Abstract
People living with HIV (PLWH) have increasingly longer life spans. This age group faces different challenges than younger PLWH, which may include increased stress and social isolation. The purpose of this study was to determine if the age and sex of PLWH is associated with measures of physiologic stress, perceived stress and social isolation. In this cross-sectional study, we enrolled 102 PLWH equally into four groups divided by age (< or > 50 years) and gender. Participants completed well-validated survey measurements of stress and isolation, and their heart rate variability over 60 minutes was measured by Holter monitor. The mean (standard deviation) Perceived Stress Scale score was 17.4 (6.94), mean visual analog stress scale score was 3.51 (2.79), and mean Hawthorne Friendship Scale score, a measure of social isolation, was 17.03 (4.84). Mean heart rate variability expressed as the standard deviation of successive N-N intervals was 65.47 (31.16) msec. In multivariable regression models that controlled for selected demographic variables, there was no relationship between the Perceived Stress Scale and age (coefficient=−0.09, p=−.23) or female gender (coefficient=−0.12, p=0.93); however, there was a modest relationship between female gender and stress using the visual analog stress scale (coefficient=1.24, p=0.05). Perceived Stress was negatively associated with the Hawthorne Friendship score (coefficient=−0.34, p=0.05). Hawthorne Friendship score was positively associated with younger age (coefficient=0.11, p=0.02). Age was the only independent predictor of physiologic stress as measured by heart rate variability (coefficient=−1.3, p<0.01). Our findings suggest that younger PLWH may experience more social isolation; however, age-related changes in heart rate variability do not appear to be related to perceived stress or social isolation. Future longitudinal research is required to more thoroughly understand this relationship and its impact on the health of PLWH.
Keywords: HIV, Psychological Stress, Aging, Social Isolation, Social Stigma
INTRODUCTION
With the development and global scale-up of potent HIV anti-retroviral therapy (ART) over the past 15 years, life expectancy for people living with HIV (PLWH) has increased dramatically. In 2001, 17% of all PLWH in the United States were older than 50 years of age. This number increased to 33% in 2009 (Prevention, 2010), and it is estimated that by 2014, half of all people living with HIV in the United States will be 50 years of age or older (High et al., 2012). AIDS-related deaths have fallen dramatically from 71% in 1988–1995 to 16% in 2005–2010 (Weber et al., 2012) while mortality from non-AIDS defining disease of aging such as cardiovascular disease and cancer is rising (Chu, et al., 2011; Engels et al., 2008; Kraft-Terry, Stothert, Buch, & Gendelman, 2010; Lescure et al., 2011). For example, cancer diagnoses among PLWH now occur approximately 20 years earlier than cancer diagnoses United States general population (Shiels, Pfeiffer, & Engels, 2010). The prevalence of neurocognitive disorders in PLWH is also high (Vance, Woodley, & Burrage, 2007). A recent study found that 52% of PLWH had some degree of neurocognitive impairment (Heaton, 2010) and that this impairment impacted their ability to work, manage financial resources, and manage activities of daily living (Gorman, Foley, Ettenhofer, Hinkin, & Gorp, 2009). It was also reported that older adults living with HIV had difficulty managing these multiple health conditions which tended to complicate key self-management tasks including medication adherence (Emlet, 2006b). These increasing chronic co-morbidities, in addition to HIV disease, might be linked to increased stress and isolation in PLWH and be exacerbated by aging.
The daily care of HIV is stressful for PLWH (Scott-Sheldon, Kalichman, Carey, & Fielder, 2008). Over the past 10 years, there has been substantial evidence linking stress and HIV disease progression (Greeson et al., 2008; Ironson & Hayward, 2008; Scott-Sheldon, Kalichman, Carey, & Fielder, 2008). The aging process has also been found to increase chronic inflammation, leading to increased disease, organ dysfunction and frailty (Butcher & Lord, 2004; Effros et al., 2008; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002). This chronic inflammatory state may be modulated by stress (Butcher & Lord, 2004). To better understand the impact of stress on the daily care of HIV, a number of stress reductions interventions have been tested in PLWH. Promisingly, stress management interventions for PLWH tend to improve coping skills, social support, and mental health, but almost all of these studies have been conducted in men under the age of 50 (Antoni, 2003; Scott-Sheldon, et al., 2008) leaving out two important populations-older adults, and women living with HIV.
Social isolation has been described as living without companionship, social support, or social connectedness (Hawthorne, 2006). It has been linked to decreased quality of life, poor health status, increased health care utilization, functional decline, and death (Perissinotto, 2012). HIV is a heavily stigmatized disease and older adults living with HIV tend to experience the dual threat of HIV stigma and ageism (Emlet, 2006b). Physiologically, in non-HIV infected populations social isolation has been linked to increased cardiovascular risk, posited to be mediated by dysregulation of the cardiovascular, neuroendocrine, inflammatory, and metabolic systems (Grant, Hamer, & Steptoe, 2009), particularly in women (Hackett, Hamer, Endrighi, Brydon, & Steptoe, 2012). Social isolation has been linked to biomarkers predictive of cardiovascular morbidity and mortality (e.g., C-reactive protein, blood pressure, Fibringin) (Shankar, McMunn, Banks, & Steptoe, 2011). Similar increases in biomarkers are associated with maladaptive behaviors including smoking and physical inactivity in adults over the age of 50 (Shankar, et al., 2011). These biomarkers are highly predictive of myocardial infarction and mortality in PLWH (Tien et al., 2010; Triant, Meigs, & Grinspoon, 2009). The allostatic load theory suggests that isolation results in a decreased response to cumulative stressful events by dysregulating the cardiovascular, neuroendocrine, inflammatory, and metabolic systems, in part explaining the consistent relationship between isolation and negative health outcomes in older adults (Juster, McEwen, & Lupien, 2010). Social isolation is increased in both older adults and PLWH, but no studies have described the possible impact of social isolation on psychological and physiologic stress in older adults living with HIV.
The purpose of this study was, therefore, to determine if age and sex of PLWH are associated with their levels of stress and social isolation. Based on available literature, we hypothesized that: 1) Older adults living with HIV would have greater associations between increased levels of stress and social isolation than younger PLWH; and 2) Women would have higher levels of stress and social isolation than men.
METHODS
Sample and Recruitment
In this cross-sectional study, we used purposive sampling to examine differences in stress and isolation between older and younger men and women living with HIV. People living with HIV were recruited from HIV clinics, HIV service organizations, and a HIV research registry into four equally sized strata (women <50 years; women >51 years; men <50; men >51 years). All subjects were recruited from November 2011 to June 2012. Inclusion criteria included: 1) documented HIV diagnosis; 2) 18 years of age or older; 3) able to understand English; and 4) currently taking highly active anti-retroviral therapy for HIV. Individuals were excluded if they: 1) were unable to give informed consent; 2) had insulin dependent diabetes; or 3) were dependent on a cardiac pacemaker. Participants with advanced diabetes and pacemakers were excluded because of the significant confounding effect on heart rate variability.
Procedures
The study was approved by the institutional review board of University Hospitals (Cleveland, OH). Written informed consent was obtained from eligible PLWH at a baseline visit. Height, weight, and vital signs were measured, and the participant was asked to rank their current level of stress on a visual analog scale that ranged from 0 to 10. Each participant then wore a Holter cardiac monitor for 1 hour at rest as described below. The participants were given a 7-day exercise and sleep diary and returned one week later to complete a survey asking about isolation, psychosocial stress, and other socio-demographic questions. Participants completed the electronic survey using iPads, to enhance the accuracy of self-report. Participants were compensated for their time with a $50 Visa cash gift card.
Measures
Demographic characteristics were obtained by self-report and medical data was abstracted directly from the participant’s medical chart. Psychosocial stress level was assessed using two methods: the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983; Cohen & Williamson, 1988) and a single item stress visual analog scale. The Perceived Stress Scale has 10 items (e.g., “In the past month, how often have you been upset because of something that happened unexpectedly?”) which are scored on a 0 (Never) to 4 (Very Often) ordered response scale. The total scale score ranges from 0–40 and is calculated by summing the item scores. A higher score indicates greater perceived stress. This widely-used scale has well-documented validity and a reliability co-efficient of 0.78 (Cohen, et al., 1983; Cohen & Williamson, 1988). The single item stress visual analog scale asked participants “On a scale of 0–10, how stressed do you feel right now?” Participants were given a 10 point scale and were asked to put an “x” to mark their response. The perceived stress scale was administered at the follow-up visit, as part of the survey. The visual analog scale was administered at the first visit, just prior to starting the Holter cardiac monitor protocol as a method to assess the participant’s perception of stress, prior to initiating the heart rate variability protocol.
Heart rate variability was measured as a surrogate marker of physiologic stress. At the first visit, participants were asked to wear a Holter cardiac monitor (Aria Holter, Spacelabs Healthcare, Hertford, UK) for 60 minutes. During this time, participants relaxed in a quiet room and were provided with reading materials. Participants were not permitted to smoke or consume caffeine during the data collection. The Holter data was first cleaned to remove artifact and then analyzed with Sentinel Impresario Holter, version 7.1.3 (Spacelabs Healthcare, Hertford, UK). A single time-domain measure of heart rate variability—standard deviation of N-N intervals (SDNN)—was chosen a priori for analyses because it is valid over short periods of time and because it has been used in previous literature examining vagal tone in conditions of psychological stress (Clays et al., 2011; Lee & Theus, 2012; Patron et al., 2012).
Social Isolation was assessed using the Hawthorne Friendship Scale (Hawthorne, 2006). This 6-item scale was developed by Hawthorne (2006) in an aging population and has been widely used to assess social isolation. Each item is scored on a 0–4 ordered response scale and the items are summed to create a total scale score. Higher scores indicate greater social connectedness, and lower scores indicate greater social isolation. Example items include “it has been easy for me to relate to others” and “I felt isolated from other people.” This scale has a reported reliability co-efficient of 0.83. We found a reliability coefficient of 0.72 in this study.
HIV stigma was assessed using the HIV Stigma Scale. This 13-item scale was developed by Sowell (1997) and revised by Emlet to measure differences in HIV stigma between older and younger adults (Emlet, 2005, 2006a). Each item is scored on a 1 (Not at all)-4 (Often) ordered response scale and the scores from all 13 items are summed to create a total scale score. Higher scores indicate more perceived stigma. Example items include: “I felt blamed for my illness” and “I felt people avoided me because of my illness”. This scale has a reported reliability coefficient of 0.83, and in this study, we found a reliability of 0.90.
Statistical Analysis
All data were directly imported from the web-based data collection system, REDCAP, into a data management program (Harris et al., 2009). All variables were summarized using appropriate descriptive statistics. Univariate comparisons between males and females were made using two-sample t-tests. Our primary hypotheses were tested using multivariable regression models. Separate models were fit for each of the three stress outcomes (Perceived Stress Scale, Visual Analog Scale, heart rate variability) and for isolation (Hawthorne Friendship Scale). Potential covariates were chosen a priori and were consistent with previous literature.
RESULTS
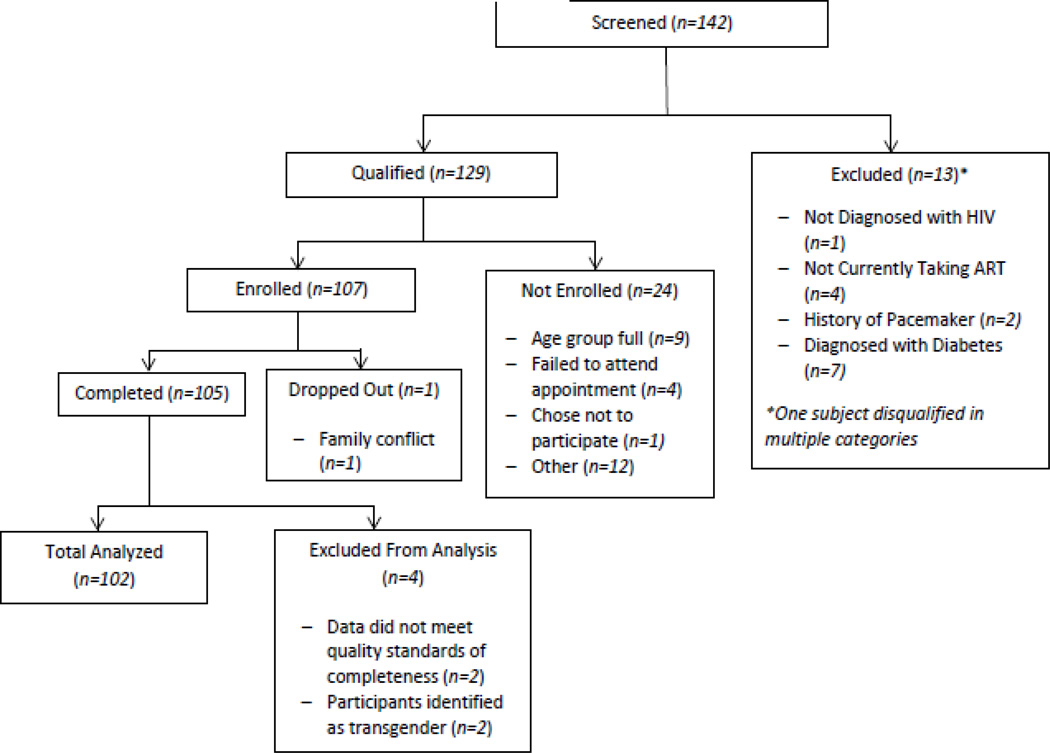
One hundred and seven participants enrolled in the study. Two participants were later found to be transgender and were removed from the primary analyses because the study aimed to compare differences between gender and age groups. Two transgender participants did not provide a large enough sample to make statistically valid claims for this group. Three participants were excluded because they were enrolled twice or dropped out before completion of the study procedures. Participant flow is described in Figure 1. Among the final sample of 102 participants, 54 were male, and 52 were over 50 years old. The mean age of all participants was 48 years, and most were African-American (83%). On average, participants had been living with HIV for 13.5 years. Mean duration of antiretroviral therapy was 11 years (range 1–27 years), and 78% of participants had undetectable viral load. Eighty percent had a co-occurring health condition, and 21% had been admitted to the emergency department in the past 12 months. Additional baseline characteristics are compared in Table 1.
Figure 1.
Recruitment and Enrollment
Table 1.
Baseline Characteristics of the Participants
| Male (n=54) | Female (n=48) | Total (n=102) | ||
|---|---|---|---|---|
| Age (years) | 47 (8.6) | 48 (9.0) | 48 (8.7) | |
| Race | ||||
| African American/Black | 42 (81%) | 43 (88%) | 85 (84%) | |
| Hispanic/Latina | 0 (0%) | 2 (4%) | 2 (2%) | |
| Native American/Indian | 1 (2%) | 0 (0%) | 1 (1%) | |
| White/Anglo (non-Hispanic) | 7 (13%) | 3 (6%) | 10 (10%) | |
| Other | 2 (4%) | 1(2%) | 3 (3%) | |
| Marital Status | ||||
| Married | 2 (4%) | 7 (14%) | 9 (9%) | |
| Single | 41 (79%) | 29 (59%) | 70 (69%) | |
| Separated | 1 (2%) | 1 (2%) | 2 (2%) | |
| Divorced | 3 (6%) | 10 (20%) | 13 (13%) | |
| Domestic Partnership | 5 (10%) | 0 (0%) | 5 (5%) | |
| Other | 0 (0%) | 2 (4%) | 2 (2%) | |
| Education Level | ||||
| 11th grade or less | 8 (15%) | 16 (33%) | 26 (26%) | |
| High school or General Equivalency Degree | 16 (31%) | 12 (25%) | 28 (28%) | |
| Some college or Associates Degree | 17 (33%) | 16 (33%) | 33 (33%) | |
| College degree (BS, BA, MS, or MA) | 12 (23%) | 4 (8%) | 16 (16%) | |
| Income | ||||
| No monthly income | 9 (17%) | 8 (16%) | 17 (17%) | |
| Less than $200 | 6 (12%) | 1 (2%) | 7 (7%) | |
| $200–$599 | 3 (6%) | 6 (12%) | 9 (9%) | |
| $600–$799 | 17 (33%) | 23 (47%) | 40 (40%) | |
| $800–$999 | 9 (17%) | 2 (4%) | 11 (11%) | |
| $1,000 or more | 9 (17%) | 8 (16%) | 17 (17%) | |
| Have Health Insurance | 47 (90%) | 48 (98%) | 95 (94%) | |
| Type of Health Insurance | ||||
| Medicaid | 21 (40%) | 30 (61%) | 51 (51%) | |
| Medicare | 15 (29%) | 13 (27%) | 28 (28%) | |
| Veteran's Benefits | 4 (8%) | 0 (0%) | 4 (4%) | |
| Private, provided by work | 2 (4%) | 2 (4%) | 4 (4%) | |
| Ryan White Care Act | 16 (31%) | 15 (31%) | 31 (31%) | |
| Have Children (yes) | 15 (29%) | 41 (84%) | 56 (56%) | |
| Mean Number of Children | 0.93 (0.88) | 0.8 (0.98) | 0.84 (0.95) | |
| Paid Employment | 6 (12%) | 8 (16%) | 14 (14%) | |
| Permanent Housing | 46 (88%) | 45 (92%) | 91 (91%) | |
| Medical History | ||||
| Years Living with HIV | 14.1 (6.4) | 13.21 (7%) | 13.57 (7%) | |
| Years since initiation of antiretroviral treatment | 11 (5.0) | 9 (4.9) | 10 (4.9) | |
| Recent CD4 T-cell count (cells/µL) | 534 (322) | 679 (385) | 602 (358) | |
| Undetectable HIV Viral Load | 40 (77%) | 40 (83%) | 80 (78%) | |
| One or more non-AIDS co-morbidities | 45 (83%) | 36 (75%) | 81 (80% | |
| Visit to the Emergency Department in the past 12 months | 9 (16%) | 12 (25%) | 21 (21%) | |
| Hospitalization in the past 12 months | 3 (5.5) | 7 (15%) | 10 (10%) | |
Data presented as mean (standard deviation) or frequency (%). Education, having health insurance, Medicaid, veterans benefits and having children statistically differed (<0.05) between men and women at baseline. Education level, income, and Medicare statistically differed (<0.05) by age at baseline.
The mean (SD) Perceived Stress Scale score was 17.4 (6.9), mean heart rate variability (SDNN) was 65.5 (31.2) msec, and mean visual analog stress scale score was 3.5 (2.8). The correlation between the Perceived Stress Scale and single item visual analog scale was 0.19 (p=0.05); between the Perceived Stress Scale and heart rate variability was 0.03 (p=0.77); and between the visual analog scale and heart rate variability was 0.16 (p=0.13). Overall, the mean Hawthorne Friendship Scale score was 17.0 (4.8) and was similar in women compared to men [17.3 (4.6) vs. 16.8 (5.0) respectively, p=0.66]. Mean HIV stigma score was 22.6 (8.9) and was also similar in women compared to men [22.6 (7.7) vs. 22.6 (9.9) respectively, p=0.99]. Perceived stress varied (p=0.06) between older participants and younger participants. Older participants had a mean score of 23.6 (6.8) and younger participants had a mean score of 21.0 (6.9). Heart rate variability significantly (p=0.02) differed by age of the participants with older participants having less heart rate variability than younger participants. Additional descriptive information on the outcomes can be found in Tables 2 and 3.
Table 2.
Descriptive Statistics of Stress, Isolation, and Stigma by Gender
| Male (n=54) | Female (n=48) | Total (n=102) | ||||||
|---|---|---|---|---|---|---|---|---|
| Scale | Possible Range |
Mean | Std | Mean | Std | Mean | Std | p- value |
| Perceived Stress Scale | 0–40 | 17.0 | 6.8 | 17.8 | 7.2 | 17.4 | 7.0 | 0.58 |
| Heart Rate Variability (SDNN, msec) | N/A | 64.9 | 31.0 | 66.1 | 31.7 | 65.5 | 31.2 | 0.85 |
| Visual Analog Stress Scale | 1–10 | 3.1 | 2.8 | 4.0 | 2.7 | 3.5 | 2.8 | 0.14 |
| Hawthorne Friendship Scale (Isolation) | 0–24 | 16.8 | 5.0 | 17.3 | 4.6 | 17.0 | 4.8 | 0.66 |
| HIV Stigma Scale | 13–52 | 22.6 | 9.9 | 22.6 | 7.7 | 22.6 | 8.9 | 0.99 |
SDNN, Standard deviation of N-N intervals
Table 3.
Descriptive Statistics of Stress, Isolation, and Stigma by Age
| ≤50 (n=49) | >49 (n=52) | |||||
|---|---|---|---|---|---|---|
| Scale | Possible Range |
Mean | Std | Mean | Std | p-value |
| Perceived Stress Scale | 0–40 | 21.0 | 6.9 | 23.6 | 6.8 | 0.06 |
| Heart Rate Variability (SDNN, msec) | N/A | 72.9 | 32.9 | 58.5 | 28.0 | 0.02 |
| Visual Analog Stress Scale | 1–10 | 3.3 | 3.05 | 3.72 | 2.53 | 0.46 |
| Hawthorne Friendship Scale (Isolation) | 0–24 | 16.5 | 5.2 | 17.5 | 4.5 | 0.33 |
| HIV Stigma Scale | 13–52 | 24.0 | 9.0 | 21.3 | 8.6 | 0.14 |
In a multivariable regression model that controlled for additional covariates of interest (Table 4) there was no relationship between the Perceived Stress Scale and either age (p=0.23) or gender (p=0.93). Female gender was associated with a 1.2 point increase in stress as measured by the visual analog scale, relative to males (p=0.05). However, age was not associated with stress, as measured by the visual analog scale (p=0.11). Age was associated with a 1.3 msec decrease in heart rate variability (p<0.01), although gender was not (p=0.50). Similarly, a 1-year increase in age predicted a 0.1 point decrease in friendship, (p=0.02); but gender did not (p=0.7). There were no observed interactions between age and gender for all four outcomes modeled (all p >0.49).
Table 4.
Multivariable Models of the Predictors of Stress and Social Isolation
| Model 1: Perceived Stress Scale |
Model 2: Stress Visual Analog Scale |
Model 3: Heart Rate Variability |
Model 4: Hawthorne Friendship Scale |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate (CI) | P Value |
Estimate (CI) | P Value |
Estimate (CI) |
P Value |
Estimate (CI) |
P Value |
|
| Age (years) | −0.09 (−0.24, 0.06) | 0.23 | −0.05 (−0.12, 0.01) | 0.11 | −1.3 (−2.1 − 0.6) | <0.01 | 0.11 (0.02, 0.20) | 0.02 |
| Gender: Female | −0.12 (−2.39, 2.69) | 0.93 | 1.24 (−0.01, 2.48) | 0.05 | 4.6 (−8.8, 18.0) | 0.50 | 0.32 (−1.41, 2.05) | 0.71 |
| BMI | 0.05 (−0.12, 0.22) | 0.55 | 0.01 (−0.07, 0.09) | 0.76 | −0.9 (−1.7, −0.1) | 0.03 | 0.00 (−0.10, 0.11) | 0.97 |
| Income >$800/month | −0.04 (−2.93, 3.01) | 0.98 | 0.41 (−0.92, 1.73) | 0.54 | 11.0 (−3.1, 25.2) | 0.14 | −0.09 (−1.92, 1.75) | 0.35 |
| Education≥ High School | −2.14 (−5.21, 0.93) | 0.17 | 0.06 (−1.30, 1.42) | 0.93 | −14.4 (−28.9, 0.2) | 0.05 | 0.89 (−0.98, 2.77) | 0.35 |
| Years since HIV Diagnosis | 0.05 (−0.17, 0.26) | 0.65 | 0.05 (−0.04, 0.15) | 0.29 | −0.30 (−0.7, 1.1) | 0.53 | −0.09 (−0.22, 0.04) | 0.19 |
| Friendship (Isolation) | −0.34 (−0.68, 0.00) | 0.05 | −0.05 (−0.20, 0.10) | 0.55 | 0.50 (−1.1, 2.1) | 0.57 | ---- | ----- |
| Stigma | 0.19 (0.00, 0.39) | 0.05 | 0.03 (−0.04, 0.15) | 0.29 | 0.20 (−0.7 −1.1) | 0.67 | −0.34 (−0.43, −0.25) | <0.01 |
Additional statistically significant or borderline predictors of heart rate variability were BMI and education level (Table 4, model 3); however, psychosocial factors such as HIV stigma and social isolation were not related to heart rate variability as measured in this study (both p >0.5). In contrast, HIV stigma was associated with both perceived stress and social isolation (Table 4, models 1 and 4). Social isolation also was associated with perceived stress independent of HIV stigma and other covariates (Table 4, model 1).
CONCLUSIONS
To the best of our knowledge, this is the first study designed to quantitatively assess the relationship between age, stress, and social isolation in adults living with HIV—a priority area defined by the NIH Office of AIDS Research HIV and Aging Working Group (High, et al., 2012). Although Perceived Stress Scale scores did not differ by age or gender, our study suggests that people living with HIV have an overall higher level of stress compared to national normative data. In a large, national study (N=2,387), the average (standard deviation) perceived stress for men was 12.1 (5.9) and for women it was 13.7 (6.6) (Cohen & Williamson, 1988). In our study, the mean perceived stress score was about 4–5 points (30–40%) higher on average Similar to the trend described by Cohen et al., older participants in our study tended to report less perceived stress. Several recent studies using the same 10-item Perceived Stress Scale have also described high levels of stress among adults living with HIV. A recent analysis of 148 PLWH found a mean score of 20.40 (6.96) (Johnson, 2012). The mean age of this sample was 45 years and over 80% were male. A study of 60 adult women living with HIV found a mean score of 30.5 (6.1), indicating very high perceived stress (Brown, Vanable, Carey, & Elin, 2011). The average age of this female sample was also 45 years. This increased stress may be related to the women’s unique social roles, increased poverty, decreased power in relationships, and increased mental health difficulties (Brown, et al., 2011). Our study supports this existing evidence that PLWH experience higher levels of stress than those not infected with HIV and that women living with HIV may experience more stress than men. Additionally, younger adults living with HIV may experience more stress than those who are older, which suggests that interventions targeting this younger group should include strategies to help minimize stress.
We also found that our participants experienced more social isolation, on average, compared to national norms and that younger PLWH were significantly more isolated than older PLWH. On average, our participants experienced significant amounts of isolation with a total mean score of 17.0 (4.8) that differed minimally by gender. These absolute scores are lower than national norms. For example, during the development of the Hawthorne Friendship scale in over 3,000 participants, 59% were very or highly socially connected with total scores ranging from 22–24 (Hawthorne, 2006), whereas only 20% of subjects in our study had scores in that range. Interestingly, we found that younger PLWH were significantly more isolated than older PLWH, a phenomenon that was also seen in HIV-uninfected populations using this scale (Hawthorne, 2006). These findings are inconsistent with conflicting data which suggests that older PLWH have “limited and fragile friend-centered social networks” (High, et al., 2012). Rather than focus exclusively on older PLWH, our data provide compelling evidence of the need to examine the different causes and consequences of increased isolation among younger PLWH. There are several possibilities for this conflicting data. First, older PLWH in this study were more experienced with HIV and over the years may have developed social networks more adapted to their health needs. Younger adults may still be struggling to accept HIV as a chronic condition and have social networks less suitable to their health needs. Second, while PLWH are living longer, it remains uncommon to see elder PLWH. The oldest participant in this study was age 64. Supportive social networks may disappear at more extreme ages than were represented in this study. As PLWH continue to age, further studies must continue observing support networks. Until then, this study suggests interventions to decrease isolation may be best targeted at younger adults.
Stigma associated with HIV was a significant predictor of perceived stress and social isolation in our study. HIV stigma continues to be a challenge for PLWH across their lifespan (Mahajan et al., 2008), but little work has focused on the effects of HIV as PLWH age. In one study, Grov, et al. (2010) found that HIV-associated stigma, increased loneliness, decreased cognitive functioning, and reduced levels of energy were significantly associated with depression in older adults living with HIV. These authors suggest that interventions focused on reducing stigma and isolation could have lasting effects on perceived mental and physical health in this population (Grov, Golub, Parsons, Brennan, & Karpiak, 2010).
Unfortunately, the existing literature provides little guidance on how to tailor individual, social, and structural stigma-reduction interventions to PLWH across the lifespan. Our study highlights the interconnectedness between HIV stigma, stress, isolation, and age in PLWH. We therefore suggest that a comprehensive, tailored, and multi-level approach may be best in dealing with these complex issues (Latkin, Weeks, Glasman, Galletly, & Albarracin, 2010; Mahajan, et al., 2008; Sengupta, Banks, Jonas, Miles, & Smith, 2011).
There are several limitations to this study. First, this was a single-site, cross-sectional study. PLWH in different geographic areas may have different experiences with stress, isolation, and stigma than those who participated in our study. We attempted to overcome this limitation with widely-used and validated measurements, thus facilitating comparison with studies using other populations of PLWH. Second, while our study provides a baseline understanding of the relationship age and stress and social isolation, further longitudinal studies are needed to understand the complex causes of stress and social isolation in PLWH of all ages. Third, we did not ask about age-related discrimination which may add to the effects of HIV-related stigma in this population. Fourth, the sample population in this study was predominantly African American which may limit the generalizability of study findings to more diverse populations or other ethnic minorities.
There is much to learn about the relationship between age, stress, and isolation in adults living with HIV. However, this study provides important contributions to this knowledge base. Given the increase in the prevalence of older adults living with HIV, it is important to better understand this relationship in order to develop interventions that will help PLWH overcome the barriers of stress and social isolation to truly live well with the disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of the women and men who participated in this study and Jan E Hanson and Lauren Starks. The project described was supported by the National Institute for Allergy and Infectious Disease through Grant P30AI36219 and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants 5KL2RR024990 and KL2TR000440. The contents of this article are solely the views of the authors and do not represent the official views of the National Institutes of Health.
References
- Antoni MH. Stress management and psychoneuroimmunology in HIV infection. CNS Spectr. 2003;8(1):40–51. doi: 10.1017/s1092852900023440. [DOI] [PubMed] [Google Scholar]
- Brown JL, Vanable PA, Carey MP, Elin L. Computerized stress management training for HIV+ women: a pilot intervention study. AIDS Care. 2011;23(12):1525–1532. doi: 10.1080/09540121.2011.569699. [DOI] [PubMed] [Google Scholar]
- Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3(4):151–160. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- Chu C, Umanski G, Blank A, Meissner P, Grossberg R, PA S. Comorbidity-Related Treatment Outcomes among HIV-Infected Adults in the Bronx, NY. Journal of Urban Health. 2011 doi: 10.1007/s11524-010-9540-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clays E, De Bacquer D, Crasset V, Kittel F, de Smet P, Kornitzer M, De Backer G. The perception of work stressors is related to reduced parasympathetic activity. International Archives Of Occupational And Environmental Health. 2011;84(2):185–191. doi: 10.1007/s00420-010-0537-z. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. [Research Support, U.S. Gov't, Non-P.H.S.] Journal of health and social behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived Stress in a Probablity Sample of the United States. In: Spacpan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Effros RB, Fletcher C, Gebo K, Halter J, Hazzard W, Horne F, High K. Aging and Infectious Diseases: Workshop on HIV Infection and Aging: What Is Known and Future Research Directions. Clinical Infectious Diseases. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlet CA. Measuring Stigma in Older and Younger Adults with HIV/AIDS: An anlysis of an HIV Stigma Scale and Initial Exploration of Subscalse. Research on Social Work Practice. 2005;15(4):291–300. [Google Scholar]
- Emlet CA. A comparison of HIV stigma and disclosure patterns between older and younger adults living with HIV/AIDS. [Comparative Study Research Support, Non-U.S. Gov't] AIDS patient care and STDs. 2006a;20(5):350–358. doi: 10.1089/apc.2006.20.350. [DOI] [PubMed] [Google Scholar]
- Emlet CA. "You're awfully old to have this disease": experiences of stigma and ageism in adults 50 years and older living with HIV/AIDS. [Research Support, N.I.H., Extramural] The Gerontologist. 2006b;46(6):781–790. doi: 10.1093/geront/46.6.781. [DOI] [PubMed] [Google Scholar]
- Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Goedert JJ. Cancer risk in people infected with human immunodeficiency virus in the United States. International Journal of Cancer. 2008;123(1):187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, Gorp WG. Functional Consequences of HIV-Associated Neuropsychological Impairment. Neuropsychology Review. 2009;19(2):186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant N, Hamer M, Steptoe A. Social isolation and stress-related cardiovascular, lipid, and cortisol responses. Ann Behav Med. 2009;37(1):29–37. doi: 10.1007/s12160-009-9081-z. [DOI] [PubMed] [Google Scholar]
- Greeson JM, Hurwitz BE, Llabre MM, Schneiderman N, Penedo FJ, Klimas NG. Psychological distress, killer lymphocytes and disease severity in HIV/AIDS. Brain Behavior and Immunity. 2008;22(6):901–911. doi: 10.1016/j.bbi.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22(5):630–639. doi: 10.1080/09540120903280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RA, Hamer M, Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology. 2012;37(11):1801–1809. doi: 10.1016/j.psyneuen.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne G. Measuring Social Isolation in Older Adults: Development and Initial Validation of the Friendship Scale. Social Indicators Research. 2006;77(3):521. [Google Scholar]
- Heaton R, Clifford D, Franklin DR, Jr, Woods S, Ake C, Vaida F, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Volberding P. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironson G, Hayward HS. Do Positive Psychosocial Factors Predict Disease Progression in HIV-1? A Review of the Evidence. Psychosom Med. 2008;70(5):546–554. doi: 10.1097/PSY.0b013e318177216c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Sevelius J, Dilworth S, Saberi P, Neilands T. Preliminary support for the construct of health care empowerment in the context of treatment for human immunodeficiency virus. Patient Preference and Adherence. 2012;6:39–404. doi: 10.2147/PPA.S30040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, McGuire L, Robles T, Glaser R. Psychological Influences on Immune Function and Health. Journal of Consulting and Clinical Psychology. 2002;70(3):537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiology of Disease. 2010;37(3):542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin C, Weeks MR, Glasman L, Galletly C, Albarracin D. A dynamic social systems model for considering structural factors in HIV prevention and detection. AIDS And Behavior. 2010;14(Suppl 2):222–238. doi: 10.1007/s10461-010-9804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EAD, Theus SA. Lower Heart Rate Variability Associated With Military Sexual Trauma Rape and Posttraumatic Stress Disorder. Biological Research For Nursing. 2012;14(4):412–418. doi: 10.1177/1099800412454453. [DOI] [PubMed] [Google Scholar]
- Lescure FX, Omland LH, Engsig FN, Roed C, Gerstoft J, Pialoux G, Obel N. Incidence and Impact on Mortality of Severe Neurocognitive Disorders in Persons With and Without HIV Infection: A Danish Nationwide Cohort Study. Clinical Infectious Diseases. 2011;52(2):235–243. doi: 10.1093/cid/ciq041. [DOI] [PubMed] [Google Scholar]
- Mahajan AP, Sayles JN, Patel VA, Remien RH, Sawires SR, Ortiz DJ, Coates TJ. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS (London, England) 2008;22(Suppl 2):S67–S79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron E, Messerotti Benvenuti S, Favretto G, Valfre C, Bonfa C, Gasparotto R, Palomba D. Association between depression and heart rate variability in patients after cardiac surgery: A pilot study. Journal of Psychosomatic Research. 2012;73(1):42–46. doi: 10.1016/j.jpsychores.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Perissinotto Cm SCICKE. Loneliness in older persons: A predictor of functional decline and death. Archives of Internal Medicine. 2012;172(14):1078–1084. doi: 10.1001/archinternmed.2012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention, Centers for Disease Control. HIV Surveillance Report, 2010. [Retrieved October 28, 2012];2010 March 2012, 2012, from http://www.cdc.gov/hiv/topics/surveillance/resources/reports.
- Scott-Sheldon LAJ, Kalichman SC, Carey MP, Fielder RL. Stress Management Interventions for HIV+ Adults. Health Psychology. 2008;27(2):129–139. doi: 10.1037/0278-6133.27.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LAJ, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: A meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychology. 2008;27(2):129–139. doi: 10.1037/0278-6133.27.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Banks B, Jonas D, Miles MS, Smith GC. HIV interventions to reduce HIV/AIDS stigma: a systematic review. AIDS And Behavior. 2011;15(6):1075–1087. doi: 10.1007/s10461-010-9847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, McMunn A, Banks J, Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011;30(4):377–385. doi: 10.1037/a0022826. 10.1037/a0022826. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Engels EA. Age at Cancer Diagnosis Among Persons With AIDS in the United States. Annals of Internal Medicine. 2010;153(7):452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, Grunfeld C. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55(3):316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Woodley RA, Burrage JR. Predictors of Cognitive Ability in Adults Aging with HIV -- A Pilot Study. Clinical Gerontologist. 2007;30(3):83–101. [Google Scholar]
- Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, Yerly S. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Medicine. 2012 doi: 10.1111/j.1468-1293.2012.01051.x. n/a-n/a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.