Abstract
Background
Cocaine pharmacotherapy trials are often confounded by considerable variability in baseline cocaine-use levels, obscuring possible medication efficacy. Testing the feasibility of using a prerandomization, abstinence-induction protocol, we screened three candidate medications to explore treatment response in patients who did, or did not, achieve abstinence during an extended baseline phase.
Method
Eligible treatment-seeking, cocaine-dependent subjects entered a 4-week baseline period (Phase I) with high-value abstinence contingent vouchers and two motivational interviewing sessions, followed by a 12-week medication trial (Phase II) with random assignment stratified on Phase I abstinence status to (1) modafinil (400mg/d), (2) levodopa/carbidopa (800/200mg/d), (3) naltrexone (50mg/d), or (4) placebo. Treatment consisted of thrice-weekly clinic visits for urine benzoylecgonine testing and weekly cognitive behavioral therapy with contingency management targeting medication compliance.
Results
Of the 118 subjects enrolled, 81 (80%) completed Phase I, with 33 (41%) achieving abstinence, defined a priori as 6 consecutive cocaine-negative urines. Tests of the interaction of each medication (active vs. placebo) by baseline status (abstinent vs. nonabstinent) permitted moderator effect analysis. Overall, baseline abstinence predicted better outcome. Cocaine-use outcomes for levodopa and naltrexone treatment differed as a function of Phase I abstinence status, with both medications producing benefit in nonabstinent but not baseline-abstinent subjects. There was no evidence of a moderator effect for modafinil.
Conclusions
The two-phase screening trial demonstrated that subgrouping of patients with respect to baseline abstinence status is feasible and clinically useful for exploring cocaine cessation and relapse-prevention effects of candidate medications.
Keywords: Contingency management, levodopa, modafinil, naltrexone, cocaine cessation, relapse prevention
1. INTRODUCTION
Numerous candidate medications have been evaluated for treating cocaine dependence, but none have shown sufficient evidence of efficacy to receive US Food and Drug Administration approval. This has prompted a shift in medication development, away from the goal of finding a single “magic bullet” medication toward phased medication treatment sequences. Broadly, two phases of cocaine cessation and relapse prevention can be identified. For patients actively using cocaine, an effective pharmacotherapy for inducing abstinence might do so by reducing the severity of cocaine withdrawal symptoms or providing partial replacement. Alternatively, for the patient who has achieved initial abstinence, pharmacotherapy for preventing relapse might work by mediating conditioned effects of stimuli previously associated with cocaine. An experimental design and paradigm for evaluating medication efficacy in cocaine cessation versus relapse prevention entails using a prerandomization lead-in period with high-value voucher contingency management (CM).
The utility of a lead-in period to establish level of cocaine use prior to randomization in a cocaine pharmacotherapy trial has been demonstrated in a series of studies by Bisaga et al. (2010, 2006, 2005). These reports indicate that approximately 44% of the participants achieve initial abstinence, defined as four or more cocaine-negative urine specimens during two weeks of lead-in with an intensive contingency reinforcement intervention. This methodology has permitted evaluation of differential medication effects of gabapentin (Bisaga et al., 2006) and memantine (Bisaga et al., 2010) in subgroups of early responders and nonresponders. Moreover, this subgrouping method responds to the FDA call for “enrichment” strategies to decrease heterogeneity in clinical-trial samples and increases the likelihood that a drug effect can be detected if one exists (FDA, 2013).
Each of the three medications selected for this study, modafinil, levodopa-carbidopa, and naltrexone, was previously evaluated for treating cocaine dependence and showed some evidence of benefit, although mixed. Determination of whether stronger treatment effects might emerge within homogeneous cocaine-dependent patient subgroups that achieved or did not achieve initial abstinence was critical. In addition to favorable safety profiles, the three medications have distinct mechanisms of action that might modulate cocaine use.
Modafinil, along with other effects, increases extracellular dopamine via transporter inhibition and has modest stimulant-like and cognitive-enhancing properties that might ameliorate cocaine-withdrawal symptoms. Initial positive clinical-trial findings (Dackis et al., 2005, 2003), while not fully confirmed in later trials (Anderson et al., 2009; Dackis et al., 2012), along with recent human laboratory research (Sofuoglu et al., 2013), suggest that modafinil may promote abstinence in chronic cocaine users by improving cognitive functions (Mereu et al., 2013) or by blunting cocaine euphoria (Dackis et al., 2003; Hart et al., 2008; Malcolm et al., 2006). Therefore, we hypothesized a stronger treatment effect of modafinil among the non-abstinent subgroup of patients.
The dopamine precursor levodopa increases central dopamine availability, which, in turn, is thought to improve brain reward circuits within a normal homeostatic range, as conceptualized by Koob et al. (Koob, 2008; Koob and Le Moal, 2008). Such actions may be particularly relevant during early recovery when a shift in attention toward nondrug rewards predicts success (Martinez et al., 2011). We reported previously that levodopa-carbidopa was associated with higher abstinence rates when administered concomitantly with abstinence-based CM, supporting the notion that this medication may have greater efficacy under conditions of reduced cocaine use or abstinence (Schmitz et al., 2008). Here we hypothesized a stronger treatment effect of levodopa among the subgroup of patients achieving initial abstinence.
Potential efficacy of naltrexone, a nonselective opioid receptor antagonist, for treating cocaine dependence is predicated on central endogenous opioid-system involvement in cocaine’s reinforcing effects (Corrigall and Coen, 1991; Ramsey and van Ree, 1991). In a preliminary study (n=85), naltrexone (50 mg/day) combined with relapse-prevention therapy was associated with reduced cocaine use in patients who completed an initial cocaine detoxification program (Schmitz et al., 2001). More recent double-blind, placebo-controlled studies reported no benefit of naltrexone at doses ≥ 50 mg/day but without regard to baseline abstinence status (Pettinati et al., 2008; Schmitz et al., 2009, 2004). Addressing these equivocal results, we hypothesized that under well-defined conditions of abstinence, stronger treatment effects of naltrexone would emerge.
In summary, the overarching aim of this study was the evaluation of a paradigm for screening multiple medications (compared with placebo) in parallel for ability to reduce cocaine use in active users (“cocaine cessation”) and maintain abstinence in recent nonusers (“relapse-prevention”). Here, the two-phase screening trial provided a vehicle to evaluate baseline abstinence status as a general predictor of treatment outcome, and more specifically, as a moderator of medication response.
2. METHODS
2.1. Study Design
Consenting subjects entered a 4-week non-medicated baseline period (Phase I), during which initiation of abstinence from cocaine was encouraged and supported with brief Motivational Interviewing (MI) sessions and CM. Achievement of abstinence during baseline was operationally defined as six consecutive cocaine-negative urines, i.e., two weeks, consistent with definitions reported in the literature (Bisaga et al., 2010; Crits-Christoph et al., 2013; McCann and Li, 2012). Comparative parallel medication evaluation (Phase II) followed stratification (abstinent/nonabstinent), randomization, and dose titration and lasted 12 weeks (Figure 1). Subjects who achieved abstinence criteria in less than four weeks entered Phase II immediately to avoid resuming cocaine use prior to starting medication treatment. During Phase II, subjects received weekly individual cognitive-behavioral therapy (CBT) and CM targeting medication compliance. Thrice-weekly clinic visits (MWF) with urine toxicology screening were required throughout both study phases. Cocaine use during treatment (urine benzoylecgonine: BE) was the primary measure of treatment outcome.
Figure 1.
Diagram of study design. UDS = urine drug screen. CM = contingency management.
a Phase I baseline period ranged from 2 to 4 weeks to allow subjects who achieved abstinence criteria in less than four weeks to be randomized and begin dose titration on assigned study medication for Phase II that lasted 12 weeks.
2.2. Subjects
The study sample included treatment-seeking, cocaine-using adults (18–55 years old) meeting Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) criteria for current cocaine dependence and submitting at least one cocaine-positive urine result during screening. Individuals with acutely unstable medical or psychiatric disorders or substance dependence aside from cocaine, cannabis, or nicotine were excluded. Also excluded were individuals currently enrolled in drug-abuse treatment and women who were pregnant, nursing, or of childbearing potential and unwilling to use acceptable birth control methods during study participation.
The single-site study took place at the outpatient Treatment Research Clinic (TRC) located at The University of Texas, Houston, Center for Neurobehavioral Research on Addiction. The TRC conducts initial eligibility screening on all individuals responding to advertisements for various treatment studies (Sayre et al., 2004). Those qualifying are further screened for one of several ongoing clinical trials. From the larger pool of individuals screened, 118 subjects were evaluated for the current trial.
The research protocol, consent form, and all assessment/advertising materials were reviewed and approved by the Committee for the Protection of Human Subjects (CPHS) of The University of Texas Medical School, Houston (Clinicaltrials.gov Identifier: NCT00218023).
2.3. Assessments
Information regarding psychiatric diagnosis and addiction severity was obtained at intake using the Structured Clinical Interview for DSM-IV (First and Pincus, 2002) and the Addiction Severity Index (McLellan et al., 1992). Prior to starting medication, all subjects underwent a medical history and physical examination, laboratory tests (blood chemistries, complete blood count, liver and thyroid function tests, urinalysis, urine pregnancy test), and cardiac evaluation (i.e., 12-lead electrocardiogram). Vital signs (including heart rate, blood pressure, weight) were obtained weekly during treatment. A side-effects checklist was completed each week, with moderate-to-severe-rated items evaluated by the study nurse and reviewed by the study physician (FGM). Serious adverse events were assessed at each clinic visit and reported to the IRB and Data Safety Monitoring Board. Medication compliance was assessed via pill-bottle openings, using the Medication Event Monitoring Systems (MEMS) and fluorescent tests for riboflavin detection in urine samples (Del Boca et al., 1996).
2.4. Treatments
2.4.1. Motivational Interviewing (MI)
The primary goal of MI was to assist patients in achieving initial abstinence by increasing motivation and commitment to change. The MI intervention, evaluated previously as part of a cocaine-detoxification program (Stotts et al., 2001), consisted of two 1-hour individual therapy sessions on the first and eighth day of Phase I. The client-centered, MI-style sessions focused on building motivation for change, exploring ambivalence, obtaining a commitment to change, making a plan for abstinence (Session 1), providing personalized feedback, reassessing commitment for change, and reevaluating the change plan (Session 2). Masters-level therapists were trained and supervised by the therapy supervisor (ALS), an expert in motivation-based therapies.
2.4.2. Contingency Management
The CM intervention used in Phase I was similar in magnitude and schedule to high-value, voucher-based interventions used in prior research (Dallery et al., 2001; Katz et al., 2002; Silverman et al., 1999). Subjects earned vouchers for cocaine-negative urine samples collected at scheduled clinic visits (M, W, F) each week. Under the escalating reinforcement schedule, voucher values began at $15 and increased by $10 for each consecutive cocaine-negative urine. Bonus vouchers worth $10 were given for three consecutive cocaine-negative urines. Provision of a cocaine-positive urine or failure to provide a scheduled sample resulted in no vouchers earned and reset the schedule to the initial value ($15). Subjects could redeem their earned vouchers for cash (≤ $25) and/or gift cards for goods and services.
The CM intervention used in Phase II targeted medication compliance and was identical to the voucher-based intervention used previously by Schmitz et al. (Schmitz et al., 2010). Medication compliance was assessed by MEMS bottle-cap openings and confirmed by urinary riboflavin test results that exceeded the cutoff level of 20 fluorescence units (Mooney et al., 2004). Voucher values started at $2.50 and increased by $1.25 for each consecutive compliant reading, with bonus vouchers ($10) awarded for evidence of three consecutive compliant readings. Missing or refused readings were considered noncompliant and reset the voucher value to $2.50. As in Phase I, vouchers earned could be exchanged at any time for cash or gift cards.
2.4.3. Cognitive-Behavioral Therapy
Subjects received weekly, 1-hour, individual CBT sessions during Phase II. This therapy component focused on coping-skills training for resisting cocaine use in high-risk situations, based on relapse-prevention theory (Larimer et al., 1999) and manual-guided techniques (Kadden, 1992). Therapy sessions were conducted by master’s-level licensed professional counselors supervised by a licensed clinical psychologist (JMS), who monitored manual adherence and competency.
2.4.4. Medication
At each clinic visit (MWF) during the 12 weeks of Phase II, subjects were administered medication at the dispensing window and given take-home doses for intervening days. Subjects in all conditions took two doses daily (morning/afternoon). All active and placebo capsules were identical in appearance, and each contained 50 mg riboflavin for subsequent evaluation of medication compliance. All investigators and staff, except the pharmacist, were blind to medication assignment. Rapid-dose-titration regimens for the active medications were consistent with our prior experience (Schmitz et al., 2010, 2012, 2001). The modafinil dose began at 200 mg (day 1) and increased to the fixed dose of 200 mg twice daily (day 2). Levodopa-carbidopa, in the sustained-release formulation (Sinemet CR), began at a dose of levodopa/carbidopa 400/100 mg (day 1) and increased to the fixed dose of 400/100 mg twice daily (day 2). Naltrexone hydrochloride doses began at 25 mg (day 1) and increased to the fixed dose of 25 mg twice daily (day 2).
2.5. Data Analysis
The primary outcome measure was cocaine use, based on thrice-weekly urine toxicology results. Benzoylecgonine (BE) concentrations ≥ 300 ng/mL were considered positive. Subjects who did versus did not achieve abstinence during Phase I were compared on baseline sociodemographic and drug-history variables, using analysis of variance for continuous data and Fisher’s exact test for categorical data. Differences in these baseline characteristics between medication treatment groups (Phase II) were evaluated as well.
The effect of Phase I abstinence status on Phase II outcome variables was analyzed separately for each medication treatment. Retention rates were compared using proportional hazards Cox regression analyses to predict time to dropout as a function of Phase I abstinence status by medication group. Treatment response was measured as the probability of cocaine-positive urines over time, and was analyzed using generalized linear mixed modeling (GLMM), an extension of the generalized linear model that can accommodate random effects, as well as the correlated nature of repeated measures data (Enders, 2010; Zuur et al., 2009). GLMM estimates the parameters of the model by maximum likelihood using all available data without imputation of values for missing data. The maximum likelihood approach has been shown to produce unbiased parameter estimates with amounts of data missing at random ≥ 25% (Collins et al., 2001). The first regression model evaluated the main effect of Phase I abstinence status as a predictor of outcome, based on the hypothesis that early responders, i.e., those achieving abstinence during Phase I, would show better overall treatment response during Phase II compared with early non-reponders. The second, a multiplicative model of abstinence status, medication group, and time, with inclusion of all lower-order effects, permitted determination of the moderating effect of Phase I abstinence. Significant interactions were followed by simple effects tests to explore medication (vs placebo) differences within each subgroup (abstinent/nonabstinent).
3. RESULTS
3.1. Subject characteristics and treatment exposure
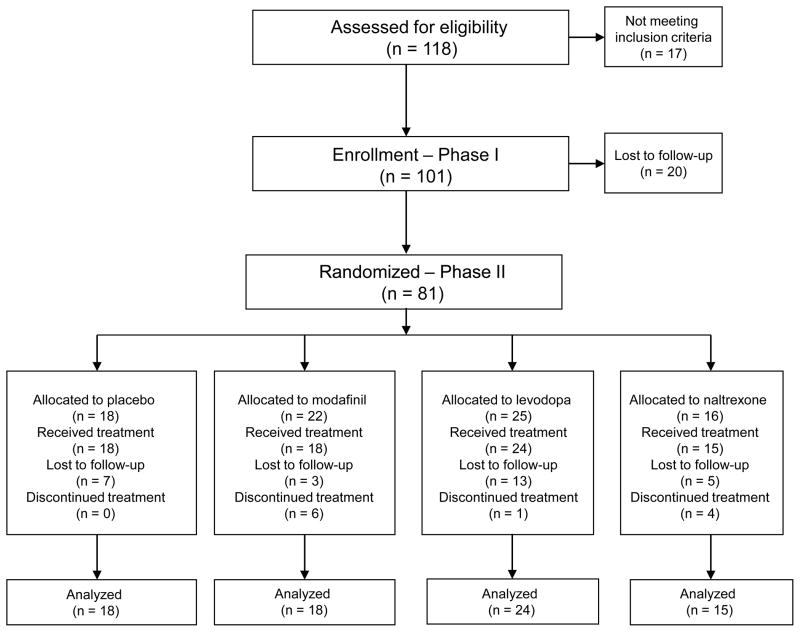
As shown in the CONSORT diagram (Figure 2), 101 of 118 candidate subjects consenting to screening satisfied inclusion criteria, provided consent to participate in this study and entered Phase I. Eighty-one (80% of 101) completed the baseline period, with 33 achieving initial abstinence (41%). Baseline characteristics of the Phase I sample by abstinence status are shown in Table 1 and reveal no significant differences.
Figure 2.
Consort diagram.
Table 1.
Subjects demographics and baseline characteristics.
| Baseline (Phase I) | ||
|---|---|---|
| Abstinent (n = 33) | Non-abstinent (n = 48) | |
| n (%) | n (%) | |
| Male | 27 (81%) | 34 (71%) |
| Race | ||
| African-American | 20 (60%) | 36 (75%) |
| Caucasian | 8 (24%) | 6 (13%) |
| Hispanic | 5 (15%) | 6 (13%) |
| Mean (SD) | Mean (SD) | |
| Age | 41.97 (9.95) | 42.90 (6.83) |
| Recent cocaine use (last 30 days) | 13.44 (7.76) | 15.88 (8.37) |
| Lifetime cocaine use (years) | 14.13 (7.93) | 14.22 (8.19) |
| Recent cannabis use (last 30 days) | 2.91 (6.23) | 5.44 (9.71) |
| Lifetime cannabis use (years) | 13.93 (10.92) | 11.68 (10.44) |
| Recent alcohol use (last 30 days) | 8.88 (8.63) | 9.54 (10.06) |
| Lifetime alcohol use (years) | 18.86 (12.76) | 18.18 (12.03) |
| n (%) | n (%) | |
| Phase II medication group | ||
| Placebo | 10 (55%) | 8 (44%) |
| Modafinil | 8 (36%) | 14 (63%) |
| Levodopa-carbidopa | 8 (32%) | 17 (68%) |
| Naltrexone | 9 (56%) | 7 (43%) |
Six subjects completing Phase I failed to return to the clinic to start Phase II, leaving 79 randomized participants who started Phase II, with 40 (50%) completing the full 12-week treatment period. Retention did not differ as a function of Phase I abstinence status by medication group for modafinil, χ2 (1) = 0.33, p ≤ 0.57, levodopa-carbidopa, χ2 (1) = 1.28, p ≤ 0.26, or naltrexone χ2 (1) = 1.80, p ≤ 0.18. The percentage of urines missing intermittently (prior to dropout) was as follows: modafinil (21%), levodopa-carbidopa (13%), naltrexone (20%), and placebo (25%).
The Phase II sample comprised mostly men (82%), 42.1 years old, and African American (70%), with a 13-year history of cocaine use and recent use reported on 15.2 of the past 30 days. There were no significant differences in baseline characteristics among the treatment groups.
3.2. Phase I abstinence status predicting cocaine use in Phase II
Phase I abstinence status predicted decreased cocaine use during Phase II under all treatment conditions: modafinil (OR = 0.003, 95% C.I. 0.0002 – 0.047, p = 0.001); levodopa-carbidopa (OR = 0.022, 95% C.I. 0.001 – 0.321, p = 0.009); and naltrexone (OR = 0.024, 95% C.I. 0.002 – 0.288, p = 0.004). Subjects achieving initial abstinence had a lower probability of cocaine use during Phase II than those failing to achieve abstinence.
3.3. Phase I abstinence status moderating medication response
Figure 3 depicts results examining the moderator effects of baseline abstinence status (Phase I), shown according to repeated time analyses of cocaine use during Phase II (left panel) and, for summary purposes, as marginal proportions of cocaine use for each medication versus placebo comparison (right panel). For modafinil (top), abstinence status failed to moderate medication effects on cocaine use, F (1,687) = 0.05, p = 0.815. For levodopa-carbidopa (middle), the interaction of abstinence status-by-medication-by-time was significant, F (1,714) = 3.99, p ≤ 0.046, supporting a moderator effect. Post-hoc analysis of simple effects revealed that among non-abstinent subjects, cocaine use was lower for levodopa-carbidopa compared to placebo, F (1,410) = 7.85, p ≤ 0.005, without evidence of change over time, F (3, 797) = 0.83, p ≤ 0.477. In the abstinent subgroup, cocaine use increased over time in the levodopa group, F (1, 6) = 52.19, p ≤ 0.0004, but not in the placebo group F (1, 9) = 0.24, p ≤ 0.634. Finally, for naltrexone (bottom), the two-way interaction of abstinence status-by-medication was significant, F (1,619) = 4.11, p ≤ 0.044, with post hoc main-effects showing naltrexone associated with reduced cocaine use in the non-abstinent, F (1, 322) = 3.94, p ≤ 0.048, but not in the abstinent subgroup, F (1, 299) = 0.85, p ≤ 0.36.
Figure 3.
Moderator effects of baseline abstinence status. Left side of panel shows predicted probability of cocaine positive urines over weeks in Phase II for each medication versus placebo comparison, with lines depicting the four stratification groups. Right side of panel shows marginal proportions (means + standard error) of cocaine use for each corresponding medication by abstinence status averaging over time. Solid bars indicate active medication. Open bars indicate placebo.
1 Week 1 timepoint represents onset of Phase II following baseline (Phase I), randomization, and dose titration.
3.4. Medication compliance and adverse events
The mean proportion of subjects coded as medication compliant, based on MEMS and fluorescence levels, was 73%, 80%, 71% and 80%, for modafinil, levodopa, naltrexone, and placebo, respectively. There were no differences in compliance as a function of medication group by time for modafinil, F (1, 1541) = 0.36, p ≤ 0.549, levodopa, F (1, 1561) = 0.04, p ≤ 0.838, or naltrexone, F (1, 1215) = 0.98, p ≤ 0.321.
Seven serious adverse events (SAEs) occurred in this study. The four events that occurred in patients randomized to levodopa-carbidopa included eye injury (torn conjunctiva), respiratory infection, arrhythmia symptoms, and suicidal tendencies. None of the SAEs was judged to be related to levodopa; however, study medication was discontinued in two of these cases (arrhythmia, suicidality). There was one SAE in the modafinil condition in which routine liver-function tests at week 4 showed an increase from baseline in hepatic enzyme values. The patient denied alcohol use and was sent out for medical evaluation but failed to return to the clinic for follow-up. One patient randomized to naltrexone experienced a work-related traumatic injury to his finger that required surgical repair. Finally, there was one SAE in the placebo condition involving a patient diagnosed with deep vein thrombosis in week 4 of the study. Study medication was discontinued, and the patient was followed until resolution of symptoms with anticoagulation therapy. All these events were reviewed and conclusions approved by the Institutional Review Board and Data Safety Monitoring Board.
4. DISCUSSION
The primary aim of this study was to examine the feasibility of using a prerandomization, abstinence-induction procedure to screen candidate cocaine-treatment medications. Results supported feasibility by showing good retention (80%) and reasonable rates of abstinence initiation (40%) during the baseline (Phase I) period of the study. Consistent with Bisaga et al. (2005), achievement of abstinence in Phase I was a robust predictor of treatment outcome. Patients who achieved two consecutive weeks of abstinence under high-value-voucher reward contingencies used less cocaine during subsequent treatment, regardless of medication received, compared with subjects who failed to achieve baseline abstinence.
The further aim of this study was screening efficacy of selected medications to reduce cocaine use in active users (“cocaine cessation”) and maintain abstinence in recent nonusers (“relapse-prevention”). By stratifying for Phase I abstinence status, we found differences in response to treatment for levodopa-carbidopa and naltrexone, with both medications showing benefit in patients who were nonabstinent at baseline but failing to show benefit in patients who achieved abstinence at baseline. In previous clinical studies neither of these medications has shown robust efficacy in relatively heterogeneous samples of treatment-seeking cocaine users (Mooney et al., 2007; Schmitz et al., 2009, 2008, 2001). The results of implementing the screening paradigm, while not conclusive, suggest efficacy of levodopa-carbidopa and naltrexone for the subgroup of nonabstinent subjects (following an initial period of treatment compliance). Most importantly, it provides a robust model for screening efficacy in abstinent and nonabstinent subjects. That the two groups of subjects showed no differences in cocaine or other drug use at treatment entry prior to baseline (Phase I), further strengthens the rationale for using a lead-in period to subgroup patients and, in doing so, identify treatment response phenotypes.
The observed ineffectiveness of levodopa-carbidopa and naltrexone in the abstinent subgroup counters initial predictions. However, it is not inconsistent with suggestions of less biological/neurochemical (and behavioral) impairment in subjects abstinent on entry who, thus, have less need for, or lower responsivity to, pharmacological manipulations (Bisaga et al., 2011; Martinez et al., 2011). Not surprisingly, it could be argued that neurotransmitter modulation and resultant behavioral change may be greatest for those with greatest impairment/dysregulation. Parallel imaging studies in the context of the screening paradigm may provide greater precision in profiling the neurobehavioral moderators of medication response.
Our screening results do not support modafinils’ efficacy for cocaine treatment. The promise of early positive results (Dackis et al., 2005, 2003) was dimmed by subsequent negative clinical trials (Anderson et al., 2009; Dackis et al., 2012). Post-hoc analyses of these negative trials, however, identified concurrent alcohol dependence (Anderson et al., 2009) and gender (Dackis et al., 2012) as sources of variability in treatment outcome. Here, using a clearly defined paradigm, bolstered by direct parallel comparison with two other agents, we can be reasonably confident of the finding.
Although it is necessary to interpret our results with caution because of the small sample size, they encourage future investigations for testing medications under well-defined baseline conditions. In our case, the efficiency of screening multiple medications against a common placebo was offset by the practical challenge of obtaining a sample size needed to provide sufficient power for testing moderator effects. At the same time, we do not believe that sample size limitations affected our ability to achieve the main aim of the study, specifically, to evaluate the feasibility of using a two-phase abstinence induction paradigm to screen candidate medications for cocaine treatment.
The utility of this screening approach may be enhanced by additional measurement of responding in Phase I. For example, in-depth analysis of withdrawal-related symptoms associated with attempts to achieve initial abstinence may have aided the interpretation of medication effects observed in Phase II. Additionally, variability in cocaine use at the onset of Phase II was unavoidable and most likely due to procedural changes during the transition, including the abrupt discontinuation of high magnitude abstinence-based CM and rapid medication titration. Future studies using this screening paradigm should consider refinements to ensure more stable baseline responding prior to evaluating the target dose of medication so that more reliable cocaine “cessation” and “relapse prevention” effects can be investigated. It is important to note that since all of the patients in this study received a moderately intense psychotherapy platform (i.e., weekly individual CBT sessions), we cannot rule out the possibility that under less intense platforms the medication effects may not have emerged. Finally, without a larger sample, it was not possible to examine the potential impact of concurrent use of other substances, such as alcohol and cannabis, on cocaine outcomes as a function of medication treatment.
Screening trials are not new to the field of cocaine treatment. Over ten years ago the Cocaine Rapid Efficacy Screening Trials (CREST) were conducted to screen a number of candidate medications in a controlled, uniform, and more efficient fashion with the goal of prioritizing development. In all, 18 medications were screened in small samples, resulting in two (reserpine and cabergoline) showing statistical superiority to placebo (Berger et al., 2005; Kampman et al., 2005; Shoptaw et al., 2005). CREST revealed problems stemming from lack of equivalence among treatment groups as a function of baseline cocaine use, further supported by Bisaga et al. (2010, 2005). The adaptive randomization method used here was recommended as a modification for improving future screening trials (Kampman et al., 2005). Enrichment strategies to increase homogeneity have also been recommended by the FDA to enhance detection of signal where it exists (FDA, 2013). What is reported here is a refinement of several models that can benefit subsequent medication development.
Acknowledgments
Role of Funding Source. Funding for this study was provided by the National Institute on Drug Abuse (NIDA) Medications Development Center Grant (P50-DA-9262). The NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors. Authors Schmitz, Grabowski, and Stotts assisted in conceptualizing and designing the study. Author Lindsay assisted in writing the protocol and overseeing the conduct of the study. Authors Green and Rathnayaka undertook the statistical analysis and preparation of results. Author Moeller provided medical oversight, interpretation of results, and manuscript review. All authors contributed to editing and re-writing of the manuscript, and provided final approval of the submission.
Conflict of Interest. All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SP, Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Leiderman DB, Montgomery MA, Goldsmith RJ, Bloch DA, Singal BM, Elkashef A. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100(Suppl 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, Nunes EV. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend. 2010;111:97–104. doi: 10.1016/j.drugalcdep.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Vosburg SK, Nunes EV. Utility of lead-in period in cocaine dependence pharmacotherapy trials. Drug Alcohol Depend. 2005;77:7–11. doi: 10.1016/j.drugalcdep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Cheng WY, Carpenter KM, Mariani JJ, Levin FR, Raby WN, Nunes EV. A placebo controlled trial of memantine as an adjunct to oral naltrexone for opioid dependence. Drug Alcohol Depend. 2011;119:e23–29. doi: 10.1016/j.drugalcdep.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6:330–351. [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology (Berl) 1991;104:167–170. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Gallop R, Gibbons MBC, Sadicario JS, Woody G. Measuring outcome in the treatment of cocaine dependence. J Alcohol Drug Depend. 2013;1:1–8. doi: 10.4172/2329-6488.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43:303–312. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O’Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Dallery J, Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: effects of reinforcer magnitude. Exp Clin Psychopharmacol. 2001;9:317–325. doi: 10.1037//1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Enders C, editor. Applied Missing Data Analysis (Methodology in the Social Sciences) Guilford Press; New York: 2010. [Google Scholar]
- FDA. Guidance for industry. [accessed January 332013];Enrichment strategies for clinical trials to support approval of human drugs and biological products. 2013 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM332181.pdf.
- First MB, Pincus HA. The DSM-IV Text Revision: rationale and potential impact on clinical practice. Psychiatr Serv. 2002;53:288–292. doi: 10.1176/appi.ps.53.3.288. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R, editors. Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. DHHS; Rockville, MD: 1992. [Google Scholar]
- Kampman KM, Leiderman D, Holmes T, LoCastro J, Bloch DA, Reid MS, Shoptaw S, Montgomery MA, Winhusen TM, Somoza EC, Ciraulo DA, Elkashef A, Vocci F. Cocaine Rapid Efficacy Screening Trials (CREST): lessons learned. Addiction. 2005;100(Suppl 1):102–110. doi: 10.1111/j.1360-0443.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- Katz EC, Robles-Sotelo E, Correia CJ, Silverman K, Stitzer ML, Bigelow G. The brief abstinence test: effects of continued incentive availability on cocaine abstinence. Exp Clin Psychopharmacol. 2002;10:10–17. doi: 10.1037//1064-1297.10.1.10. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic homeostatic dysregulation as a driver of drug-seeking behavior. Drug Discov Today Dis Models. 2008;5:207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, Khan R, Mojsiak J, Myrick DL, Hedden S, Cochran K, Woolson RF. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32:577–587. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18:414–418. doi: 10.1111/j.1755-5949.2011.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 2013;229:415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Sayre SL, Green C, Rhoades H, Schmitz JM. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addict Disord Treat. 2004;3:165–173. [Google Scholar]
- Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa-carbidopa treatment for cocaine dependence: two double-blind, randomized, clinical trials. Drug Alcohol Depend. 2007;88:214–223. doi: 10.1016/j.drugalcdep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Xie H, Dackis C, Rabinowitz AR, O’Brien CP. A double blind, placebo-controlled trial that combines disulfiram and naltrexone for treating co-occurring cocaine and alcohol dependence. Addict Behav. 2008;33:651–667. doi: 10.1016/j.addbeh.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, van Ree JM. Intracerebroventricular naltrexone treatment attenuates acquisition of intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1991;40:807–810. doi: 10.1016/0091-3057(91)90090-o. [DOI] [PubMed] [Google Scholar]
- Sayre SL, Evans M, Hokanson PS, Schmitz JM, Stotts AL, Averill P, Grabowski J. “Who gets in?” Recruitment and screening processes of outpatient substance abuse trials. Addict Behav. 2004;29:389–398. doi: 10.1016/j.addbeh.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Lindsay JA, Green CE, Herin DV, Stotts AL, Moeller FG. High-dose naltrexone therapy for cocaine-alcohol dependence. Am J Addict. 2009;18:356–362. doi: 10.3109/10550490903077929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Lindsay JA, Stotts AL, Green CE, Moeller FG. Contingency management and levodopa-carbidopa for cocaine treatment: a comparison of three behavioral targets. Exp Clin Psychopharmacol. 2010;18:238–244. doi: 10.1037/a0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Rathnayaka N, Green CE, Moeller FG, Dougherty AE, Grabowski J. Combination of modafinil and d-amphetamine for the treatment of cocaine dependence: a preliminary investigation. Front Psychiatry. 2012;3:77. doi: 10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Grabowski J. Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. Am J Addict. 2004;13:333–341. doi: 10.1080/10550490490480982. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, Ling W. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;100(Suppl 1):78–90. doi: 10.1111/j.1360-0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology (Berl) 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotts AL, Schmitz JM, Rhoades HM, Grabowski J. Motivational interviewing with cocaine-dependent patients: a pilot study. J Consult Clin Psychol. 2001;69:858–862. doi: 10.1037//0022-006x.69.5.858. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Leno EN, Walker N, Saveliev AA, Smith GM, editors. Mixed Effects Models and Extensions in Ecology with R. Springer; New York: 2009. [Google Scholar]