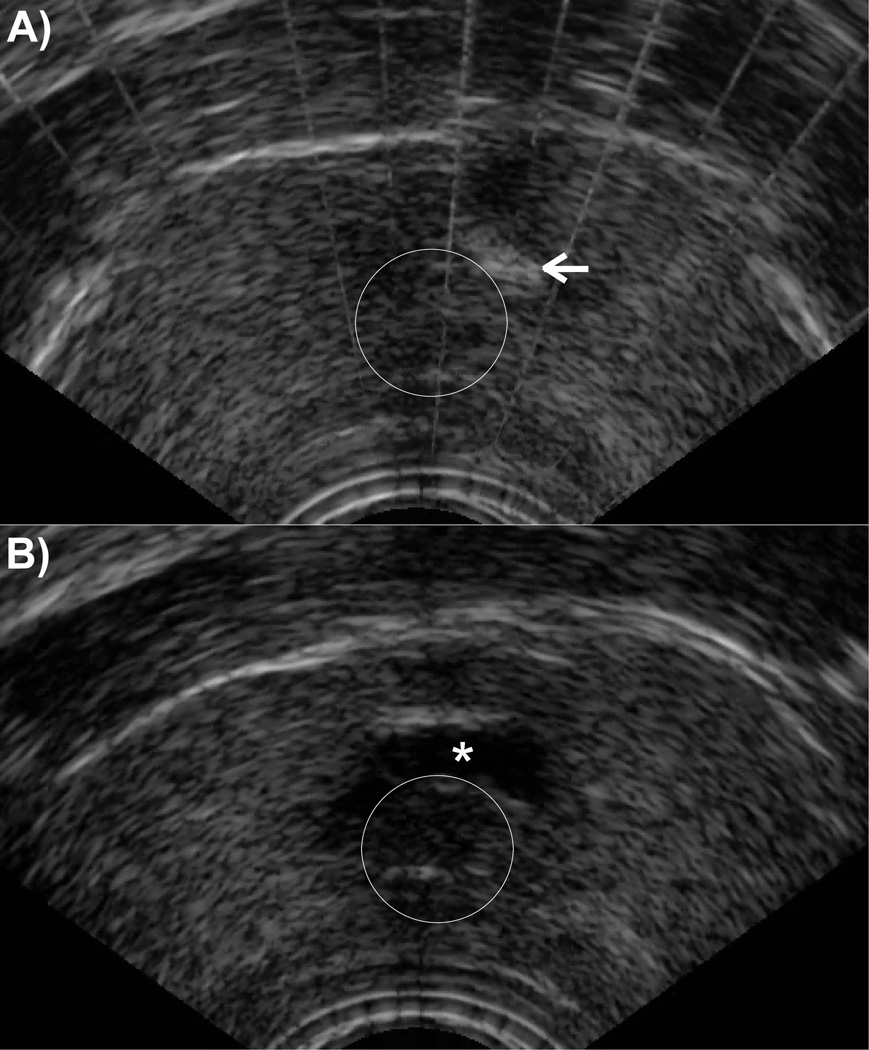
A total of 34 prostate histotripsy treatments were performed in 21 canine subjects. Mean preprocedure prostate volume was 22.2 (Range 10.5 –52.8) cc. During treatment, the cavitation bubble cloud was well visualized on TRUS (Figure 2) and the targeted periurethral parenchymal tissue became progressively hypoechoic while the prostatic urethra appeared structurally intact throughout treatment despite intraluminal cavitation. Immediately following histotripsy (POD0), endoscopic assessment of the prostatic urethra/treatment plane demonstrated punctate hemorrhage, shaggy urothelium, tissue flaps and in some cases gross disintegration. In subjects undergoing 2 treatments, there was an intervening band of normal appearing prostatic urethra between treatments. Acute (POD0) gross urethral disintegration was endoscopically apparent in only 4/34 (11.8%) treatment zones immediately after treatment, but developed in 27/34 (79.4%) by POD14.
Figure 2.
Transrectal ultrasound appearance of prostate histotripsy therapy: Early in treatment the histotropsy bubble cloud (arrow) is visualized adjacent to the urethra (outlined circle) (A). During treatment the targeted region becomes progressively hypoechoic producing a hypoechic cavity (*) adjacent to the urethra (B) at the conclusion of treatment.
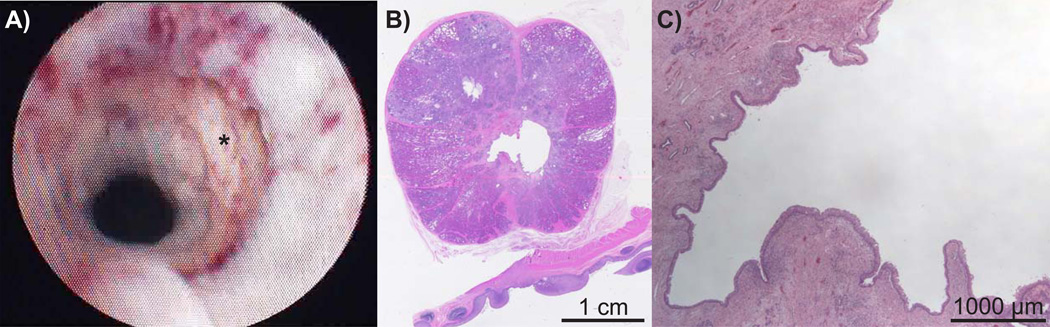
Treatment planes that did not develop gross urethral disintegration by POD 14 were seen on TRUS to have persistent internal echoes and/or gravity fluid/debris levels within the targeted adjacent periurethral parenchyma suggesting undrained homogenate. Conversely, planes with cavities communicating with disintegrated urethra had absent or minimal internal echoes consistent with drainage of the homogenized material via the urethra. These findings were confirmed histologically (Figure 3).
Figure 3.
Endoscopic appearance of urethral disintegration on POD 14 (A) with confirmatory low (B) and high-power (C) micrographs demonstrating drainage of the homogenized adjacent prostatatic parenchyma with minimal inflammation and urothelialization of the cavity.
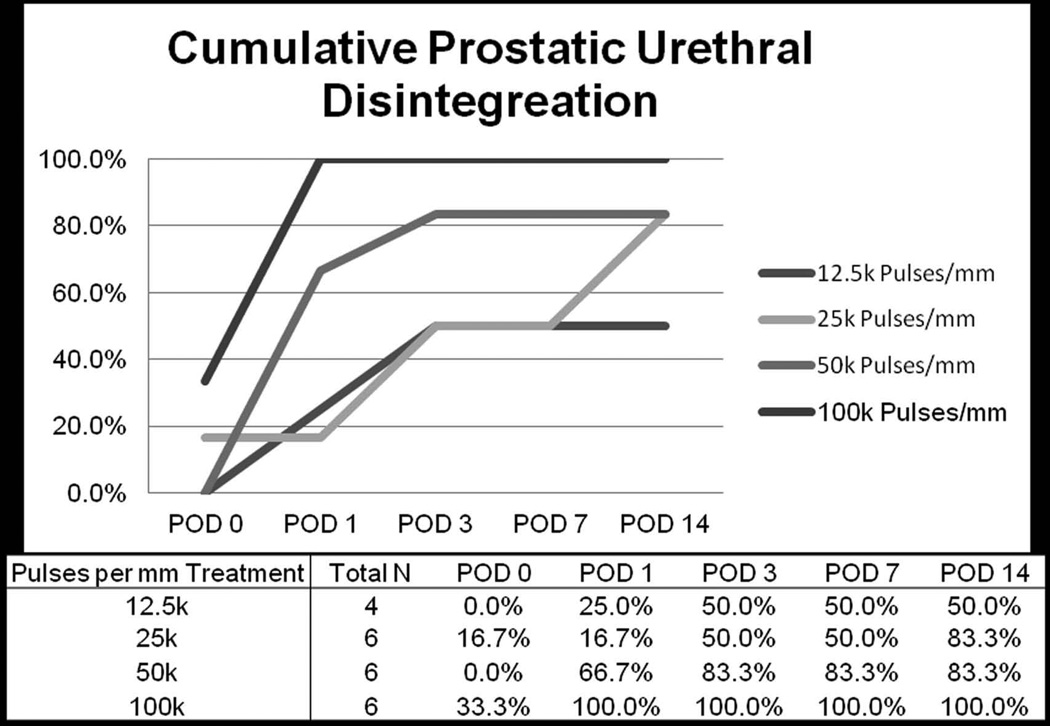
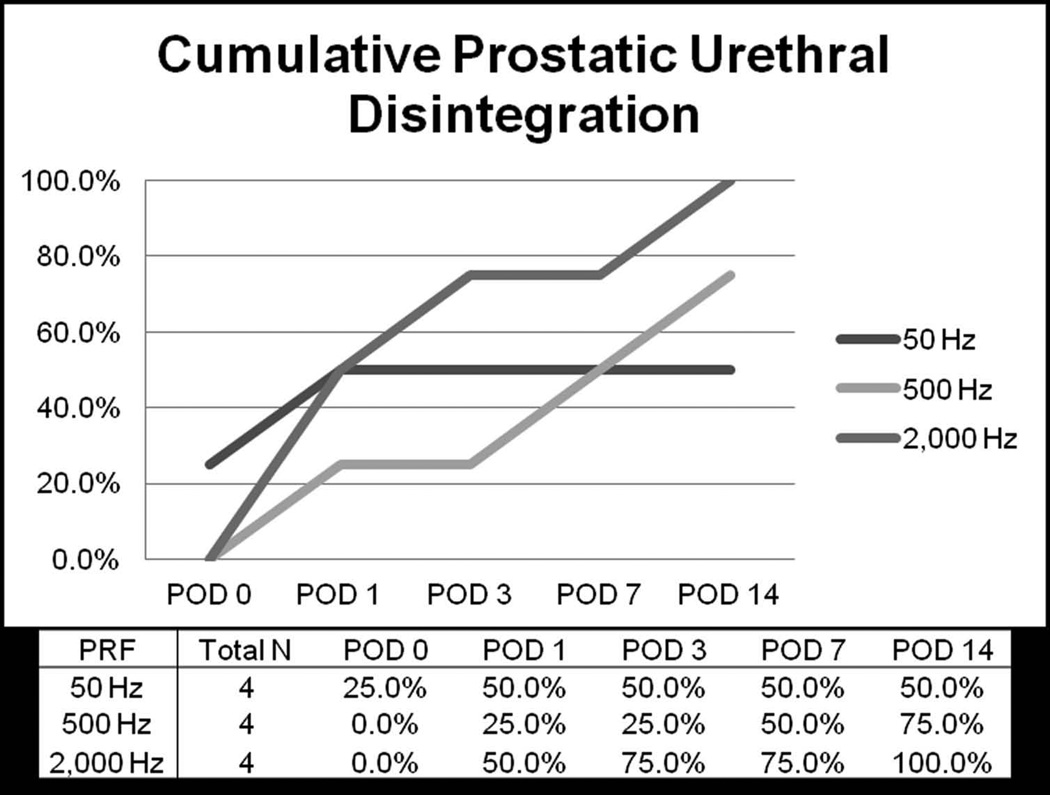
Application of greater number of histotripsy pulses (at PRF of 500 Hz) resulted in increased likelihood of urethral disintegration and generally earlier presentation of urethral disintegration (Figure 4). Specifically, by POD14 urethral disintegration occurred in 50%, 83%, 83%, and 100% of subjects receiving 12.5k, 25k, 50k, and 100k pulses per mm of treatment path, respectively. While immediate prostatic urethral disintegration was rare it did occur within 24 hours of treatment in all subjects receiving 100k pulses per mm treatment. With respect to histotripsy pulse delivery at varying PRF (50, 500, and 2,000 Hz) and constant pulse number (25,000 pulses per mm), increasing treatment PRF to 2000 Hz was associated with 100% urethral disintegration by POD14, compared to 75% for 500 Hz and 50% for 50 Hz (Figure 5).
Figure 4.
Cumulative development of prostatic urethral disintegration with histotripsy treatment with 12.5k to 100k pulses with constant PRF (500 Hz)
Figure 5.
Cumulative development of prostatic urethral disintegration at PRF of 50, 500, and 2,000 Hz with constant treatment dose (25k pulses per mm)
Complications included rectoprostatic fistula in two subjects and development of intraperitoneal urinoma in two subjects secondary to prostatic urethral and capsular perforation.
Discussion
Over the last several decades, minimally-invasive ablative therapies have been introduced in an effort to reduce the morbidity associated with TURP. Previous attempts to accomplish this goal with transurethral needle ablation (TUNA) and microwave forms of energy failed to replicate the efficacy and durability of TURP (possibly due to lack of tissue debulking).(18, 19) HIFU has been extensively investigated in Europe and Asia for transrectal treatment of disorders of the prostate. HIFU delivers long (3–5 sec) pulses of acoustic energy to thermally coagulate the targeted tissue volume.(20) While outcomes are encouraging for prostate cancer(21, 22); previous work attempting to utilize HIFU for treatment of BPH has ultimately proven to be unsuccessful as nearly 45% of patients required TURP within 4 years of HIFU treatment.(23) In this clinical setting, TRUS confirmed TURP-like defects in only 25–30% of cases following HIFU(24), concerning for inadequate tissue debulking. The authors postulated that three factors may have contributed to the poor durability: 1) inadequate volume of targeted tissue necrosis, 2) necrotic treatment area evolved into scar possibly increasing fibrosity within the prostate and preventing improvement of urinary symptoms, and 3) obstructive tissue at the bladder neck was not sufficiently treated to relieve obstruction.(23)
In preclinical studies, histotripsy has been shown to homogenize and debulk prostate tissue in the in vivo canine model.(11–13) Based on these results, creating a TURP like defect with histotripsy appears feasible, however, the optimal treatment strategy to reliably and efficiently achieve this goal is unknown in part because the prostatic urethra is more resilient to mechanical tissue effects than prostate parenchyma.(13) To best explore prostatic urethral tissue response to a range of acoustic pulse parameters histotripsy was applied along a linear path perpendicularly traversing the urethra. This simplified the treatment environment (minimizing the impact of factors such as acoustic shielding from the pubis and os penis), and allowed for multiple treatments per prostate in appropriate subjects. Since the size of the cavitation bubble cloud varies with tissue medium, acoustic aperture, and pulse repetition frequency (2) this treatment strategy does not allow calculation of treatment density (pulses/volume treatment) making comparisons of treatment densities in this study to previous work difficult. Instead, “histotripsy dose” was reported as number of pulses applied per mm of transverse treatment path.
Using this treatment strategy with a PRF of 500 Hz, histotripsy doses of 100,000 pulses/mm treatment were required to achieve urethral disintegration within 24 hours of treatment in all subjects and doses ≥25,000 pulses/mm treatment were required to achieve urethral disintegration in at least 80% of subjects within two weeks of treatment. Qualitatively, these treatment doses were well above the threshold to induce homogenization of the adjacent periurethral prostatic parenchyma which consistently demonstrated increased sensitivity to histotripsy treatment effects compared to the urethra on intra-treatment TRUS. Histology confirmed this observation with the presence of undrained homogenized prostatic tissue in all cases which failed to achieve disintegration of the adjacent prostatic urethra consistent with previous data.(13, 17) Further, in such cases, the intact adjacent urethra appeared completely normal despite the adjacent parenchymal damage.
While direct tissue homogenization is clearly of importance to the mechanism of action of histotripsy (3, 6, 7), factors other than treatment dose likely contribute to successful tissue homogenization. One such factor is PRF as data suggest modifying PRF during histotripsy treatment may alter the efficiency of tissue erosion.(25) Interestingly, in this study, altering PRF did not appear to substantially impact the development of urethral disintegration within 24 hours of treatment. However, there was an increase in urethral disintegration14 days after treatment as PRF was increased from 50 Hz to 500 Hz to 2,000 Hz (50% vs. 75% vs. 100%, respectively). This observation, if confirmed by larger studies, was unexpected based on in vitro data suggesting that increased PRF is associated with decreased efficiency of tissue homogenization, sporadic cavitary damage, and increased number of pulses to achieve equivalent damage in both myocardium and liver.(16, 26) One possible explanation for the greater rate of delayed urethral disintegration at higher PRF is that the larger bubble-cloud produced by increasing PRF may lead to a larger field of incompletely homogenized, yet lethal tissue damage, that when remodeled in the chronic setting leads to overall higher rates of urethral disintegration.
While the prostatic urethra is more resistant to histotripsy than the parenchyma/adenoma, the etiology of its resistance is unknown. In vitro studies, examining lesion size in agar have demonstrated that lesion size is directly related to duration of treatment (number of pulses) and inversely related to “tissue” stiffness.(15) Histologically, the prostatic urethra (and its periurethral fibromuscular) stroma contains more collagen than the surrounding prostatic parenchyma suggesting increased tissue strength and “stiffness” compared to the parenchyma. As a result, the increased collagen content of the prostatic urethra may contribute to the higher threshold for histotripsy induced tissue disintegration, a hypothesis that is actively being evaluated. Aside from decreased sensitivity of the urethra to the effects of histotripsy, an additional possible explanation of failure to disintegrate the urethra is inadequate urethral targeting. However, using real time cystourethroscopy we have previously demonstrated the presence of histotripsy induced intraluminal cavitation using the same treatment strategy(17) suggesting adequate urethral targeting.
Although results from this study will help guide future histotripsy research, they are not without limitations, most importantly their generalizability to humans. However, the prostate is very similar between both species with the arterial supply originating from the internal pudendal artery, and branches perforating the capsule to supply the stroma and glandular tissues. Structurally, while the canine prostate is bilobed without zonal structures, the human prostate is subdivided into several zones, with potentially different treatment thresholds, with the transition zone being most relevant to BPH.(27) Additionally, the intraperitoneal location of the canine prostate lends itself to a transabdominal histotripsy approach, whereas structural modeling suggests that the human prostate will likely be more amenable to a transperineal approach (28). The transabdominal approach combined with small prostate size likely contributed to the complications observed in this study – the cigar shaped histotripsy bubble cloud was perpendicular to the axis of the urethra making adequate targeting of the urethra while attempting to maintain the bubble cloud within the parenchyma along the midline sulcus difficult in some subjects, thus increasing the risk of rectal injury and prostatic capsular perforation. Ultimately, human studies are needed to determine both the clinical efficacy and optimal treatment algorithm for prostate histotripsy for BPH.
In summary, extracorporeal application of histotripsy to the prostate is capable of homogenizing targeted prostate parenchyma with subsequent creation of a TURP like defect. Increasing the number of histotripsy pulses and/or increasing the PRF of these pulses applied to the urethra appears to improve the rate and efficiency of prostatic urethral disintegration in the canine model, which may facilitate drainage of the homogenate and enhance prostate debulking. This understanding will prove useful in developing treatment strategies for prostate histotripsy treatment of BPH in human trials.
Acknowledgments
Funding: NIH RO1DK087871
Footnotes
Disclosures:
WWR and TLH have equity, royalty, and consulting interests in HistoSonics, Inc
Conflict of Interest:
Drs. Hall and Roberts report grants from National Institutes of Health, during the conduct of the study; personal fees and other from HistoSonics, Inc., outside the submitted work; In addition, Dr. Hall and Roberts have a patent US 8,057,408,B2 licensed to HistoSonics, and a patent PCT/2115-003767/POA pending to HistoSonics. Drs. Schade, Styn and Ives have nothing to disclose.
References
- 1.Kieran K, Hall TL, Parsons JE, Wolf JS, Jr, Fowlkes JB, Cain CA, et al. Refining histotripsy: defining the parameter space for the creation of nonthermal lesions with high intensity, pulsed focused ultrasound of the in vitro kidney. J Urol. 2007 Aug;178(2):672–676. doi: 10.1016/j.juro.2007.03.093. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z, Hall TL, Fowlkes JB, Cain CA. Effects of acoustic parameters on bubble cloud dynamics in ultrasound tissue erosion (histotripsy) J Acoust Soc Am. 2007 Jul;122(1):229–236. doi: 10.1121/1.2735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Raghavan M, Hall TL, Chang CW, Mycek MA, Fowlkes JB, et al. High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy--histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2007 Oct;54(10):2091–2101. doi: 10.1109/TUFFC.2007.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Hall TL, Fowlkes JB, Cain CA. Optical and acoustic monitoring of bubble cloud dynamics at a tissue-fluid interface in ultrasound tissue erosion. J Acoust Soc Am. 2007 Apr;121(4):2421–2430. doi: 10.1121/1.2710079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Raghavan M, Hall TL, Mycek MA, Fowlkes JB. Evolution of bubble clouds induced by pulsed cavitational ultrasound therapy - histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2008 May;55(5):1122–1132. doi: 10.1109/TUFFC.2008.764. [DOI] [PubMed] [Google Scholar]
- 6.Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 2003 Oct;50(10):1296–1304. doi: 10.1109/tuffc.2003.1244746. [DOI] [PubMed] [Google Scholar]
- 7.Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: the role of dynamic interaction between insonation and microbubble activity. J Acoust Soc. Am. 2005 Jan;117(1):424–435. doi: 10.1121/1.1828551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Fan Z, Hall TL, Winterroth F, Fowlkes JB, Cain CA. Size measurement of tissue debris particles generated from pulsed ultrasound cavitational therapy-histotripsy. Ultrasound Med Biol. 2009 Feb;35(2):245–255. doi: 10.1016/j.ultrasmedbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall TL, Fowlkes JB, Cain CA. A real-time measure of cavitation induced tissue disruption by ultrasound imaging backscatter reduction. IEEE Trans Ultrason Ferroelectr Freq Control. 2007 Mar;54(3):569–575. doi: 10.1109/tuffc.2007.279. [DOI] [PubMed] [Google Scholar]
- 10.Wang TY, Xu Z, Winterroth F, Hall TL, Fowlkes JB, Rothman ED, et al. Quantitative ultrasound backscatter for pulsed cavitational ultrasound therapy-histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2009 May;56(5):995–1005. doi: 10.1109/tuffc.2009.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lake AM, Hall TL, Kieran K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008 Sep;72(3):682–686. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hempel CR, Hall TL, Cain CA, Fowlkes JB, Xu Z, Roberts WW. Histotripsy fractionation of prostate tissue: local effects and systemic response in a canine model. J Urol. 2011 Apr;185(4):1484–1489. doi: 10.1016/j.juro.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall TL, Hempel CR, Wojno K, Xu Z, Cain CA, Roberts WW. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009 Oct;74(4):932–937. doi: 10.1016/j.urology.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheat JC, Hall TL, Hempel CR, Cain CA, Xu Z, Roberts WW. Prostate histotripsy in an anticoagulated model. Urology. 2010 Jan;75(1):207–211. doi: 10.1016/j.urology.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Bigelow TA. Experimental investigation of the effect of stiffness, exposure time and scan direction on the dimension of ultrasound histotripsy lesions. Ultrasound Med Biol. 2011 Nov;37(11):1865–1873. doi: 10.1016/j.ultrasmedbio.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol. 2006 Jan;32(1):115–129. doi: 10.1016/j.ultrasmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Schade GR, Styn NR, Hall TL, Roberts WW. Endoscopic assessment and prediction of prostate urethral disintegration after histotripsy treatment in a canine model *. J Endourol. 2012 Feb;26(2):183–189. doi: 10.1089/end.2011.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Guidline on the Managment of Benign Prostatic Hyperplasia (BPH) American Urologic Association. 2010 [cited 2011 April 5, 2011]; Available from: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph.
- 19.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011 May;185(5):1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 20.Hynynen K, Darkazanli A, Damianou CA, Unger E, Schenck JF. Tissue thermometry during ultrasound exposure. Eur Urol. 1993;23(Suppl 1):12–16. doi: 10.1159/000474673. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed HU, Freeman A, Kirkham A, Sahu M, Scott R, Allen C, et al. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol. 2011 Apr;185(4):1246–1254. doi: 10.1016/j.juro.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 22.Crouzet S, Rebillard X, Chevallier D, Rischmann P, Pasticier G, Garcia G, et al. Multicentric oncologic outcomes of high-intensity focused ultrasound for localized prostate cancer in 803 patients. Eur Urol. 2010 Oct;58(4):559–566. doi: 10.1016/j.eururo.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 23.Madersbacher S, Schatzl G, Djavan B, Stulnig T, Marberger M. Long-term outcome of transrectal high-intensity focused ultrasound therapy for benign prostatic hyperplasia. Eur Urol. 2000 Jun;37(6):687–694. doi: 10.1159/000020219. [DOI] [PubMed] [Google Scholar]
- 24.Madersbacher S, Kratzik C, Susani M, Marberger M. Tissue ablation in benign prostatic hyperplasia with high intensity focused ultrasound. J Urol. 1994 Dec;152(6 Pt 1):1956–1960. doi: 10.1016/s0022-5347(17)32278-4. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, et al. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004 Jun;51(6):726–736. doi: 10.1109/tuffc.2004.1308731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TY, Xu Z, Hall TL, Fowlkes JB, Cain CA. An efficient treatment strategy for histotripsy by removing cavitation memory. Ultrasound Med Biol. 2012 May;38(5):753–766. doi: 10.1016/j.ultrasmedbio.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans HE. Miller's Anatomy of the Dog. Philadelphia, PA: W.B. Saunders; 1993. pp. 514–516. [Google Scholar]
- 28.Hall TL, Hempel CR, Sabb BJ, Roberts WW. Acoustic access to the prostate for extracorporeal ultrasound ablation. J Endourol. 2010 Nov;24(11):1875–1881. doi: 10.1089/end.2009.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]