Abstract
The lipolytic enzyme phospholipase A2 (PLA2) is involved in the degradation of high-molecular weight phospholipid aggregates in vivo. The enzyme has very high catalytic activities on aggregated substrates compared with monomeric substrates, a phenomenon called interfacial activation. Crystal structures of PLA2s in the absence and presence of inhibitors are identical, from which it has been concluded that enzymatic conformational changes do not play a role in the mechanism of interfacial activation. The high-resolution NMR structure of porcine pancreatic PLA2 free in solution was determined with heteronuclear multidimensional NMR methodology using doubly labeled 13C, 15N-labeled protein. The solution structure of PLA2 shows important deviations from the crystal structure. In the NMR structure the Ala1 alpha-amino group is disordered and the hydrogen bonding network involving the N-terminus and the active site is incomplete. The disorder observed for the N-terminal region of PLA2 in the solution structure could be related to the low activity of the enzyme towards monomeric substrates. The NMR structure of PLA2 suggests, in contrast to the crystallographic work, that conformational changes do play a role in the interfacial activation of this enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax A., Ikura M. An efficient 3D NMR technique for correlating the proton and 15N backbone amide resonances with the alpha-carbon of the preceding residue in uniformly 15N/13C enriched proteins. J Biomol NMR. 1991 May;1(1):99–104. doi: 10.1007/BF01874573. [DOI] [PubMed] [Google Scholar]
- Brady L., Brzozowski A. M., Derewenda Z. S., Dodson E., Dodson G., Tolley S., Turkenburg J. P., Christiansen L., Huge-Jensen B., Norskov L. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature. 1990 Feb 22;343(6260):767–770. doi: 10.1038/343767a0. [DOI] [PubMed] [Google Scholar]
- Brunie S., Bolin J., Gewirth D., Sigler P. B. The refined crystal structure of dimeric phospholipase A2 at 2.5 A. Access to a shielded catalytic center. J Biol Chem. 1985 Aug 15;260(17):9742–9749. [PubMed] [Google Scholar]
- Brzozowski A. M., Derewenda U., Derewenda Z. S., Dodson G. G., Lawson D. M., Turkenburg J. P., Bjorkling F., Huge-Jensen B., Patkar S. A., Thim L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature. 1991 Jun 6;351(6326):491–494. doi: 10.1038/351491a0. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M. Structures of larger proteins in solution: three- and four-dimensional heteronuclear NMR spectroscopy. Science. 1991 Jun 7;252(5011):1390–1399. doi: 10.1126/science.2047852. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Robien M. A., Gronenborn A. M. Exploring the limits of precision and accuracy of protein structures determined by nuclear magnetic resonance spectroscopy. J Mol Biol. 1993 May 5;231(1):82–102. doi: 10.1006/jmbi.1993.1259. [DOI] [PubMed] [Google Scholar]
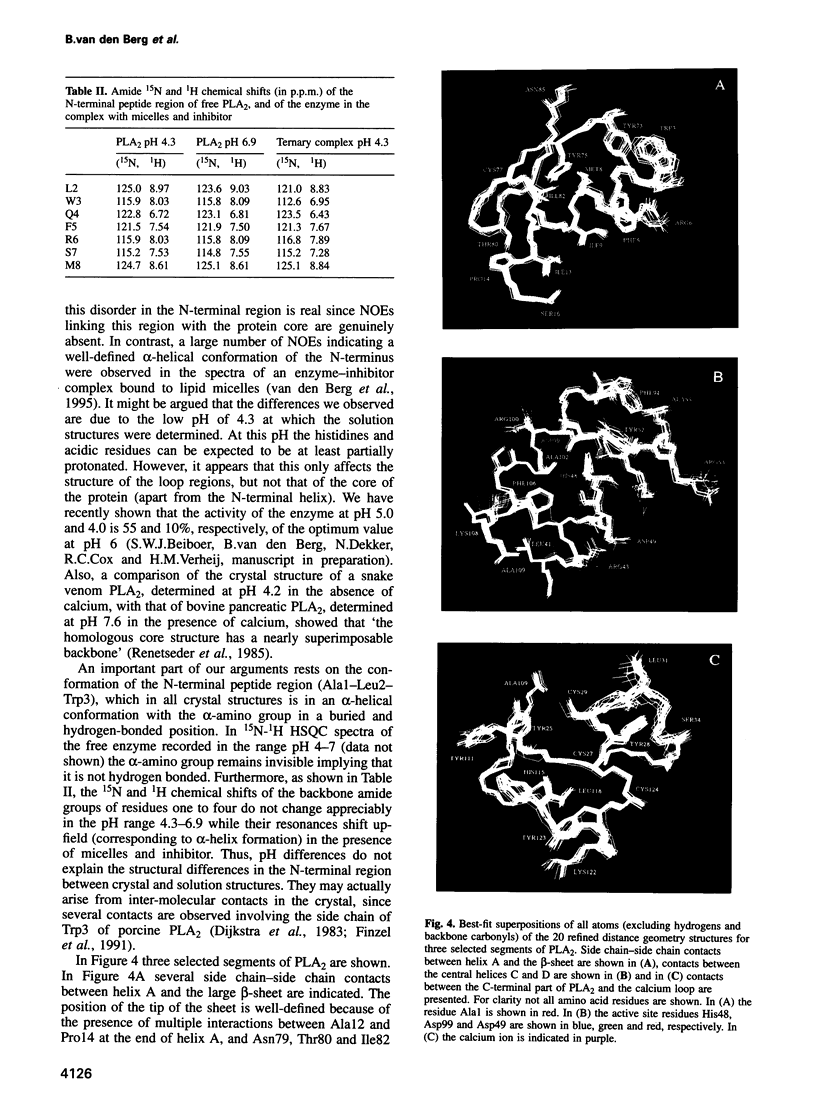
- Dekker N., Peters A. R., Slotboom A. J., Boelens R., Kaptein R., de Haas G. Porcine pancreatic phospholipase A2: sequence-specific 1H and 15N NMR assignments and secondary structure. Biochemistry. 1991 Mar 26;30(12):3135–3146. doi: 10.1021/bi00226a022. [DOI] [PubMed] [Google Scholar]
- Demaret J. P., Brunie S. Molecular dynamics simulations of phospholipases A2. Protein Eng. 1990 Dec;4(2):163–170. doi: 10.1093/protein/4.2.163. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Kalk K. H., Drenth J., de Haas G. H., Egmond M. R., Slotboom A. J. Role of the N-terminus in the interaction of pancreatic phospholipase A2 with aggregated substrates. Properties and crystal structure of transaminated phospholipase A2. Biochemistry. 1984 Jun 5;23(12):2759–2766. doi: 10.1021/bi00307a035. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Kalk K. H., Hol W. G., Drenth J. Structure of bovine pancreatic phospholipase A2 at 1.7A resolution. J Mol Biol. 1981 Mar 25;147(1):97–123. doi: 10.1016/0022-2836(81)90081-4. [DOI] [PubMed] [Google Scholar]
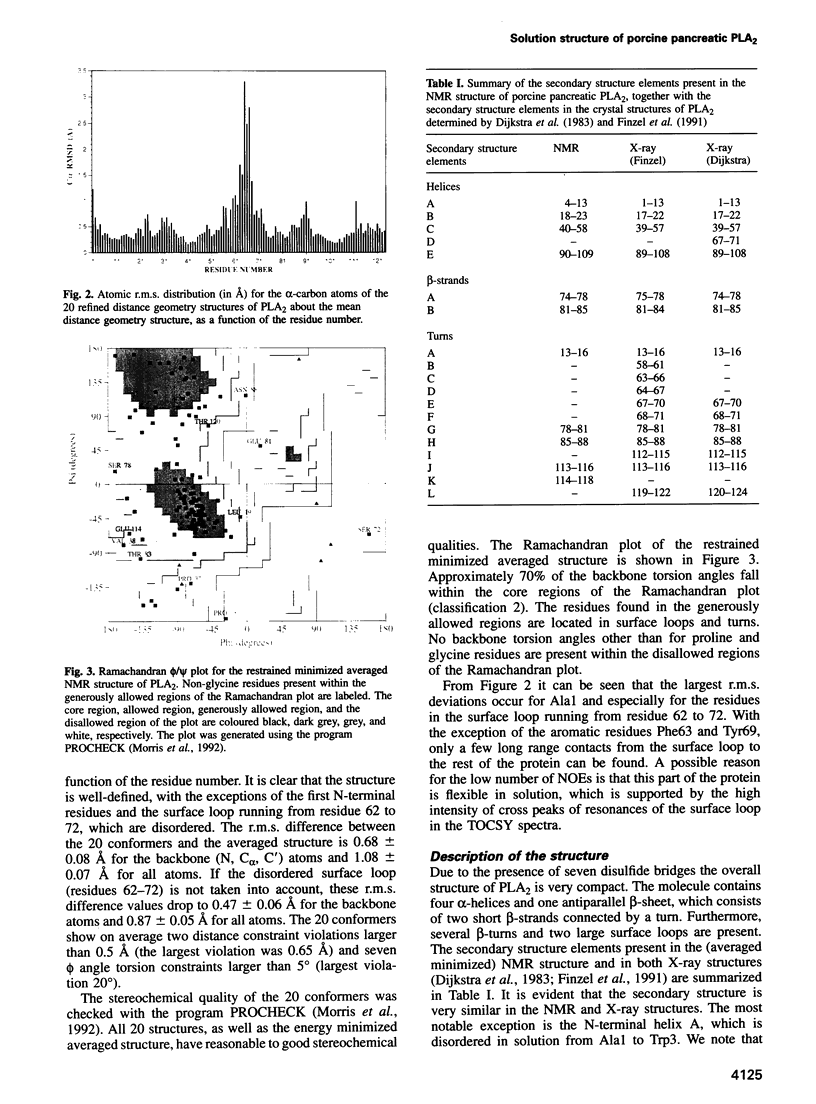
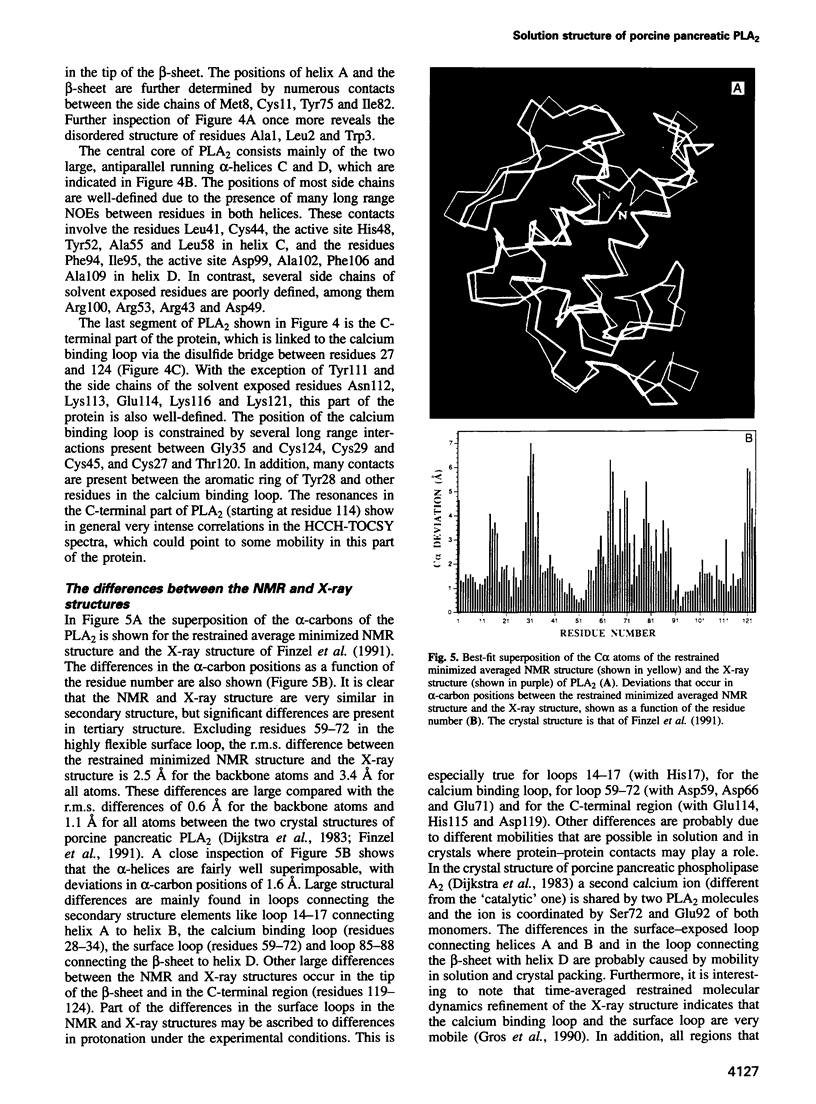
- Dijkstra B. W., Renetseder R., Kalk K. H., Hol W. G., Drenth J. Structure of porcine pancreatic phospholipase A2 at 2.6 A resolution and comparison with bovine phospholipase A2. J Mol Biol. 1983 Jul 25;168(1):163–179. doi: 10.1016/s0022-2836(83)80328-3. [DOI] [PubMed] [Google Scholar]
- Finzel B. C., Ohlendorf D. H., Weber P. C., Salemme F. R. An independent crystallographic refinement of porcine phospholipase A2 at 2.4 A resolution. Acta Crystallogr B. 1991 Aug 1;47(Pt 4):558–559. doi: 10.1107/s0108768190012939. [DOI] [PubMed] [Google Scholar]
- Fisher J., Primrose W. U., Roberts G. C., Dekker N., Boelens R., Kaptein R., Slotboom A. J. 1H NMR studies of bovine and porcine phospholipase A2: assignment of aromatic resonances and evidence for a conformational equilibrium in solution. Biochemistry. 1989 Jul 11;28(14):5939–5946. doi: 10.1021/bi00440a034. [DOI] [PubMed] [Google Scholar]
- Gros P., van Gunsteren W. F., Hol W. G. Inclusion of thermal motion in crystallographic structures by restrained molecular dynamics. Science. 1990 Sep 7;249(4973):1149–1152. doi: 10.1126/science.2396108. [DOI] [PubMed] [Google Scholar]
- Grzesiek S., Döbeli H., Gentz R., Garotta G., Labhardt A. M., Bax A. 1H, 13C, and 15N NMR backbone assignments and secondary structure of human interferon-gamma. Biochemistry. 1992 Sep 8;31(35):8180–8190. doi: 10.1021/bi00150a009. [DOI] [PubMed] [Google Scholar]
- Havel T. F. An evaluation of computational strategies for use in the determination of protein structure from distance constraints obtained by nuclear magnetic resonance. Prog Biophys Mol Biol. 1991;56(1):43–78. doi: 10.1016/0079-6107(91)90007-f. [DOI] [PubMed] [Google Scholar]
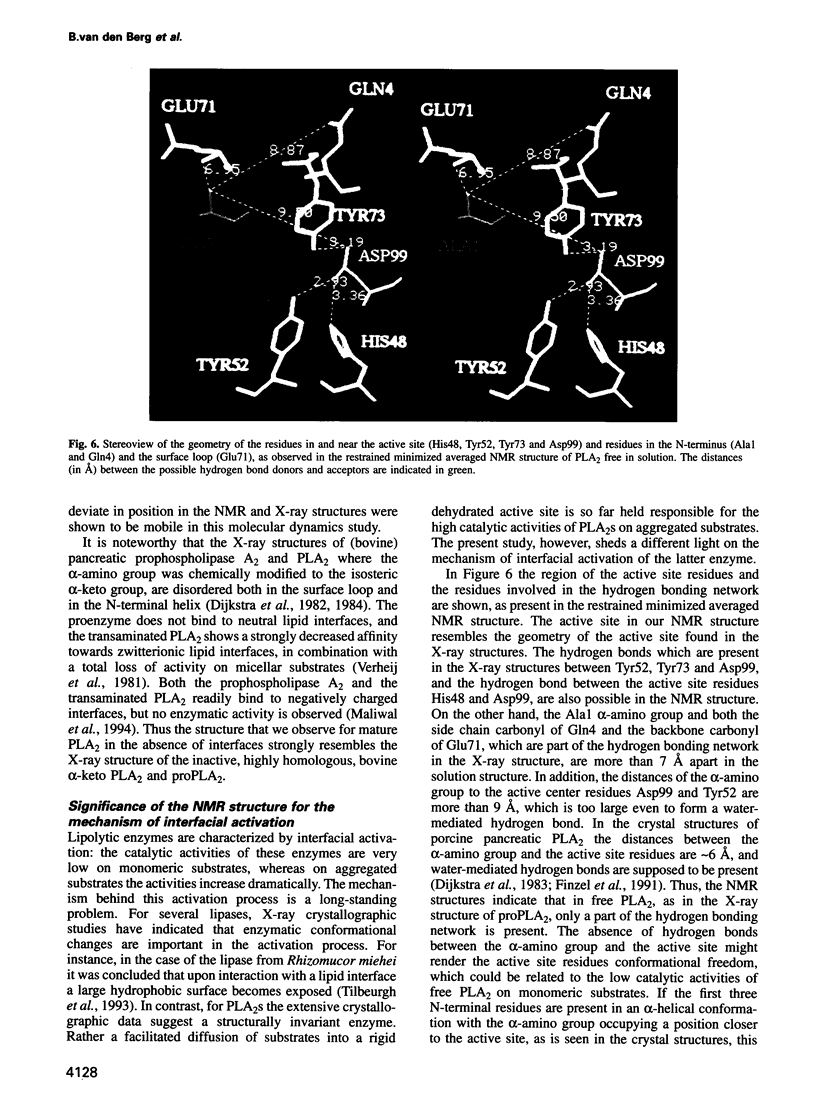
- Ikura M., Kay L. E., Bax A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990 May 15;29(19):4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- Kuipers O. P., Thunnissen M. M., de Geus P., Dijkstra B. W., Drenth J., Verheij H. M., de Haas G. H. Enhanced activity and altered specificity of phospholipase A2 by deletion of a surface loop. Science. 1989 Apr 7;244(4900):82–85. doi: 10.1126/science.2704992. [DOI] [PubMed] [Google Scholar]
- Maliwal B. P., Yu B. Z., Szmacinski H., Squier T., van Binsbergen J., Slotboom A. J., Jain M. K. Functional significance of the conformational dynamics of the N-terminal segment of secreted phospholipase A2 at the interface. Biochemistry. 1994 Apr 19;33(15):4509–4516. doi: 10.1021/bi00181a010. [DOI] [PubMed] [Google Scholar]
- Marion D., Driscoll P. C., Kay L. E., Wingfield P. T., Bax A., Gronenborn A. M., Clore G. M. Overcoming the overlap problem in the assignment of 1H NMR spectra of larger proteins by use of three-dimensional heteronuclear 1H-15N Hartmann-Hahn-multiple quantum coherence and nuclear Overhauser-multiple quantum coherence spectroscopy: application to interleukin 1 beta. Biochemistry. 1989 Jul 25;28(15):6150–6156. doi: 10.1021/bi00441a004. [DOI] [PubMed] [Google Scholar]
- Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- Morris A. L., MacArthur M. W., Hutchinson E. G., Thornton J. M. Stereochemical quality of protein structure coordinates. Proteins. 1992 Apr;12(4):345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Kunze H., de Haas G. H. Phospholipase A2 (phosphatide acylhydrolase, EC 3.1.1.4) from porcine pancreas. Methods Enzymol. 1974;32:147–154. doi: 10.1016/0076-6879(74)32018-6. [DOI] [PubMed] [Google Scholar]
- Pardi A., Billeter M., Wüthrich K. Calibration of the angular dependence of the amide proton-C alpha proton coupling constants, 3JHN alpha, in a globular protein. Use of 3JHN alpha for identification of helical secondary structure. J Mol Biol. 1984 Dec 15;180(3):741–751. doi: 10.1016/0022-2836(84)90035-4. [DOI] [PubMed] [Google Scholar]
- Peters A. R., Dekker N., van den Berg L., Boelens R., Kaptein R., Slotboom A. J., de Haas G. H. Conformational changes in phospholipase A2 upon binding to micellar interfaces in the absence and presence of competitive inhibitors. A 1H and 15N NMR study. Biochemistry. 1992 Oct 20;31(41):10024–10030. doi: 10.1021/bi00156a023. [DOI] [PubMed] [Google Scholar]
- Powers R., Garrett D. S., March C. J., Frieden E. A., Gronenborn A. M., Clore G. M. The high-resolution, three-dimensional solution structure of human interleukin-4 determined by multidimensional heteronuclear magnetic resonance spectroscopy. Biochemistry. 1993 Jul 6;32(26):6744–6762. doi: 10.1021/bi00077a030. [DOI] [PubMed] [Google Scholar]
- Renetseder R., Brunie S., Dijkstra B. W., Drenth J., Sigler P. B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox venom. J Biol Chem. 1985 Sep 25;260(21):11627–11634. [PubMed] [Google Scholar]
- Scott D. L., Sigler P. B. Structure and catalytic mechanism of secretory phospholipases A2. Adv Protein Chem. 1994;45:53–88. doi: 10.1016/s0065-3233(08)60638-5. [DOI] [PubMed] [Google Scholar]
- Scott D. L., White S. P., Browning J. L., Rosa J. J., Gelb M. H., Sigler P. B. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science. 1991 Nov 15;254(5034):1007–1010. doi: 10.1126/science.1948070. [DOI] [PubMed] [Google Scholar]
- Scott D. L., White S. P., Otwinowski Z., Yuan W., Gelb M. H., Sigler P. B. Interfacial catalysis: the mechanism of phospholipase A2. Science. 1990 Dec 14;250(4987):1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Thunnissen M. M., Ab E., Kalk K. H., Drenth J., Dijkstra B. W., Kuipers O. P., Dijkman R., de Haas G. H., Verheij H. M. X-ray structure of phospholipase A2 complexed with a substrate-derived inhibitor. Nature. 1990 Oct 18;347(6294):689–691. doi: 10.1038/347689a0. [DOI] [PubMed] [Google Scholar]
- Tomoo K., Ohishi H., Doi M., Ishida T., Inoue M., Ikeda K., Mizuno H. Interaction mode of n-dodecylphosphorylcholine, a substrate analogue, with bovine pancreas phospholipase A2 as determined by X-ray crystal analysis. Biochem Biophys Res Commun. 1992 Sep 16;187(2):821–827. doi: 10.1016/0006-291x(92)91270-z. [DOI] [PubMed] [Google Scholar]
- Verger R. Interfacial enzyme kinetics of lipolysis. Annu Rev Biophys Bioeng. 1976;5:77–117. doi: 10.1146/annurev.bb.05.060176.000453. [DOI] [PubMed] [Google Scholar]
- Wery J. P., Schevitz R. W., Clawson D. K., Bobbitt J. L., Dow E. R., Gamboa G., Goodson T., Jr, Hermann R. B., Kramer R. M., McClure D. B. Structure of recombinant human rheumatoid arthritic synovial fluid phospholipase A2 at 2.2 A resolution. Nature. 1991 Jul 4;352(6330):79–82. doi: 10.1038/352079a0. [DOI] [PubMed] [Google Scholar]
- Westerlund B., Nordlund P., Uhlin U., Eaker D., Eklund H. The three-dimensional structure of notexin, a presynaptic neurotoxic phospholipase A2 at 2.0 A resolution. FEBS Lett. 1992 Apr 20;301(2):159–164. doi: 10.1016/0014-5793(92)81238-h. [DOI] [PubMed] [Google Scholar]
- White S. P., Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990 Dec 14;250(4987):1560–1563. doi: 10.1126/science.2274787. [DOI] [PubMed] [Google Scholar]
- van Tilbeurgh H., Egloff M. P., Martinez C., Rugani N., Verger R., Cambillau C. Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography. Nature. 1993 Apr 29;362(6423):814–820. doi: 10.1038/362814a0. [DOI] [PubMed] [Google Scholar]
- van den Berg B., Tessari M., Boelens R., Dijkman R., Kaptein R., de Haas G. H., Verheij H. M. Solution structure of porcine pancreatic phospholipase A2 complexed with micelles and a competitive inhibitor. J Biomol NMR. 1995 Feb;5(2):110–121. doi: 10.1007/BF00208802. [DOI] [PubMed] [Google Scholar]